Introduction
SEND: The Standard for Exchange of Nonclinical Data Implementation Guide (SENDIG) is based on the SDTM and guides users on the organization, structure, and format of standard nonclinical study tabulation datasets for exchange between organizations or to be submitted to a regulatory authority. The following videos introduce you to the SENDIG and the SDTM, which used together serve as a map that orients you on how your data fits into the standard.
Versions
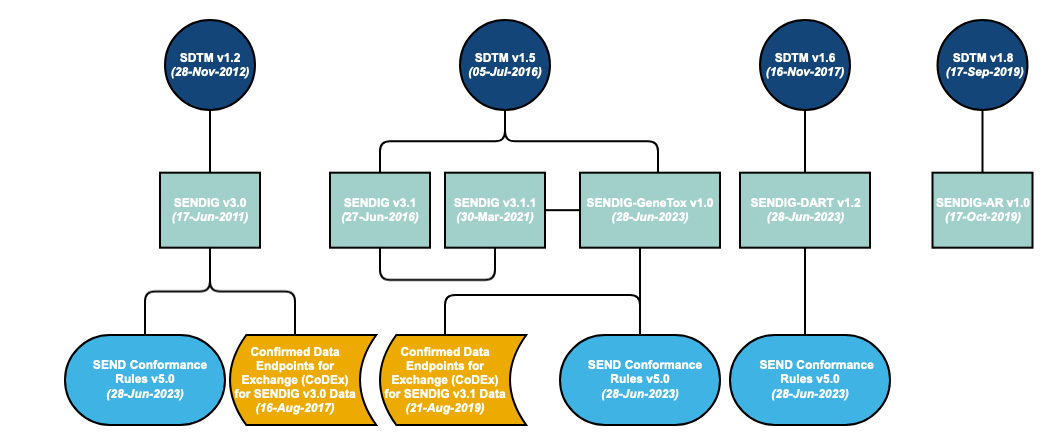
A Standard for Exchange of Nonclinical Data Implementation Guide (SENDIG) is developed in reference to a specific SDTM model. However, the SDTM is cumulative – each new release builds on the previous model. Therefore, the models are backward compatible. The SENDIG is designed to support data typically found in single-dose general toxicology, repeat-dose general toxicology, and carcinogenicity studies, as well as respiratory and cardiovascular testing conducted during safety pharmacology studies.
Additional SENDIGs have been developed to support other study types. For example, SENDIG-DART v1.2 defines recommended standards for the submission of certain types of data collected in DART studies, in particular embryo-fetal development (EFD) studies and toxicity studies conducted on juvenile animals. SENDIG-AR v1.0 supports the submission of data from studies conducted under the Animal Rule. In addition to models and implementation guides, conformance rules have been developed, which help to ensure that generated data structures conform to the standards. These rules aim to identify all conformance rules and case logic from the SENDIG, classifying and codifying them in a form that supports quality processes and tool development.
Therapeutic Area User Guides
Controlled Terminology
Controlled Terminology is the set of codelists and valid values used with data items within CDISC-defined datasets. The following video introduces you to CDISC Controlled Terminology and how it is used with CDISC standards.
Traceability
Regulatory Requirements
CDISC standards are required or recommended by several global regulatory agencies. Standardized data enables regulators to streamline the review process with a more consistent use of analysis tools to better view drug data and highlight areas of concern. The following video introduces you to regulatory requirements and the use of CDISC standards.
Team Guiding Principles
Standards in Development
Foundational
For current versions of the standards, please visit the Standards Home Page.
| Standard Sort descending | Release Notes | Projected Publication |
|---|---|---|
| ADaM and IG v3.0 | In Development |
2026 |
| CDASH v2.0 | In Development |
2026 |
| CDASHIG v3.0 | In Development |
2026 |
| SDTM v3.0 | Resolving Internal Review comments. |
2026 |
| SDTMIG v4.0 | Resolving Internal Review comments. |
2026 |
| SENDIG v4.0 | In Public Review |
2026 |
Data Exchange
For current versions of the Data Exchange standards, please visit the Data Exchange Page.
| Standard Sort descending | Release Notes | Projected Publication |
|---|---|---|
| Controlled Terminology Package 60 | Resolving Public Review Comments |
2025 |