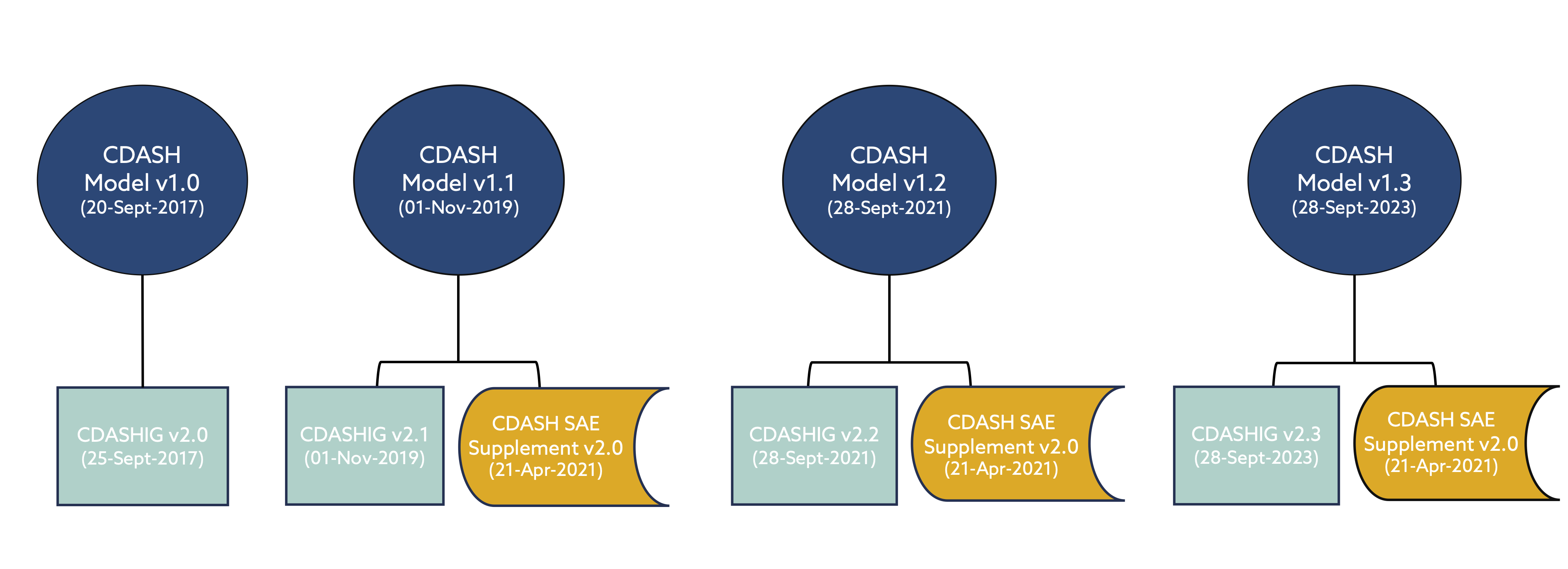
The latest versions of the Clinical Data Acquisition Standards Harmonization Implementation Guides (CDASHIGs) have been developed in reference to a specific CDASH model. However, the CDASH model is cumulative – each new release builds on the previous model. Therefore, the models are considered backward compatible.
CDASH Courses
Articles
Examples
CDASH: The Clinical Data Acquisition Standards Harmonization Implementation Guide (CDASHIG) establishes a standard way to collect data consistently across studies and sponsors so that data collection formats and structures provide clear traceability of submission data into the SDTM, delivering more transparency to regulators and others who conduct data review. The following videos introduce you to the CDASH model and the CDASHIG.
CDASHIG metadata will be developed using the same Observation Classes and Domains as SDTMIG.
Using the same domain names for collection and tabulation builds in high-level traceability and gives implementers the opportunity to automate some SDTMIG dataset creation.
Regulatory reviewers appreciate traceability. Implementers appreciate efficiency and the ability to automate.
When concepts are related but not identical, or when values are not identical (except for case), the names will be similar and shall indicate a degree of relatedness. For example, substance use duration may be collected using the CDASHIG variables SUCDUR and SUCDURU with the respective values of "5" and "YEARS", but submitted as SUDUR = "P5Y".
Using the same variable name for data collection and data tabulation allows implementers to directly populate identical collection and tabulation variables.
Efficiency and clear traceability.
When developing the standard, consider the needs of all stakeholders (e.g., clinical team members, sites, clinical data managers, SDTM programmers, statisticians and statistical programmers) and balance the needs of each.
CDISC develops standards that benefit all stakeholders. Decisions made in developing CDASH must weigh the cost-benefit ratio for all stakeholders.
- Optimizing CDASH for all stakeholders supports wider adoption.
- Weighing all viewpoints helps to respond to inquiries from stakeholder representatives during public review and beyond.
CDASH will share value lists with SDTM controlled terminology codelists, using the CDISC Submission Values as much as possible, or synonyms where the CDISC Submission Values are not practical for data collection.
Using the same (or synonymous) values for data collection and submission results in greater transparency, traceability, and efficiency.
Efficiency and clear traceability.
CDASH creates standardized questions that match the CDASH definition of the variable.
The basis for semantic interoperability is that every time we use the variable/question it means the same thing.
Semantic interoperability allows us to combine data across studies and get a meaningful result, and also makes data useful outside the context of a single study.