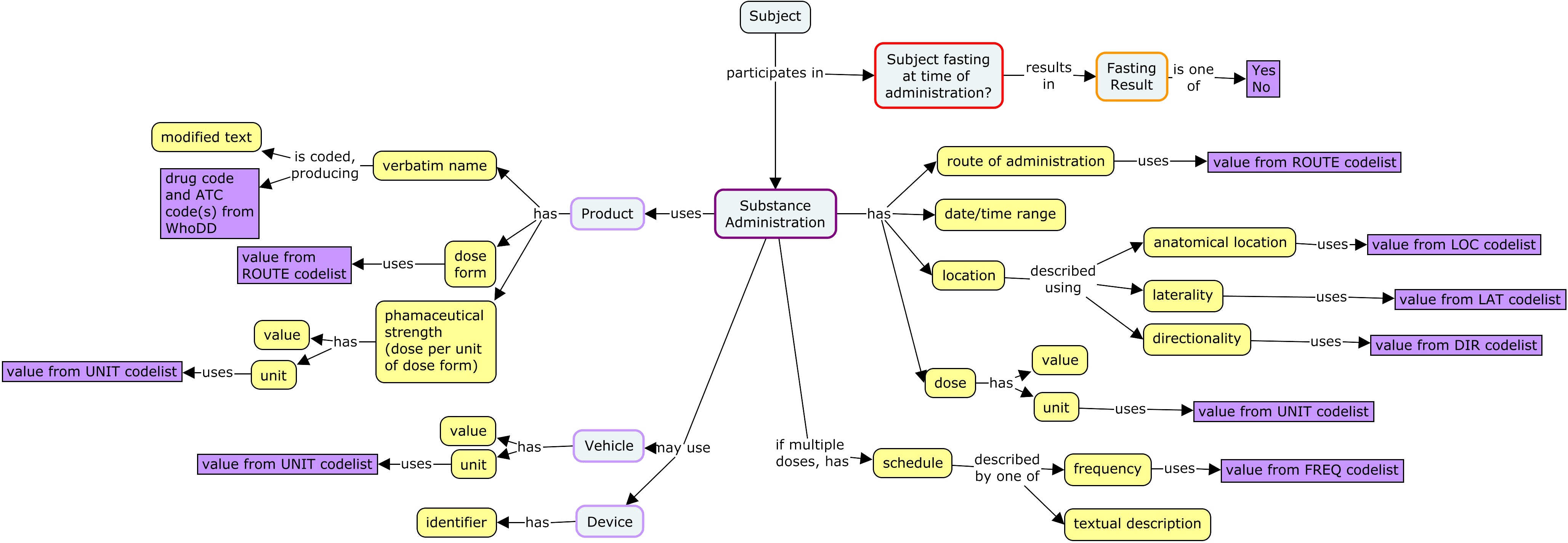
In some situations, there is a need to describe the vehicle in which the dose is delivered. Sometimes a device used in administration may be recorded. Additional data that may be collected includes location of administration and fasting status. For many routes of administration, the location of administration is implicit in the route, and collection of location of administration is not necessary.
SDTM includes a large number of variables to describe dosing. This is due to a number of options for describing substance administrations. The concept map above does not show all these possibilities.
An SDTM interventions record can describe a single administration or a series of periodic administrations.
- A record for a single administration will have the dose for that administration.
- A record for a series of period administrations needs to include
- A description of the administrations within the period, as either
- the frequency for periodic administrations
- a description of the schedule of administrations.
- Doses, which may be described with
- the dose for a single administration
- the total daily dose
- a textual description
- A description of the administrations within the period, as either
A product can have one active ingredient or multiple active ingredients.
- If a product has a single active ingredient, dose can be described using
- the amount of product (e.g., number of tablets, volume of liquid)
- the amount of active ingredient (e.g., mg)
- the amount of active ingredient expressed relative to the subject's body size (e.g. mg/m2 or mg/kg)
- If a product has multiple active ingredients, then an SDTM record for the combination product can only express dose as the amount of product.