
CDISC 360i is transforming clinical research by digitalizing study design through analysis making metadata interoperable across the entire study lifecycle.
With connected standards and end-to-end automation, 360i eliminates manual tasks, enables AI, ensures traceability and consistency, and empowers the industry to deliver results faster, with better quality, and at a lower cost accelerating the delivery of new therapies to patients.
360i Progress Report: 2025 Achievements
At the US Interchange in October 2025, we shared our vision for seamlessly integrating CDISC Standards into platforms to pave the way for true automation. With support from PwC, CDISC developed a “clickable prototype,” a wireframe for a potential software tool to help visualize the future of end-to-end standards.
To illustrate this concept, we used an Oncology Study as an example, connecting the prototype to the CDISC Library with standardized, digitized assets from the Breast Cancer Therapeutic Area User Guide (TAUG). We demonstrated how users can start their study by easily selecting endpoints, then quickly moving on to configure study details. Biomedical concepts link directly to the Schedule of Activities, making it easy to edit, add, or import information from other studies. Next, users move on to configure analyses, preview outputs, review datasets, and even interact with a traceability map. Throughout the process, AI works behind the scenes to verify choices in real time, ensuring accuracy while keeping the “human in the loop.”
The prototype illustrates what becomes possible with just a few clicks when CDISC Standards are implemented as linked metadata, harnessing automation, and increasing efficiency. By delivering a visual representation of what is achievable, we delivered a touchstone for a fully connected future.
In 2025, together with our global community, we launched and advanced the 360i vision through the tactical work of the 360i Operational Team, the “Art of the Possible” prototype, an AI Innovation Challenge, and our Technology Vendor Roundtable.
The 360i Initiative officially launched in March 2025 and quickly gained momentum. The 360i Team fostered extensive industry collaboration, bringing together over 80 individuals across several project teams (Design, Build, and Run, along with an Operational Steering Committee, Reviewers, and Parallel groups to work on key outcomes, including CDISC open rules, biomedical concepts, and analysis concepts).
The 360i Team set out to transform the clinical research process into a dynamic, non-linear model - one where steps and phases can run in parallel rather than in sequence, and data flows seamlessly from one stage to the next. Along the way, we gathered valuable lessons. Where did we make progress? What insights did we gain? And, how far have we come?
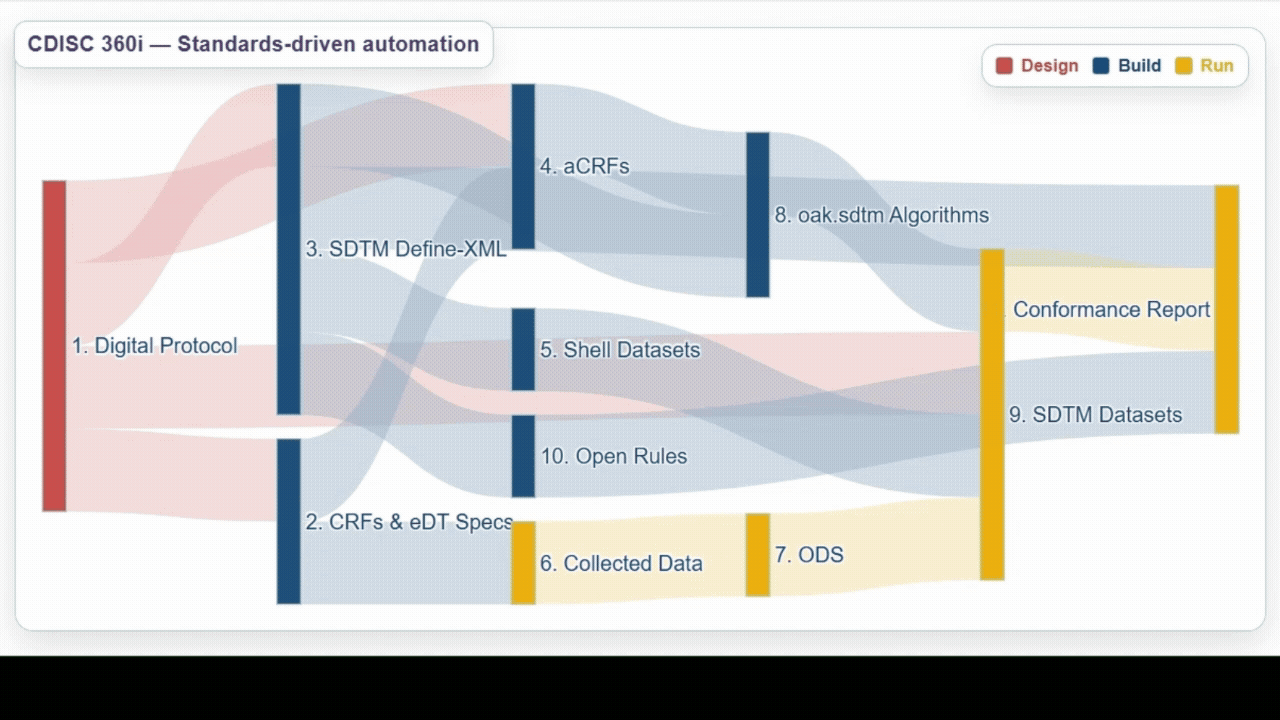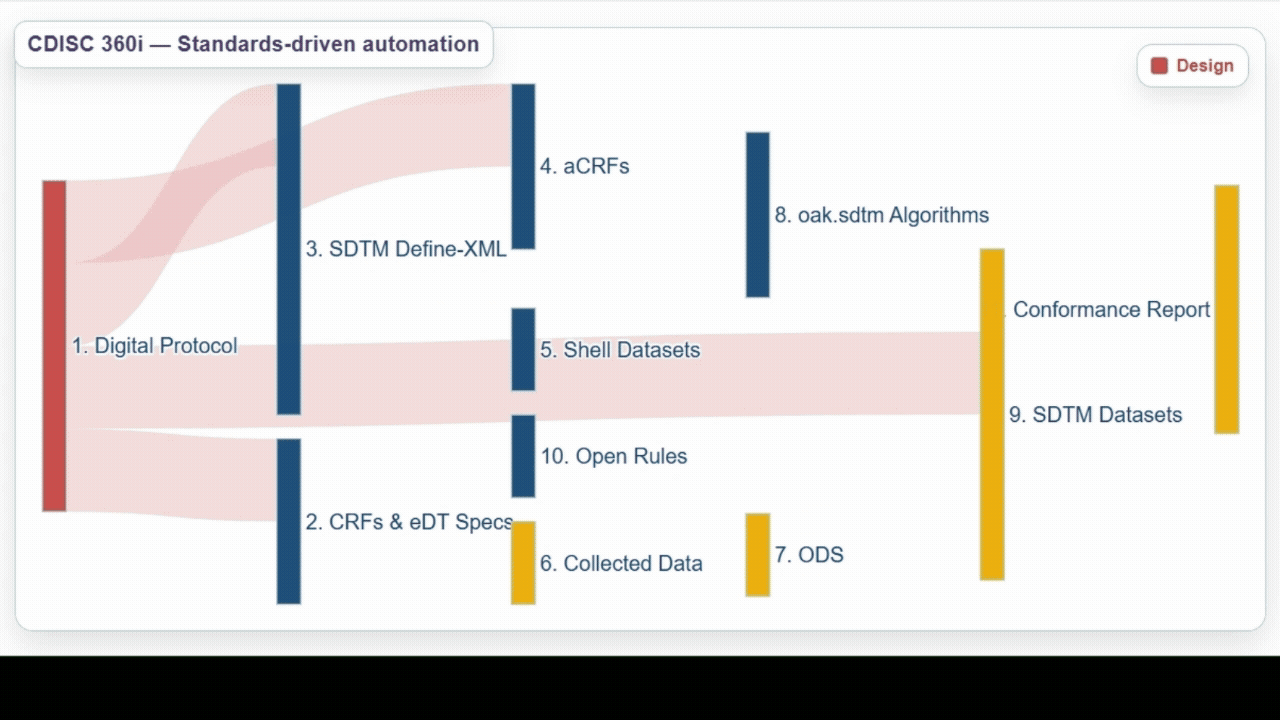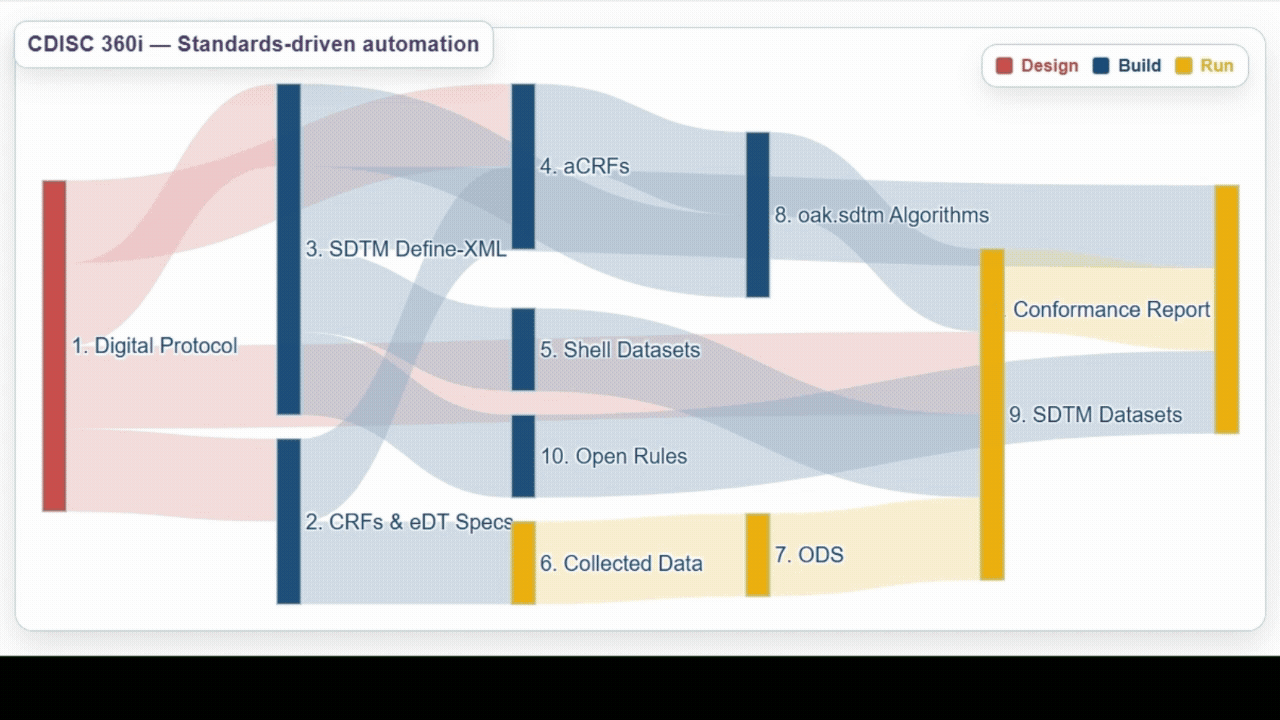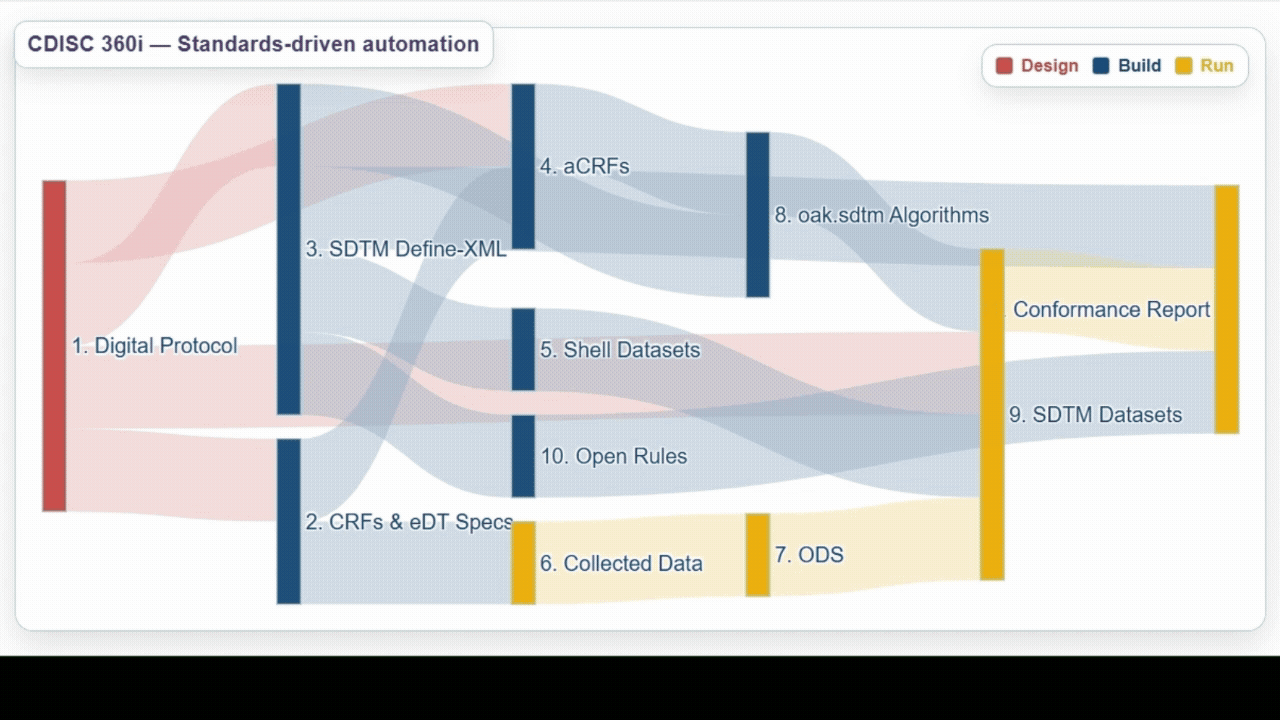
| Team | Achievements | Lesson Learned |
Design | Developed several detailed user stories spanning from study design through study amendment - an important step toward aligning roles across our industry.
From these user stories and through the use of open source tools and utilities, generated a USDM-JSON 4.0 file with associated biomedical concepts.
This confirmed that standardizing study definitions linked to biomedical concepts is highly effective, and provides clear evidence of how these connections enable downstream use cases. |
|
Build | Successfully utilized biomedical concepts linked to USDM to generate eCRF specifications, ODM.xml, SDTM annotated CRFs (aCRFs), SDTM dataset specializations, SDTM trial design domains, define.xml, and SDTM shell data sets. |
|
Run | Demonstrated ability to process data from a wide range of sources and automate generation and validation of SDTM datasets, as well as exploring the use of AI/ML to help support development. |
|
The 360i Team produced a series of video demonstrations of key 360i deliverables showing how this really works!
The full end-to-end automation was pulled together using a Google Collab notebook that is available in GitHub: CDISC 360i Notebook
The GitHub repositories used in the videos are public and use open-source libraries
The repository is under continued development; follow repository for latest releases
There are multiple notebooks available within the repository
Raise any issues encountered while using notebooks within the repository
The process for using these libraries is consistent:
Fork the original public repository into a personal GitHub repository
Create new branch in your personal GitHub repository
Edit code to meet individual requirements, add features, or fix issues
Commit changes to personal branch
If proposing change to parent (original) branch to share with wider community:
Raise a Pull Request with original repository owner
Original repository owner will review the Pull Request and work with requestor to evaluate and test Pull Request
Merge new code in Pull Request with original parent branch
The 2025 CDISC AI Innovation Challenge, a global contest launched in June 2025, directly supported the 360i initiative by serving as a catalyst for its vision of an automated, modern clinical research lifecycle. Accelerating the digitization and automation of clinical research using AI, Machine Learning, and CDISC Standards, the Challenge focused on three targeted AI/ML use cases to advance the digitization and automation of clinical research using CDISC standards.
Use Case #1
Protocol Library: Participants created a USDM-centric repository by extracting legacy protocol content using AI/ML, with thirteen submissions making it the most popular use case.
Winner: Faro’s AI-powered protocol digitization solution transforms static clinical study protocols into structured, reusable data that drives efficiency across the clinical development lifecycle.
Runner Up: Zifo’s solution transforms protocols from static documents into reusable, machine-readable assets - accelerating study design, standardization, and reuse.
Use Case #2
BC Acceleration: This challenge showcased AI/ML-driven approaches to accelerate the development and curation of Biomedical Concepts, receiving five strong submissions.
Winner: The Smart BC suite, submitted by Saama, is a sophisticated framework designed to extract, standardize, and link Biomedical Concepts from clinical documents using an advanced AI-driven approach.
Runner Up: Lindus Healths’ BC Registry Framework registers new BCs, checks against local registries and the CDISC Library for matches, then falls back to an advanced NCIT ontology search.
Use Case #3
Automated Traceability: Participants demonstrated semantic traceability from statistical analyses back to source data using CDISC standards, resulting in four compelling submissions for this complex use case.
Winner: Merck's solution is an open-source traceability engine that automatically pulls together study files like protocols, CRFs, SDTM, ADaM, and TLFs. The engine then builds one clear “lineage graph” that shows how results, variables, and endpoints connect back from where they came
Runner Up: Zifo establishes end-to-end traceability across trial artifacts by applying AI-driven metadata extraction and CDISC Standards, enabling dependency queries and impact analysis from study design through statistical outputs.
The response to the 2025 CDISC AI Innovation Challenge underscored growing momentum and creativity surrounding the use of AI and ML in clinical research. Participants from across the globe demonstrated technical excellence, forward thinking, and a shared commitment to advancing standards-driven innovation for the benefit of the entire research ecosystem.
CDISC AI Innovation Challenge: | CDISC AI Innovation Challenge: | CDISC AI Innovation Challenge: |
As we kick-off Phase 2 in 2026, The AI Innovation Challenge will be exploring new use cases, which will be shared in early to mid year.
This collaborative group brings together leading technology vendors from the CDISC member community to accelerate the development and adoption of CDISC Standards, advancing the 360i vision for a connected and modern clinical research lifecycle. By fostering innovation and interoperability, the roundtable ensures that CDISC standards remain practical, scalable, and aligned with emerging digital workflows.
Throughout 2025, the roundtable convened multiple times, virtually and in person at the US Interchange in Nashville, creating a forum for dialogue, problem-solving, and strategic alignment.
These sessions play a pivotal role in shaping the future of clinical trials by enabling vendors and developers to build tools that integrate seamlessly with CDISC’s structured standards metadata and digital protocol models. The outcome is a stronger ecosystem of solutions designed to support automation, improve data quality, and reduce inefficiencies across the research continuum.
To join the member roundtable, please complete this form.
360i Resources
Upcoming Webinars
Pagination
- Page 1
- Next page
360i Webinar Archive
Upcoming Events
