Introduction
CDASH: The Clinical Data Acquisition Standards Harmonization Implementation Guide (CDASHIG) establishes a standard way to collect data consistently across studies and sponsors so that data collection formats and structures provide clear traceability of submission data into the SDTM, delivering more transparency to regulators and others who conduct data review. The following videos introduce you to the CDASH model and the CDASHIG.
Versions
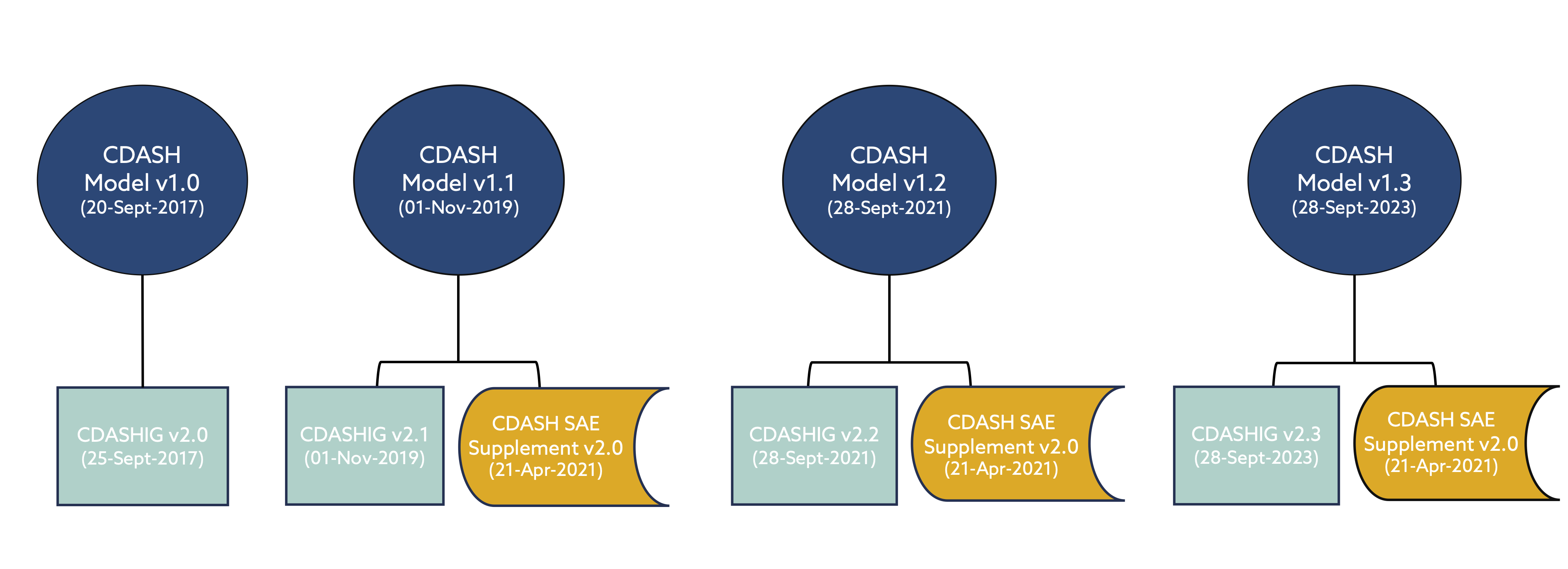
The latest versions of the Clinical Data Acquisition Standards Harmonization Implementation Guides (CDASHIGs) have been developed in reference to a specific CDASH model. However, the CDASH model is cumulative – each new release builds on the previous model. Therefore, the models are considered backward compatible.
Therapeutic Area User Guides
Therapeutic Area User Guides (TAUGs) extend the Foundational Standards to represent data that pertains to specific disease areas. TAUGs include disease-specific metadata, examples and guidance on implementing CDISC standards for a variety of uses, including global regulatory submissions. The following video will introduce you to TAUGs and how they relate to CDISC Foundational Standards.
Controlled Terminology
Controlled Terminology is the set of codelists and valid values used with data items within CDISC-defined datasets. The following video introduces you to CDISC Controlled Terminology and how it is used with CDISC standards.
Traceability
Traceability is a fundamental element of data quality and a requirement for studies submitted to regulatory authorities. From data collection to final analysis, traceability plays a crucial role in ensuring the integrity of source data and in reinforcing clinical research results. The following video provides an introduction to implementing traceability in CDISC-compliant studies.
Regulatory Requirements
CDISC standards are required or recommended by several global regulatory agencies. Standardized data enables regulators to streamline the review process with a more consistent use of analysis tools to better view drug data and highlight areas of concern. The following video introduces you to regulatory requirements and the use of CDISC standards.
Team Guiding Principles
Standards in Development
Foundational
For current versions of the standards, please visit the Standards Home Page.
| Standard Sort descending | Release Notes | Projected Publication |
|---|---|---|
| ADaM and IG v3.0 | In Development |
2026 |
| CDASH v2.0 | In Development |
2026 |
| CDASHIG v3.0 | In Development |
2026 |
| SDTM v3.0 | Resolving Internal Review comments. |
2026 |
| SDTMIG v4.0 | Resolving Internal Review comments. |
2026 |
| SENDIG v4.0 | In Public Review |
2026 |
Data Exchange
For current versions of the Data Exchange standards, please visit the Data Exchange Page.
| Standard Sort descending | Release Notes | Projected Publication |
|---|---|---|
| Controlled Terminology Package 60 | Resolving Public Review Comments |
2025 |