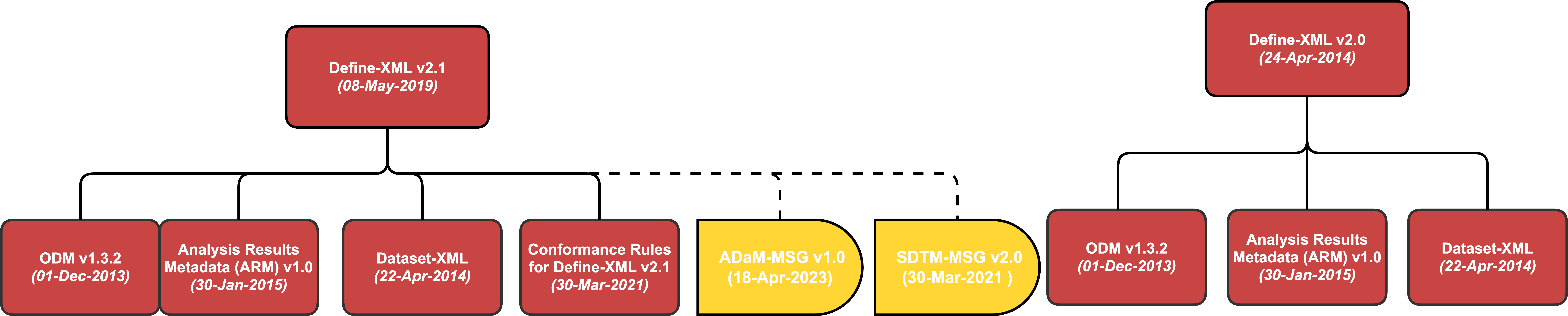
Define-XML Courses
Articles
Supporting automation for implementation of CDISC Foundational standards is a fundamental requirement for Data Exchange Standards development.
From the beginning, the Data Exchange Standards have been harmonized with CDISC Foundational standards.
Reduces cost and improves efficiency for CDISC implementers.
Data Exchanges Standards should reuse XML components from the ODM and other CDISC extensions when usage is aligned with the semantics.
The ODM has already been aligned with BRIDG.
Parsimonious models are more efficient.
The Data Exchange Standards are developed following an agile development process.
Agile is widely recognized as a software development best practice.
- New versions are more frequent and with fewer changes.
- Effort for adopting a new version will be less.
- New versions reflect most urgent needs identified by implementers.
Define as much of the standard through the XML Schema as possible.
Leverage XML technology as much as possible. This may include related technologies like Json.
Enables implementers to leverage widely available low-cost tools and encourages collaboration with organizations working with other health care/biomedical science standards.
Ensure our Data Exchange Standards are clearly documented.
Many programmers will need to implement XML Technology standards without attending a training class.
The quality of documents following the Data Exchange Standards will be consistent. This is important for encouraging new implementations.