CDISC, in collaboration with the FDA’s Center for Tobacco Products (CTP), is developing non-proprietary, consensus-based standards for use in studies of tobacco products. One of CTP’s Key Strategic Priorities is Pre & Post-Market Controls: Regulations & Product Reviews, which includes identification of need, development, testing, adoption, implementation, and maintenance of data standards required for the efficient and effective review of research, regulatory submissions, and compliance and enforcement.
The standards will be delivered in a Tobacco Implementation Guide (TIG) to facilitate tobacco research, scientific review, harm reduction, and information exchange. Version 1.0 of the Tobacco Implementation Guide will follow the CDISC Standards Development Process and build on existing CDISC standards to provide a comprehensive resource that is freely available on the CDISC website for sponsors, academics, and regulators to leverage in tobacco research. The Tobacco Implementation Guide v1.0 will be available in CDISC Library to enable and automate standards’ implementation in user systems.
The Tobacco Implementation Guide v1.0 will include the following:
- “End-to-end” Biomedical Concept data standards that address relevant concepts for tobacco research from data collection through analysis for clinical and nonclinical research.
- Metadata table examples of common clinical concepts.
- CDASH-compliant eCRFs and associated metadata to enable consistent data collection. eCRFs will be available and downloadable via the eCRF Portal for implementation in tobacco research.
- New SDTM domains and variables identified during development
- Controlled Terminology indexed in National Cancer Institute - Enterprise Vocabulary Service.
- Conformance Rules to allow users to evaluate conformance of the data structure to the standard. An emerging Industry best practice is to use Conformance Rules on an ongoing basis, throughout the study, to keep the data as close to submission ready as possible and to ensure quality in all data exchange scenarios.
- Questionnaires, Ratings and Scales supplements as identified and prioritized by CTP
- Educational content to support correct and consistent implementation of the Implementation Guide.
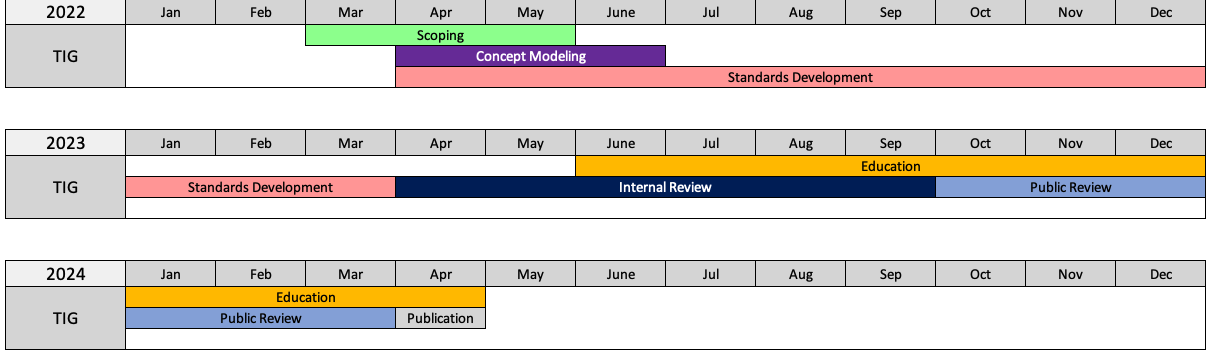
Project Timelines
We anticipate release of the Tobacco Implementation Guide v1.0 in Q2 2024.
How to Participate
Please visit the Participate tab to learn how you can get involved.
This project is supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award [FAIN] totalling $460,419 with 100 per cent funded by FDA/HHS. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA/HHS, or the U.S. Government.
If you would like participate in this important initiative, please follow the instructions below:
Internal and Public Reviewers:
- Development of the Tobacco Implementation Guide will follow the CDISC Standards Development Process, including Internal and public Reviews. Internal Review took place in Q2 2023 and Public Review is anticipated Q3 2023.
- As a Targeted Reviewer (Internal/Public) we estimate 15-20 hours per review.
- Sign up on the CDISC Volunteer page and indicate Tobacco Implementation Guide as the Standards Development team. Please enter Targeted Internal and Public Reviewer in the box "Specify in which capacity you want to participate".
Participation in Standards Development:
- To be part of the Standards Development team, we anticipate that you should be available 5-10 hours per month, including weekly team meetings as well as off-line review and input during the standards development phase (Q1 2022 to Q4 2022).
- In additional we anticipate your involvement during the Internal and Public Review stages to assist in resolving comments. Internal Review is anticipated Q1-2023 and Public Review is anticipated Q2-2023.
- Sign up on the CDISC Volunteer page and indicate Tobacco Implementation Guide as the Standards Development team. In the specify box enter in what capacity you feel you can contribute to the team and the amount of time you can contribute to the project.
Volunteering
- Targeted Reviewer (Internal/Public): 15-20 hours per review.
- Supporting the development team (in a review or SME capacity): 5-10 hours per month.
- Participate as a developer: FTE 40% or more.
- Yes, please follow the instructions on the Participate tab.
- No, volunteers on all CDISC teams are welcome from member and non-member organizations.
Relationship to other Standards
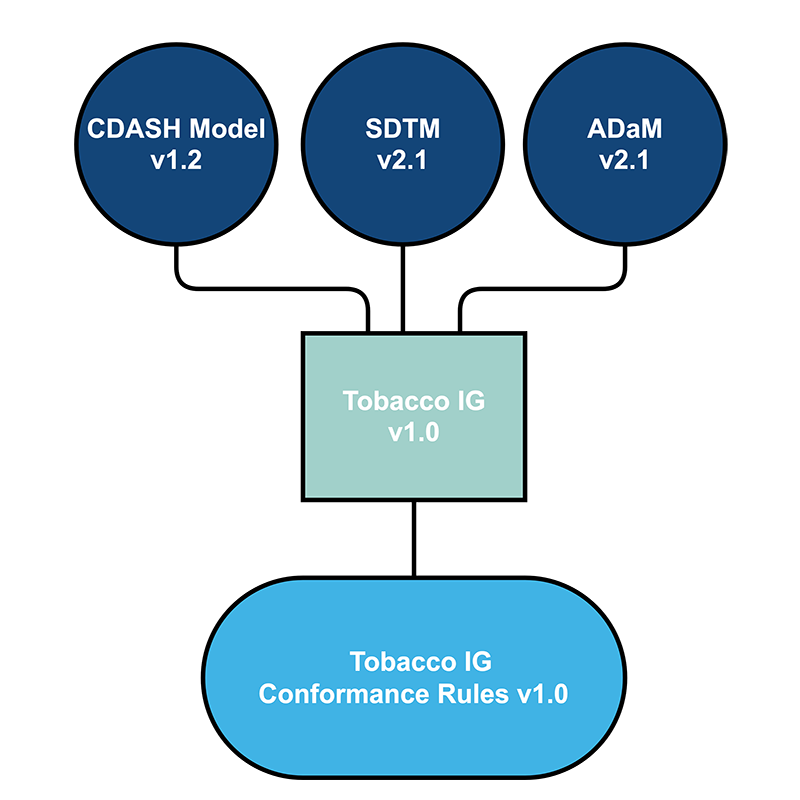
- The Tobacco Implementation Guide v1.0 will sit along side the other CDISC Implementation Guides that are supported by the SDTM model (e.g., SDTMIG, SENDIG).
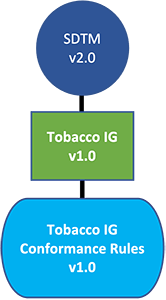
- The Tobacco Implementation Guide v1.0 will facilitate tobacco research, scientific review, harm reduction, and information exchange. Existing standards will be reused where appropriate; the new standards will not replace existing Foundational Standards.
- Yes, it is anticipated that in addition to normative domain specifications, assumptions, and conformance rules, the Tobacco Implementation Guide v1.0 will also contain informative CDASH, SDTM, and ADaM examples
Scope
- Non-clinical studies are in scope for this project. During the scoping phase the team will evaluate the extent of non-clinical concepts to be included in the Tobacco Implementation Guide v1.0.