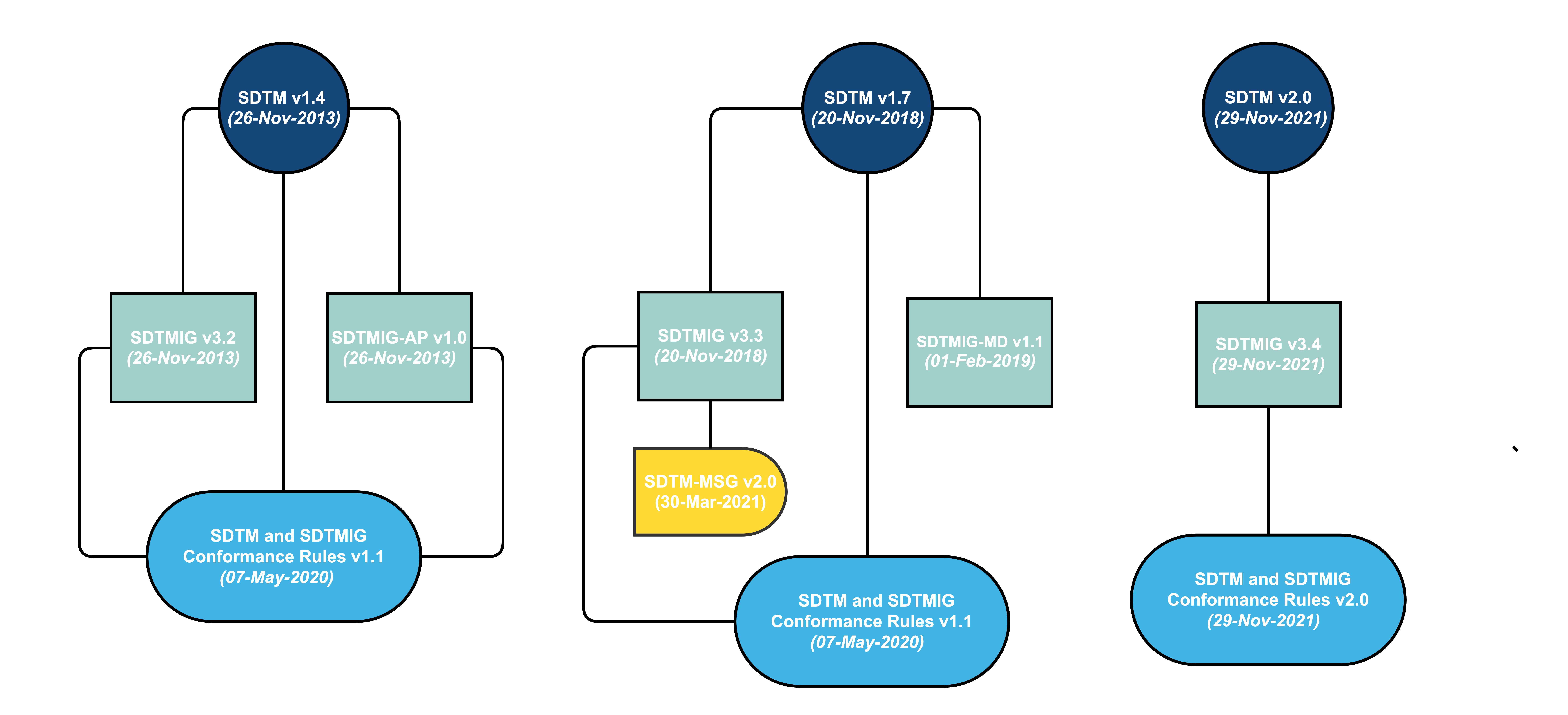
A Study Data Tabulation Model Implementation Guide (SDTMIG) is developed in reference to a specific SDTM model. However, the SDTM is cumulative – each new release builds on the previous model. Therefore, the models are backward compatible. For example, SDTMIG-AP v1.0 was developed in reference to SDTM v1.4, but it may be used in a submission that uses SDTM v1.7.
Implementers should be aware that if they are referencing a model for which the IG was not originally developed, variables may have been added or deprecated from the model. In addition to models and implementation guides, conformance rules have been developed, which help to ensure that generated data structures conform to the standards. These rules aim to identify all conformance rules and case logic from the SDTM and SDTMIG, classifying and codifying them in a form that supports quality processes and tool development.
Articles
Examples
Public Review - SDTMIG
SDTMIG: The Study Data Tabulation Model Implementation Guide (SDTMIG) for human clinical trials guides users on the organization, structure, and format of standard clinical study tabulation datasets for interchange between organizations or to be submitted to a regulatory authority. The following videos introduce you to the SDTMIG and the SDTM, which used together serve as a map that orients you on how your data fits into the standard.
Coordinate across standards teams and within SDS. Refer to guiding priniciples, published models, implementation guides (IGs), and therapeutic area user guides (TAUGs) when debating how to model data.
Decision consistency during consultation and development leads to the production of higher quality products. Inconsistent decision making leads to incorrect usage or inconsistent use of standards. This, in turn, leads to confusion within the user community.
- Ensures consistency across IGs, conformance rules, and TAUGs
- Facilitates alignment of decisions made by different foundation and therapeutic area teams
Follow published rules; be mindful of precedent setting.
Provide timely support for developing new projects and when teams approach SDS for consultation.
Timely support during consultation and development leads to the production of higher quality products.
Allows predictability and alignment with project timelines and publications.
- Be accountable to complete support steps for given projects and consultation.
- Read pre-reads as available for full-team meeting.
- Bear in mind time provided for a topic of discussion.
- Determine short- and long-term solutions as needed.
- Form subteams as needed to address issues, but set goals and time limits for the decisions to be made.
Coordinate across standards teams and within SDS. Be mindful of the impact of changes to the SDTM and SDTMIG and other CDISC standards.
Changes and updates may affect the work of implementers. Significant alterations to the SDTM and SDTMIG used by multiple user communities should be communicated to all stakeholders.
- Decreases the amount of changes the user community has to implement
- Decreases the cost in time and money of versioning
- Decreases the introduction of errors within company standards
- Minimizes unnecessary or unproductive changes
- Bear in mind the processes in place for requesting new domains or changes to the standard.
- Refer to the SDS Decision Tree for the appropriate steps to follow when considering new activities (e.g, an idea, a new project or proposal).
- Change requesters should always check the most recent model, IG, and non-standard variable registry before submitting change requests.
- Change requesters should check with the appropriate foundational teams to determine impact. Feedback should be solicited from all foundational teams.