
What is CDISC 360i?
CDISC 360i is a multi-year initiative with the aim to transform the way we develop and use standards within clinical research creating connected and interoperable information enabling automation, enhancing data integrity, and accelerating innovation.
Building on the CDISC 360 proof of concept launched a few years ago to gain community involvement, 360i (“i” for Implementation) is designed to close gaps in data standards by adding the required semantic layer to enable end-to-end automation. This initiative facilitates seamless data flow from study design through analysis, helping researchers, sponsors, and regulators drive efficiency, improve data quality, and accelerate research timelines.
The Need for Change
Clinical research today faces significant challenges due to fragmented systems, isolated operational silos and data, and time consuming manual processes that slow decision-making, increase costs, and delay access to life saving therapies. Despite existing data standards, inconsistencies and gaps within and across the standards hinder automation, collaboration, and efficiency. As science progresses, research data and standards are becoming more intricate, and current systems and workflows struggle to keep up. This makes it harder to maintain traceability, integrate information, and respond to modern research needs.
In a time when speed, precision, and innovation are critical, the current approach simply isn’t sustainable. To advance clinical research, we must adopt a smarter, more connected approach to create a standards-driven, automated workflow that eliminates redundant, manual processes while ensuring clinical data is consistent, traceable, and reusable. By improving efficiency, consistency, and quality of data workflows, this initiative will help reduce time and costs in clinical research and position the industry to handle the growing complexity of modern trials.
Key Benefits of a Connected Research Ecosystem: What is 360i’s Value?
- Patients: Easier access to structured, interpretable data for better engagement
- Sponsors: Protocol driven research automating the information pipeline reducing time to study results and increasing quality
- Regulators: Reduced variability and clickable traceability from analysis to the collected data increasing confidence in decisions
- Researchers: Reduce barrier to entry and cost for standards through ready to use implementable standards and open-source tools
- Technologists: Provide machine readable and interoperable inputs and outputs for easier adoption by software solutions
What Will 360i Deliver? | CDISC 360i Goals: |
 |
|
Join industry leaders currently engaged in 360i!
CDISC 360i is actively seeking contributors to help advance our 360i initiative. Whether you have expertise in data standards, programming, or emerging technologies, there are many ways to get involved.
Key Areas for Contribution
Standards Development
Support USDM, Biomedical Concepts, CDASH, SDTM, ADaM, HL7 FHIR, and more.Technology & Programming
Work with Dataset-JSON, Define-XML, ODM, and automation tools.Clinical Data & Systems
Share expertise in EDC, real-world data, HL7/FHIR, patient apps, safety data, and external transfers.Research Lifecycle Support
Contribute to study design, data management, statistical analysis, regulatory submissions, and data sharing.
Next Steps:
Sign up as a 360i Contributor via the Become a Volunteer webpage.
Take the Contributor Survey to tell us about your skills and interests so we can match you with the right opportunities.
Participation Levels:
Co-Lead (~40% commitment) facilitating and leading one of the 360i teams.
Contributor (~20% commitment) providing expertise and hands on work.
Reviewer (~5% commitment) providing input and feedback to team deliverables.
We welcome contributors at all levels. Your participation will drive innovation, collaboration, and impact in clinical research!
April 2021 - Europe Virtual Interchange
October 2020 - US Virtual Interchange
Stage 3: Project Evaluation
- Introduction, Future State, Process and Architecture of the PoC
- PoC for Study Design and Configuration using CDISC 360 Concept-Based Standards
- Automation of SDTM & ADaM Generation and Artifacts using CDISC 360 Enriched Standards
- Automation of ADaM & TFL Generation using CDISC 360 Enriched Standards
- Biomedical Concepts: What Did We Learn?
- Concluding Remarks and Next Steps
May 2020 - October 2020
Stage 3: Bringing the Work Together
- Developed metadata content per concept model and flowed to sandbox library
- Flowed metadata across the use cases
- Auto-generated study metadata and data artifacts
April 2020 - EU Virtual Interchange
Stage 2: Project Evaluation - Refined Scope Deliverables
- Beyond Biomedical Concepts: How Study Management Concepts Fill the Gaps
- CDISC 360 Metadata-Driven Data Transformation Engine for Automation and Transparency
October 2019 - March 2020
Stage 2: Team Maturation, Deeper Substance Development
- Changed emphasis from workstream to task-team centric
- Matured concept model
- Tooled process concept-based standards metadata to auto-create study artifacts
October 2019 - US Interchange
Stage 1: Project Evaluation – Next Six-month Plan
- CDISC 360: Preparing for a Bright Future
- CDISC 360 Update: Evolution of the CDISC Standards
- CDISC Library: Integrating and Surfacing 360 Content
- CDISC 360: Art of the Possible Initial Concepts
- CDISC 360 Use Cases -Industry Perspectives Workstream 4 -DEFINE Use Case 1: End to Start Standards Specification
- CDISC 360 Use Cases: Industry Perspectives Workstream 5 -Build(Use Case 2) Configure study specification and create artifacts
- CDISC 360 Use Cases -Industry Perspectives Use Case 3 (Workstream 6): Automated Data Processing
May 2019 - Oct 2019
Stage 1: Workstream Organization, Moving up the Learning Curve
- Performed Workstream-centric Activities
- Carried Out Workstream Logistics and Early Substance Development
- Established Collaboration Technology Platform
Webinars:
Previous webinars (Archived)
28 February 2025: 360i Program Kickoff: Enabling Standards Driven Automation from Study Design Through Results
White Papers:
The 2024 Data Standards White Paper highlights the need to transition from static, PDF-based Study Data Reviewer's Guides (SDRGs) to structured, machine-readable formats, facilitating automation, reducing manual effort, and enhancing data traceability. The white paper was authored by a group of industry experts from the pharmaceutical sector, including representatives from major pharmaceutical companies and regulatory agencies.
The 2021 CDISC 360 White Paper describes the output of the CDISC 360 Proof of Concept as well as the technical prototypes developed using CDISC standards as linked metadata. The CDISC 360 standards content provides the additional semantics needed to support metadata-driven automation across the end-to-end clinical research data lifecycle.
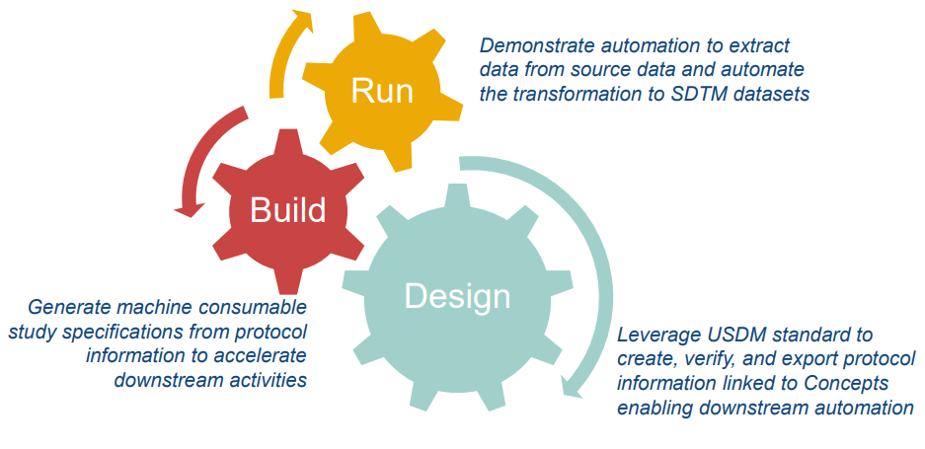
Phase 1: Laying the Foundation for Automation (12-18 Months Focus)
CDISC 360i’s Phase 1 focuses on:
Create connected standards (packages) from study endpoints through SDTM and demonstrate automation
Build and broaden the Biomedical Concepts community to operationalize curation and accelerate content
Establish industry think tank to align on analysis concept definition and scope for implementation in 2026
Deliver on CDISC Open Rules scope, Engine 1.0, and plan for inclusion of conformance rules in all future standards
Continue TransCelerate and industry collaboration to drive adoption and conformance
Define roadmap and scope for digitizing the Trial Master File (TMF)