
Therapeutic Area Data Standards User Guide for Nutritional Research
Version 1.0 (Provisional)
Notes to Readers
- This is the provisional version 1.0 of the Therapeutic Area User Guide for Nutrition.
- This document is based on CDASH Model v1.0 and CDASHIG v2.0, SDTM v1.7 and SDTMIG v3.3, and SDTMIG-PGx v1.0.
Revision History
| Date | Version |
|---|---|
| 2019-09-17 | 1.0 Provisional |
See Appendix E for Representations and Warranties, Limitations of Liability, and Disclaimers.
Contents
- 1 Introduction
- 2 Overview of Nutrition
- 3 Food and Fluid Intake
- 4 Infant Nutrition
- 5 Stool Samples and Stool Assessments
- 6 Questionnaires, Ratings, and Scales
- 7 Appendices
- Appendix A: Nutrition Team
- Appendix B: Glossary and Abbreviations
- Appendix C: Non-Standard Variables
- Appendix D: References
- Appendix E: Representations and Warranties, Limitations of Liability, and Disclaimers
1 Introduction
This Therapeutic Area Data Standards User Guide for Nutritional Research (TAUG-Nutrition) was led by nutrition companies under the Clinical Data Interchange Standards Consortium (CDISC) umbrella.
The goal of the Nutrition Therapeutic Area Data Standards project is to accelerate clinical research and nutrition product development by facilitating the creation and maintenance of data standards, tools, and methods for conducting research in therapeutic areas important to public health.
The purpose of the TAUG-Nutrition is to describe how to use CDISC standards to represent data pertaining to nutrition studies. This provisional Version 1.0 focuses on infant nutrition, food and fluid intake, stool sampling, and questionnaires (specific to clinical studies in nutrition), and includes concepts for both adult and pediatric populations.
Nutrition concepts covered in this guide were selected from concepts identified by one or more nutrition stakeholders as important, and which were not addressed or not completely addressed by existing CDISC standards and implementation guides. This TAUG does not provide guidance on what data is needed for regulatory submission or approval; it only provides advice on how to represent data in a standard form. The examples included are intended to show how particular kinds of data can be represented using CDISC standards. Note: Examples are only examples and should not be over-interpreted.
This first version of the TAUG-Nutrition focuses on how to represent data using the Study Data Tabulation Model (SDTM). There are some examples of Clinical Data Acquisition Standards Harmonization (CDASH) implementation. Future versions of this guide may include additional CDASH and Analysis Data Model (ADaM) representations.
1.1 How to Read this Document
- First, read the CDASH Implementation Guide (CDASHIG), the SDTM and the SDTM Implementation Guide for Human Clinical Trials (SDTMIG), and the SDTM Implementation Guide for Pharmacogenomics/Genetics (SDTMIG-PGx) v1.0 to gain some familiarity with the models and their general implementation in human clinical trials. These standards are available on the CDISC website, at: http://www.cdisc.org/cdash, http://www.cdisc.org/sdtm and https://www.cdisc.org/standards/foundational/pgx, respectively.
- Next, read Introduction to Therapeutic Area Standards to be sure to know what to expect from a TAUG.
- Read this guide all the way through (without skipping any sections) at least once.
- Finally, revisit any sections of particular interest.
Draft standards of interest to this document are listed at: https://wiki.cdisc.org/x/w42zAg).
Some things to bear in mind while reading this document:
- This document does not replace or supersede the foundational CDISC standards or their implementation guides, and should not be used as a substitute for any other CDISC standard.
- This document does not repeat content already published in another CDISC standard.
- This document is not and does not try to be an exhaustive documentation of every possible kind of data that could be collected in relation to nutritional research.
- The advice and examples presented in this document are influenced by ongoing internal standards development at CDISC. If a modeling approach seems inconsistent with a published standard, it may be a genuine error, but it also could be a reflection of potential or upcoming changes to the standard.
- The examples in this document use CDISC Controlled Terminology where possible; however, some values that seem to be controlled terminology may still be under development at the time of publication, or even especially plausible "best-guess" placeholder values. Do not rely on any source other than the CDISC value set in the National Cancer Institute Terminology Resources (available at http://www.cancer.gov/research/resources/terminology/cdisc ) for controlled terminology.
1.2 Organization of this Document
This document is divided into the following sections:
- Section 1, Introduction, provides an overall introduction to the purpose and goals of the Nutrition project.
- Section 2, Overview of Nutrition, provides a general overview ot the concepts contained in this document.
- Section 3, Food and Fluid Intake, covers an overview of food and fluid intake in nutrition studies. SDTM modeling is not included in this TAUG as this will be covered in the Combination Therapy User Guide.
- Section 4, Infant Nutrition, covers data that are usually related to dispensing of infant nutrition during studies as well as examples of data collected on daily feeding activities.
- Section 5, Stool Samples and Stool Assessments, covers data related to baseline and on-study stool sample collection, processing, and characteristics, and bowel associated symptoms.
- Section 6, Questionnaires, Ratings, and Scales, highlights these items as they are related to nutrition studies.
- Appendices provide additional background material and describe other supplemental material relevant to nutritional research.
1.3 CDASH Metadata and Annotated CRFs
CDASH examples include both metadata tables and sample case report forms (CRFs). Each table of CDASH metadata corresponds to an example annotated CRF (aCRF), built directly from the metadata. The annotations show the variables associated with each field in the context of data collection (CDASH) and submission (SDTM). The color of the variable name denotes the applicable context. SDTMIG variable names are shown in RED. If the same variable name is used for both the SDTMIG variable and the CDASHIG variable, only the SDTMIG variable is shown. If the CDASHIG variable differs from the one defined in the SDTMIG, the CDASHIG variable is also shown in GRAY. Data collected but not submitted in SDTM-based datasets are denoted as. NOT SUBMITTED
The following diagram illustrates how to interpret the annotations.
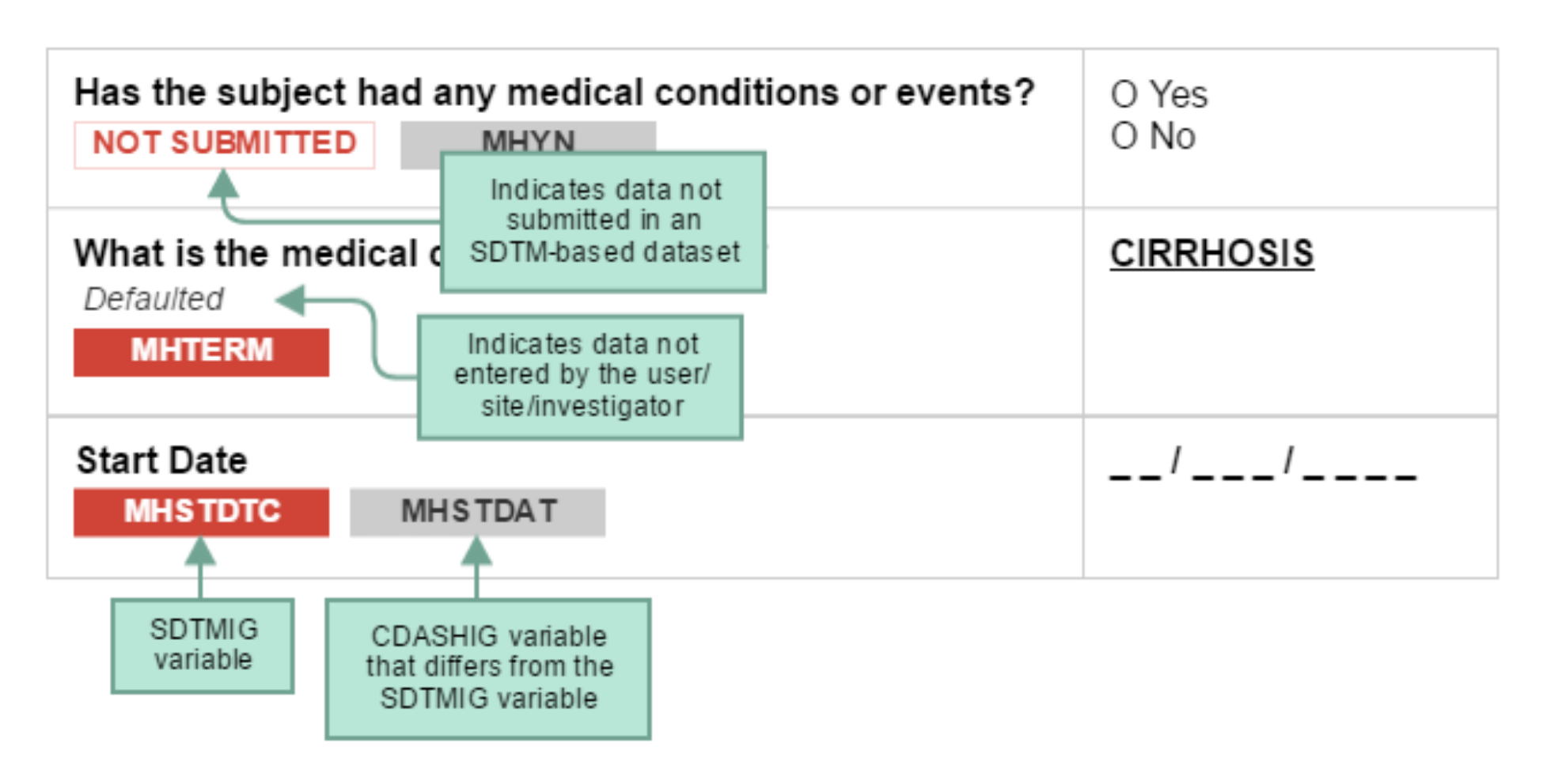
When viewing sample aCRFs, bear in mind that:
- Example CRFs are provided to illustrate data collection instruments. They are only examples and are not meant to imply that any particular layout is preferable to another.
- Example CRFs are best understood in conjunction with their respective metadata tables and/or the CDASH Domain Metadata Tables.
- Most example CRFs do not include the Highly Recommended header variables. The population of these values is usually determined by the sponsor's data management system.
- Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
1.4 Known Issues
-
Non-standard variables (NSVs): To make examples easier to understand, NSVs are shown as though they were appended to a dataset rather than being represented as supplemental qualifiers. This is also consistent with a proposed future structure for representing NSVs, a modification of the NSV proposal that went for public review and which is still under development (available in draft form at: http://wiki.cdisc.org/x/Ui68AQ). A list of all NSVs used in this document, and the variable-level metadata that might become normative for the NSVs should they be promoted to standard variables, is provided in Appendix C, Non-Standard Variables.
- Stool color consistency and weight: Discussions have been ongoing about the modeling of data such as characteristics of stool samples (e.g., stool color, stool consistency and stool weight) and if these data should be modeled in the Laboratory Test Results (LB) domain or the Biospecimen Findings (BS) domain. The following recommendations were provided by CDISC and reviewed by the CDISC Global Governance Group:
- The LB domain should be used to represent data about specimens that are removed from the body and undergo an observation or test that informs about the state of the subject (e.g., color, volume, pH, measurement of an analyte).
- In contrast, the BS domain should be used to represent information about the sample that is not meant to inform about the health of the subject (e.g., tracking, sample condition, amount of specimen available for testing—when that information is used to keep track for testing, test run dates).
-
Timing variables: The time when a subject completes diary information may not be defined precisely by the sponsor. Instructions to subjects filling out a diary card might refer to calendar days (e.g., "Complete this diary with any relevant information from 00:00 on Day 1 to 11:59 on Day 1"), or might ask the subject to complete the diary card at the end of the day before retiring for the night (in which case any diary information after they retired for the night would be captured on the next day's diary). In the examples in this document, the --TPT value "END OF DIARY DAY X" is used to represent imprecise timing of diary data collection. The variables --EVLINT and --EVINTX are used to capture the evaluation interval. However, values such as -P1D are not intended to imply that data are collected in strict 24-hour intervals (e.g., 21:30 on one day to 21:30 on the next day).
-
Modeling of meal and nutrient data: The Combination Therapy subteam is currently looking at the modeling of "product" and "ingredient" data in order to provide a consistent modeling approach across various projects. The modeling of meal, ingredients, and nutrient content as well as feeding recipes used in infant nutrition falls under the scope of the CT team. Modeling of these types of data is therefore not shown in this version of the TAUG-Nutrition.
Proposed amendment to the Drug Accountability domain: A proposal has been submitted to change the domain description from "drug accountability" to "product accountability" in order to extend the use of this domain. It is expected that this will be implemented in a future version of the SDTMIG. At the time of publication of this document, a draft domain has been created to document these changes. Please refer to Product Accountability (DA) for more information.
-
Representing total number of breastfeeding feeds per day: This example uses the NSV EXNADEVI to represent the number of breastfeeding feeds per day. The modeling of this example is still under discussion and users are warned that this modeling may be subject to change.
-
Modeling of --ORRES/--STRESC when the CRF collection is not controlled terminology: There is no clear guidance on how to model findings data that is not collected according to controlled terminology. For example if "Yes/No" is collected on the CRF, should the value of --ORRES be as collected (i.e., "Yes" or "No" and the value of --STRESC be "Y" or "N"), or should both --ORRES and --STRESC both be "Y" or "N"? This issue has been raised with the CDISC Submission Data Standards (SDS) team and at the time of publication of this document is still under discussion.
Therefore, in this TAUG, these assessments have been represented using the LB domain because the tests were performed in order to inform about the state of the subject.
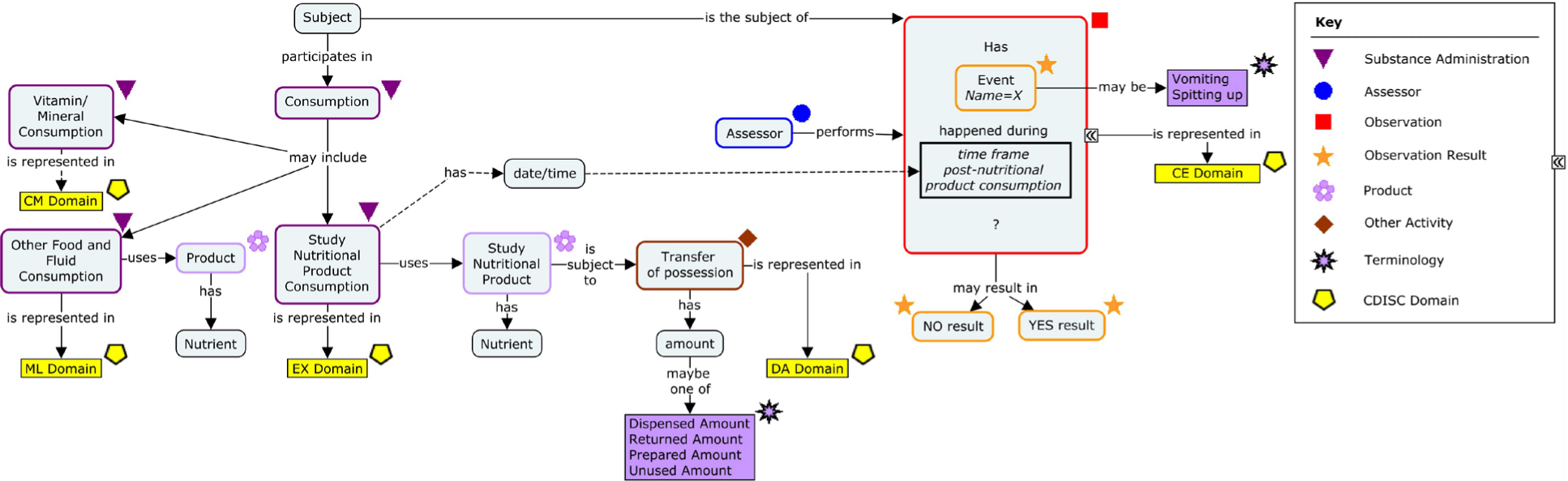
2 Overview of Nutrition
The concepts in the following figure were identified by one or more nutrition stakeholders as important to nutritional research. These concepts fall into 4 main categories:
1. Food and fluid Intake
2. Infant nutrition
3. Stool sampling and assessments
4. Questionnaires specific to clinical studies in nutrition
The concepts were developed to include both adult and pediatric populations.
Concept Map: Overview of Nutrition
3 Food and Fluid Intake
Food and fluid intake data represent nutrients (ingredients) in a consumed meal (regimen) composed of food and other sources of nutrition, including beverages. The nutrient amounts are derived from collected data on the quantities of products within a meal, and data on the nutrients within products.
Currently, there is no consensus regarding the best way to represent food and fluid intake data in the SDTM. Various strategies have been used across published standards to represent this type of "parent" and "child" data. The Combination Therapy subteam is focused on finding a unified approach that can be applied across all disease areas and will take account of this nutrition concept.
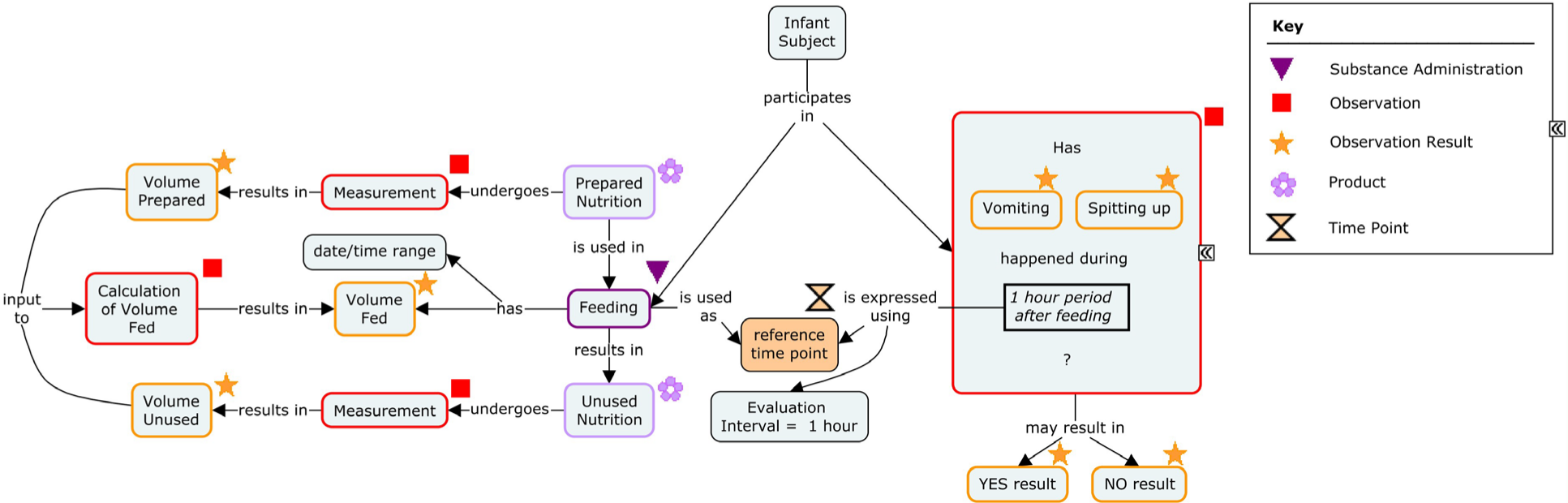
4 Infant Nutrition
Clinical research on infant nutrition is conducted in full conformance with the principles of the World Medical Association Declaration of Helsinki [1] and the International Conference on Harmonisation (ICH) guidelines for Good Clinical Practice [2], as appropriate for nutritional products. The clinical effects of a product may be investigated in observational or interventional studies to determine the safety and/or efficacy of the product.
The World Health Organization recommends exclusive breastfeeding for the first 6 months of life, followed by breastfeeding with complementary feeding up to 2 years of age and beyond. [3] Breastfeeding subjects are often included in the study protocol and used as a reference.
The subjects in a pre-term study can also receive fortified or unfortified human milk from the mother or from a donor. Modeling of pre-term infant nutrition is being handled by the Combination Therapy subteam, focusing on finding a unified approach that can be applied across all disease areas and which will take account of this nutrition concept; it is therefore not included in this document.
The following figure represents the concepts of interest in this document, which relate to feed preparation, administration, and events related to feeding.
Concept Map: Infant Nutrition
4.1 Amount of Study Product Dispensed and Returned
In some nutrition studies, a supply of study product may be provided to the infant's caregiver, to be used between 2 scheduled visits. The data collected on this can be represented using the Drug Accountability (DA) domain.
Example 1
In this study, the sponsor dispensed a number of cans to the subject to use between the study visits. In this study, it was important to note the number of opened and unopened cans returned (i.e., the volume remaining in the unopened cans was not required to be collected); the variable DACAT was used to represent "Opened" and "Unopened" cans returned.
| Row 1: | Shows that at the first visit on day 1, 30 cans of study product were dispensed to the subject. |
|---|---|
| Row 2: | Shows that at the second visit on day 21, 9 cans of unopened study product were returned by the subject. |
| Row 3: | Shows that at the second visit on day 21, 1 can of opened study product was returned by the subject. |
da.xpt
| Row | STUDYID | DOMAIN | USUBJID | DASEQ | DASPID | DATESTCD | DATEST | DACAT | DAORRES | DAORRESU | DASTRESC | DASTRESN | DASTRESU | VISITNUM | VISIT | DADTC | DADY |
| 1 | ABC | DA | 101 | 1 | 1 | DISPAMT | Dispensed Amount | Study Product | 30 | CAN | 30 | 30 | CAN | 1 | VISIT 1 | 2017-05-01 | 1 |
| 2 | ABC | DA | 101 | 2 | 1 | RETAMT | Returned Amount | Unopened Study Product | 9 | CAN | 9 | 9 | CAN | 2 | VISIT 2 | 2017-05-21 | 21 |
| 3 | ABC | DA | 101 | 3 | 1 | RETAMT | Returned Amount | Opened Study Product | 1 | CAN | 1 | 1 | CAN | 2 | VISIT 2 | 2017-05-21 | 21 |
4.2 Daily Feeding
In most nutrition studies, the daily intake is recorded by the caregiver in a diary. It is very common for young infants to spit up or vomit some of the formula; the occurrence of spitting up or vomiting may be recorded. This is sometimes limited to a certain period after the feeding. Spitting up is generally effortless, where the contents of the feed rolls out of the mouth, sometimes with a burp. Vomiting is generally a more forceful ejection of the stomach contents. Guidance on the definitions of spitting up and vomiting may be provided in the study protocol.
Example 1
In this example, 2 types of blinded premixed infant formula were administered. For each feed, the total volume of blinded infant formula administered was collected. The Exposure as Collected (EC) domain was used to represent data on administration of infant formula as collected (e.g., in a blinded fashion). The information about each feed was collected on a new line in a daily diary, with each day's feeds being recorded on a separate page of the diary. The line numbers from the daily diary, which restarted at 1 on each page, were stored in the ECSPID variable. As the line numbers recorded in the ECSPID variable would not uniquely identify each feed, a combination of day number and line number was used in the ECLNKID variable to identify linked records.
| Row 1: | Shows the volume of blinded study Formula A consumed by the infant on the first feed of the diary (day 1). |
|---|---|
| Row 2: | Shows the volume of blinded study Formula B consumed by the infant on the second feed of the diary (day 1). |
ec.xpt
| Row | STUDYID | DOMAIN | USUBJID | ECSEQ | ECSPID | ECLNKID | ECTRT | ECDOSE | ECDOSU | ECDOSFRM | ECROUTE | ECSTDTC | ECENDTC | ECSTDY | ECENDY |
| 1 | ABC | EC | 101 | 1 | 1 | D1-1 | FEEDING FORMULA A | 50 | mL | SUSPENSION | ORAL | 2017-05-19T13:00 | 2017-05-19T13:20 | 1 | 1 |
| 2 | ABC | EC | 101 | 2 | 2 | D1-2 | FEEDING FORMULA B | 60 | mL | SUSPENSION | ORAL | 2017-05-19T19:00 | 2017-05-19T19:35 | 1 | 1 |
The Clinical Events (CE) domain was used to record the occurrence of any "spitting up" or "vomiting" that occurred within 1 hour of the end of each feed. The value of CELNKID was the same as ECLNKID to allow for linking of the CE data to a particular feed record in EC.
| Rows 1, 2: | Show the response to the "spitting up" and "vomiting" questions on the first feed of the diary (day 1). |
|---|---|
| Rows 3, 4: | Show the response to the "spitting up" and "vomiting" questions on the second feed of the diary (day 1). |
ce.xpt
| Row | STUDYID | DOMAIN | USUBJID | CESEQ | CEGRPID | CELNKID | CETERM | CEPRESP | CEOCCUR | CEDTC | CEDY | CEEVINTX |
| 1 | ABC | CE | 101 | 1 | 1 | D1-1 | Vomiting | Y | N | 2017-05-19 | 1 | Within 1 hour after feeding |
| 2 | ABC | CE | 101 | 2 | 1 | D1-1 | Spitting up | Y | Y | 2017-05-19 | 1 | Within 1 hour after feeding | 3 | ABC | CE | 101 | 3 | 2 | D1-2 | Vomiting | Y | N | 2017-05-19 | 1 | Within 1 hour after feeding |
| 4 | ABC | CE | 101 | 4 | 2 | D1-2 | Spitting up | Y | Y | 2017-05-19 | 1 | Within 1 hour after feeding |
Once the study was unblinded, the unblinded exposure data were represented using the Exposure (EX) domain. The value of EXLNKID was the same as ECLNKID to allow for linking of the EX data to a particular blinded feed record in EC. Note that if this was an unblinded study, then the data may have been represented directly in EX without the need to use the EC domain. Please refer to SDTMIG v3.3 Section 6.1 (available at: https://www.cdisc.org/standards/foundational/sdtmig ), which describes the use of the EC and EX domains and the requirements to document derivations used to show data in EX in the Define-XML file.
| Row 1: | Shows the volume of study formula consumed by the infant on the first feed of the diary (day 1). |
|---|---|
| Row 2: | Shows the volume of study formula consumed by the infant on the second feed of the diary (day 1). |
ex.xpt
| Row | STUDYID | DOMAIN | USUBJID | EXSEQ | EXLNKID | EXTRT | EXDOSE | EXDOSU | EXDOSFRM | EXROUTE | EXSTDTC | EXENDTC | EXSTDY | EXENDY |
| 1 | ABC | EX | 101 | 1 | D1-1 | INFFEED PRE MIX | 50 | mL | SUSPENSION | ORAL | 2017-05-19T13:00 | 2017-05-19T13:20 | 1 | 1 |
| 2 | ABC | EX | 101 | 2 | D1-2 | INFFEED-PLUS PRE-MIX | 60 | mL | SUSPENSION | ORAL | 2017-05-19T19:00 | 2017-05-19T19:30 | 1 | 1 |
The RELREC dataset was used to record the relationship between the data on exposure to infant formula and associated clinical events.
relrec.xpt
| Row | STUDYID | RDOMAIN | USUBJID | IDVAR | IDVARVAL | RELTYPE | RELID |
| 1 | ABC | EC | ECLNKID | ONE | 1 | ||
| 2 | ABC | CE | CELNKID | MANY | 1 | ||
| 3 | ABC | EX | EXLNKID | ONE | 1 |
In some studies, the volume prepared and the volume remaining after feeding are collected and can be represented in the SDTM using the DA (Product Accountability) domain. See Section 1.4, Known Issues, for information on a proposal to extend the use of the DA domain to include cover "product" accountability.
Example 2
The infant nutrition product as described in the following example was provided by the sponsor to the the caregiver. This was a blinded infant formula powder for term infants provided in cans. To prepare the formula, the caregiver made up a bottle of formula using a set number of spoons of formula with water. The volume of formula prepared and volume of formula left after feeding were recorded in a diary provided by the sponsor. For the purposes of this example, only the first 3 feeds are shown. DAGRPID was used to group each pair of records associated with a given feed (prepared amount/remaining amount). DASPID represented the line number from diary.
| Rows 1-2: | Show the volume of formula prepared and the volume of formula left after feeding for the first feed of the diary (day 1). |
|---|---|
| Rows 3-4: | Show the volume of formula prepared and the volume of formula left after feeding for the second feed of the diary (day 1). |
| Rows 5-6: | Show the volume of formula prepared and the volume of formula left after feeding for the third feed of the diary (day 2). |
da.xpt
| Row | STUDYID | DOMAIN | USUBJID | DASEQ | DAGRPID | DASPID | DATESTCD | DATEST | DACAT | DAORRES | DAORRESU | DASTRESC | DASTRESN | DASTRESU | DADTC | DADY |
| 1 | ABC | DA | 101 | 1 | 1 | 1 | PREPAMT | Prepared Amount | STUDY PRODUCT | 100 | mL | 100 | 100 | mL | 2017-05-19 | 1 |
| 2 | ABC | DA | 101 | 2 | 1 | 1 | REMAMT | Remaining Amount | STUDY PRODUCT | 15 | mL | 15 | 15 | mL | 2017-05-19 | 1 |
| 3 | ABC | DA | 101 | 3 | 2 | 2 | PREPAMT | Prepared Amount | STUDY PRODUCT | 100 | mL | 100 | 100 | mL | 2017-05-19 | 1 |
| 4 | ABC | DA | 101 | 4 | 2 | 2 | REMAMT | Remaining Amount | STUDY PRODUCT | 25 | mL | 25 | 25 | mL | 2017-05-19 | 1 |
| 5 | ABC | DA | 101 | 5 | 3 | 1 | PREPAMT | Prepared Amount | STUDY PRODUCT | 100 | mL | 100 | 100 | mL | 2017-05-20 | 2 |
| 6 | ABC | DA | 101 | 6 | 3 | 1 | REMAMT | Remaining Amount | STUDY PRODUCT | 10 | mL | 10 | 10 | mL | 2017-05-20 | 2 |
The actual amount of unblinded study product administered to the infant was represented using the EX domain. The sponsor chose to represent this as a weight (g) of study product actually consumed by the infant. Note that in this study 2 spoons of study product (15g each) were always used to prepare 100mL of feed. The actual weight (g) of study product consumed was calculated as the total weight of study product in the prepared volume (two 15g spoons = 30g) multiplied by the proportion of the prepared volume that was consumed ((total volume prepared minus total volume remaining) divided by total volume prepared). For example, if 100 mL of feed was prepared using 30g of study product (two 15g spoons) and the subject only consumed 85mL of the feed (i.e., 15mL was remaining), then the actual weight (g) of study product consumed was calculated as ((85/100)*30) = 25.5g of study product.
The SDTMIG indicates this type of derived exposure information can be represented in SDTM using the EX domain. Please refer to SDTMIG v3.3 Section 6.1 (available at: https://www.cdisc.org/standards/foundational/sdtmig ), which describes the use of EC and EX domains and the requirement to describe the derivations used to show data in EX in the Define-XML document.
| Row 1: | Shows the weight of study formula consumed by the infant on the first feed of the diary (day 1). |
|---|---|
| Row 2: | Shows the weight of study formula consumed by the infant on the second feed of the diary (day 1). |
| Row 3: | Shows the weight of study formula consumed by the infant on the third feed of the diary (day 2). |
ex.xpt
| Row | STUDYID | DOMAIN | USUBJID | EXSEQ | EXLNKID | EXTRT | EXDOSE | EXDOSU | EXDOSFRM | EXROUTE | EXSTDTC | EXENDTC | EXSTDY | EXENDY |
| 1 | ABC | EX | 101 | 1 | 1 | INFFEED | 25.5 | g | POWDER, FOR SOLUTION | ORAL | 2017-05-19 | 2017-05-19 | 1 | 1 |
| 2 | ABC | EX | 101 | 2 | 2 | INFFEED | 22.5 | g | POWDER, FOR SOLUTION | ORAL | 2017-05-19 | 2017-05-19 | 1 | 1 |
| 3 | ABC | EX | 101 | 3 | 3 | INFFEED | 27 | g | POWDER, FOR SOLUTION | ORAL | 2017-05-20 | 2017-05-20 | 2 | 2 |
The relationship between the product accountability and administration information was represented in the RELREC dataset below.
relrec.xpt
| Row | STUDYID | RDOMAIN | USUBJID | IDVAR | IDVARVAL | RELTYPE | RELID |
| 1 | ABC | DA | DAGRPID | MANY | 1 | ||
| 2 | ABC | EX | EXLNKID | ONE | 1 |
In some clinical studies, breastfeeding may be used as a comparator to study products and therefore may need to be represented as "exposure" information. These data may be collected in a variety of formats (e.g., start and stop times of individual feeds, duration of each breastfeeding episode/number of minutes on the breast, total breastfeeding time for a day). Breastfeeding may also be collected as “background” data, in which case it would be represented using the Meal Data (ML) domain. If represented in ML, the data structure would look the same as the Exposure (EX) examples apart from using the ML domain code/variables.
Example 3
This example shows the representation of breastfeeding in EX with individual start and stop times per feed. Two CRFs ("Breast Milk Fed from Breast" and "Breast Milk Fed from Bottle") were used to collect the information. EXCAT was used to differentiate data from the 2 CRFs.
| Row 1: | Shows an example of breastfed milk. Note that there is no EXDOSE because the actual amount of milk fed cannot be determined when feeding is direct from the breast. |
|---|---|
| Row 2: | Shows an example of expressed breast milk that is fed using a bottle. The amount of milk consumed is represented in EXDOSE, as this was collected based on the volume fed from the bottle. |
ex.xpt
| Row | STUDYID | DOMAIN | USUBJID | EXSEQ | EXTRT | EXCAT | EXDOSE | EXDOSU | EXDOSFRM | EXROUTE | EXSTDTC | EXENDTC |
| 1 | ABC | EX | 101 | 1 | BREAST MILK | BREAST MILK FED FROM BREAST | NOT APPLICABLE | ORAL | 2017-05-19T13:00 | 2017-05-19T13:20 | ||
| 2 | ABC | EX | 101 | 2 | BREAST MILK | BREAST MILK FED FROM BOTTLE | 50 | mL | NOT APPLICABLE | ORAL | 2017-05-19T17:00 | 2017-05-19T17:15 |
Example 4
This example shows the representation of breastfeeding in EX with the duration in minutes of each breastfeeding episode shown in the EXDUR variable. The individual start and stop times of feeding were not collected; therefore, only the date of feeding was represented in the EXSTDTC and EXENDTC variables.
Note: The SDTM variable EXDUR variable should only be used if specific duration data are collected on the CRF and should not be derived from start and stop times. If duration needs to be calculated from start and stop times, this should be done in an ADaM dataset. Individual feeds were recorded sequentially on a diary and the diary line number was recorded in EXSPID to differentiate the records.
ex.xpt
| Row | STUDYID | DOMAIN | USUBJID | EXSEQ | EXSPID | EXTRT | EXDOSE | EXDOSU | EXDOSFRM | EXROUTE | EXSTDTC | EXENDTC | EXDUR |
| 1 | ABC | EX | 101 | 1 | 1 | BREAST MILK | NOT APPLICABLE | ORAL | 2017-05-19 | 2017-05-19 | PT20M | ||
| 2 | ABC | EX | 101 | 2 | 2 | BREAST MILK | NOT APPLICABLE | ORAL | 2017-05-19 | 2017-05-19 | PT15M |
Example 5
This example shows the representation of breastfeeding as a total number of feeds per day.
ex.xpt
| Row | STUDYID | DOMAIN | USUBJID | EXSEQ | EXTRT | EXDOSE | EXDOSU | EXDOSFRM | EXROUTE | EXSTDTC | EXENDTC | EXEVINTX | EXNADEVI | |
| 1 | ABC | EX | 101 | 1 | BREAST MILK | NOT APPLICABLE | ORAL | 2017-05-19 | 2017-05-19 | CALENDAR DAY | 4 |
EX NSV Metadata
| Variable | Label | Type | Role | Codelist | Origin |
| EXNADEVI | Number of Administrations in Eval. Int. | integer | Non-standard Record Qualifier | CRF |
5 Stool Samples and Stool Assessments
In both adult and infant nutrition studies, stool and gastrointestinal (GI) symptoms can be important outcome and safety parameters. These data may then be used to assess if the study product has had any effect on stool frequency/consistency by comparing information collected at baseline with data collected during the study.
Stool assessments can be divided into two categories: (1) bowel movements and (2) bowel-associated symptoms.
• Bowel movement data are related both to assessment of the bowel movement (e.g., frequency of bowel movements) and to assessment of the stool sample (e.g., stool color and consistency, laboratory measurements from feces samples such as pH and bacterial composition).
•Bowel associated symptoms represent the assessment of symptoms related to bowel movement (e.g., flatulence, abdominal distension, constipation).
The following concept map shows the various assessments that may be performed on bowel movements and stool samples.
Concept Map: Stool Assessments
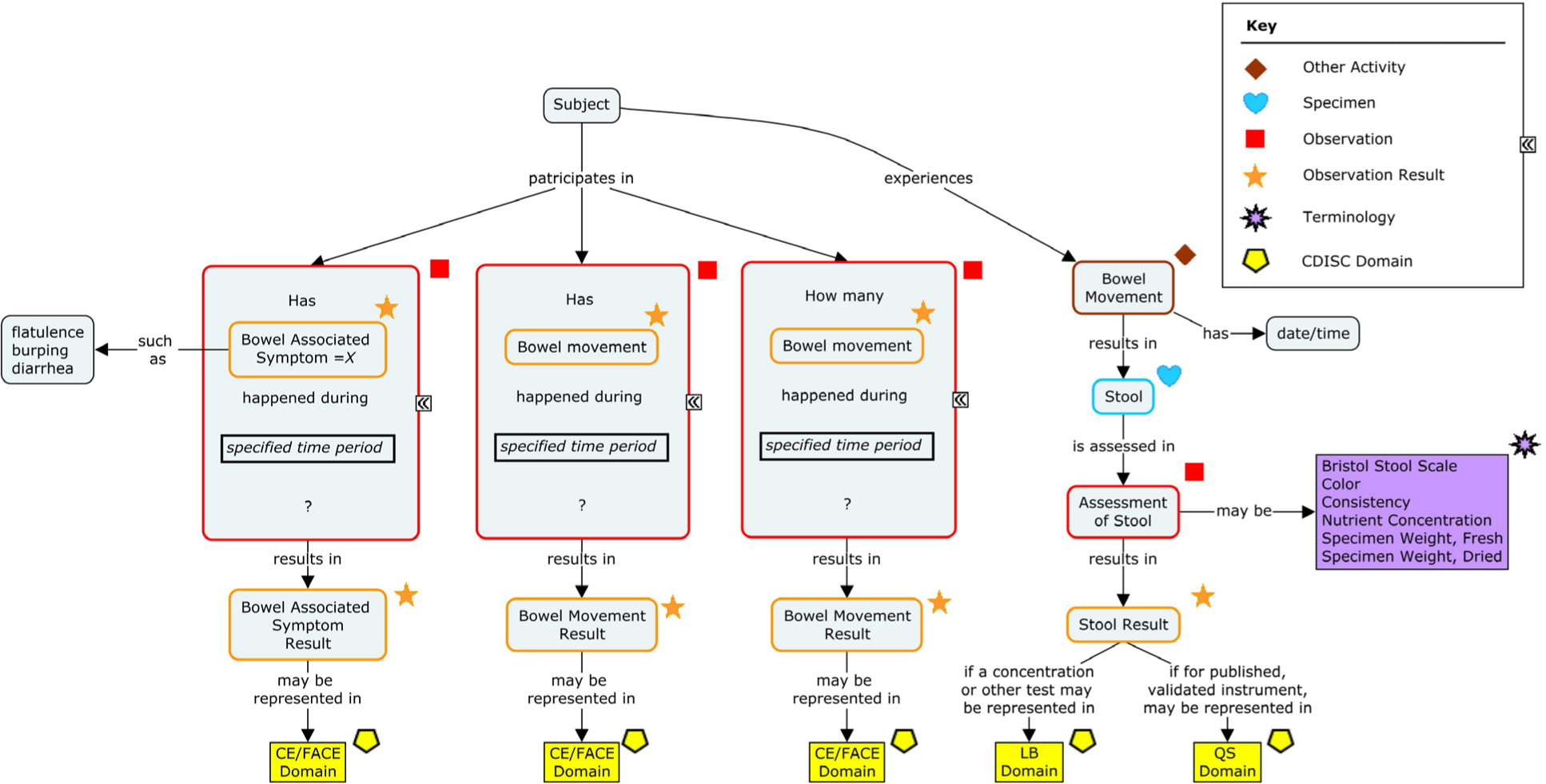
5.1 Types of Diary Collection for Stool Samples and Stool Assessments
Data collection on bowel movements is usually conducted using diaries completed by a subject or other evaluator (e.g., caregiver). Fecal samples may also be taken by the subject or caregiver, or they may be taken at hospitals or clinics.
Figure 5.1.1 presents 3 types of diary data collection:
- • Subject recall: At the visit, the subject is asked to recall from memory data such as the average number of stool episodes per day, or typical consistency of the stool over a set number of days prior to the visit. The subject is not required to keep detailed information on the number of episodes/type of consistency, but estimates these based on memory recall.
• End-of-day diary completion: The subject is asked to complete a diary at the end of each day with the total number of stool episodes and typical consistency over the day.
• Per-episode diary completion: The subject is asked to complete a diary with the time of each stool episode and the typical consistency of that stool episode.
Figure 5.1.1 Types of Diary Collection
When providing diaries for a subject to complete, it is important to provide the subject with instructions on how and when to complete the diary; those instructions must be understood by all functions within the study team. Although this may differ from protocol to protocol, detailed, unambiguous instructions will help ensure that data are collected in a consistent format across all subjects. This may be particularly important when analyzing the data. Some diary completion instructions may be as simple as "Please record all bowel movements that occurred between 00:00 and 23:59 on this day." It also may be important to provide information on how subjects should record data if, for example, they retire to bed before or after midnight and then they have a bowel movement prior to or after midnight. Providing instructions on which diary day to record these episodes will help ensure that data are recorded consistently across all subjects. Note: Some sponsors may use a standard instrument to collect information on the color/consistency of stool samples. SDTM modeling of these concepts should therefore follow the separate supplements for these scales. See Section 6, Questionnaires, Ratings, and Scales, for scales that have been identified for nutritional research. The following examples show how subject recall, end of day, and each episode diary data may be collected using CDASH standards and represented in SDTM standards. Example 1 This example shows how subject-recalled information may be collected and represented. At the baseline visit, the subject was asked to recall: • What was the average number of bowel movements per day over the 3 days prior to the visit? • What was the typical consistency of the bowel movements over the 3 days prior to the visit? See Section 1.3, CDASH Metadata and Annotated CRFs for explanation of annotations. CRF Metadata Recalled average number of bowel movements over a 3-day period prior to the visit was represented in the FACE dataset. FAEVAL was used to represent that the average number of stools was assessed by the subject. The non-standard variable (NSV) FACOLSRT was used to represent that the collected result type was an average. The NSV FASOURCE was used to indicate that the results were obtained using subject recall at the visit, as opposed to information gathered from a data collection tool (e.g., a patient daily diary). face.xpt FACE NSV Metadata Recalled typical consistency of the stool over a 3-day period prior to the visit was represented using the Laboratory Test Results (LB) domain. The consistency of the stool was assessed by the subject; therefore, LBEVAL was used to represent this. The NSVs LBCOLSRT and LBSOURCE were used to represent that the collected result was typical and that this was information recalled from the subject. Certain Expected variables have been omitted in consideration of space and clarity. lb.xpt LB NSV Metadata The sponsor chose to provide an explicit relationship between the event frequency and typical stool consistency, representing this in the following RELREC dataset. relrec.xpt Information on the number and consistency of stools can be collected on a daily basis using patient diaries. In this case, the calculation of the average number of stools or the typical stool consistency over the 3-day period may be performed in an ADaM dataset using the source diary data. Example 1 The sponsor chose to use a paper diary to collect the information. Each subject was given a diary with instructions on how to complete it at the end of the day on each of the 3 days before the next visit. At that next visit, the subject returned the completed diary and the collected information was entered into the electronic data capture (EDC) system. See Section 1.3, CDASH Metadata and Annotated CRFs for explanation of annotations. CRF Metadata Because these data were based on a diary and the assessments were not completed at a specified protocol visit, the sponsor chose to leave the VISITNUM variable blank. The total daily number of bowel movements for each of the 3 days of the diary was represented in the FACE dataset. FAEVAL was used to represent that the daily total number of bowel movements was assessed by the subject. face.xpt The typical stool consistency of bowel movements for each of the 3 days of the diary was represented using the Laboratory Test Results (LB) domain. The consistency of the stool was assessed by the subject at the end of the day; therefore, LBEVAL was used to represent this. If a caregiver were assessing the stool sample (e.g., for an infant), then LBEVAL would be "CAREGIVER". Certain Expected variables have been omitted in consideration of space and clarity.
lb.xpt LB NSV Metadata
The sponsor chose to provide an explicit relationship between the event frequency and typical stool consistency, representing this in the following RELREC dataset. relrec.xpt In some cases, patient diaries can be used to collect information on individual stool episodes and consistency within each day. In this case, the average number of stools or the typical stool consistency over the 3-day period may be calculated in an ADaM dataset using this source diary data. Example 1 The sponsor chose to use a paper diary to collect the information. Each subject's caregiver was given a diary with instructions on how to record details of each of the subject's bowel movements during the 3 days before the next visit. At that next visit, the subject's caregiver returned the completed diary and the collected information was entered into the EDC system. See Section 1.3, CDASH Metadata and Annotated CRFs, for explanation of annotations. CRF Metadata • Because these data were based on a diary and the assessments were not completed at a specified protocol visit, the sponsor chose to leave the VISITNUM variable blank.
• Because data were collected on individual bowel movement episodes, these were considered clinical events and therefore data for the occurrence of each episode were represented using the CE domain.
• The consistency and color of each episode were represented using the Laboratory Test Results (LB) domain.
• The CESTDTC variable was used to represent the start date and time of the bowel movement, which was a combination of the date of diary completion and the start time of the individual bowel movement that was recorded by the caregiver.
• CEREFID was populated by the sponsor to represent the diary day number and the individual bowel movement (e,g,. D-3_1 represents bowel movement 1 on diary day -3). The same format was used for LBREFID on the LB records showing the consistency and color of the stool. The CE and LB datasets can therefore be linked based on --REFID variables.
• Bowel movements were assessed by the infant's caregiver and therefore the non-standard variable (NSV) CEEVAL was used to represent this. (Note that in SDTM v1.7, --EVAL cannot be used as a parent variable in Events domains therefore CEEVAL was represented as an NSV.) If a subject was assessing their own stool sample, then CEEVAL would be "STUDY SUBJECT". ce.xpt CE NSV Metadata The following example shows the consistency and color of the stool associated with each bowel movement shown in the preceding CE data. Note that there is no LB consistency/color data for the last 2 rows of the CE dataset because these rows represent (1) a day when there were no bowel movement episodes and (2) a day when the infant's caregiver forgot to complete the diary. LBEVAL was used to represent that the consistency of the stool was assessed by the infant's caregiver. If a subject was assessing their own stool sample, then LBEVAL would be "STUDY SUBJECT". LBDTC was populated using the same value as CESTDTC because the sponsor considered that the start date/time of the bowel movement equated to the sample collection date/time. Certain Expected variables have been omitted in consideration of space and clarity. lb.xpt The sponsor chose to provide an explicit relationship between the bowel movement occurrence and stool consistency/color by representing this in the following RELREC dataset. relrec.xpt In nutrition studies, subjects are often provided with a diary in order to provide daily information on GI symptoms (e.g., nausea, vomiting, burping, abdominal distension, flatulence, colic/cramps, regurgitation, diarrhea, constipation). Example 1 In this example, the subject was given a diary at visit 1 to record GI symptoms starting from the day after visit 1 to the day before visit 2. The diary was then collected at visit 2 and a new diary was given to the subject. See Section 1.3, CDASH Metadata and Annotated CRFs,for explanation of annotations. CRF Metadata Summarizing data of the daily diaries is generally done during the analysis stage and therefore only daily records are represented here. For the purposes of this example, only the first 2 days of the diary are shown. • Because the diary data were not visit-related, the sponsor chose to leave the VISITNUM variable blank.
• The subject was instructed to complete the diary at the end of the day and to record the occurrence/severity of the symptoms over that day. The FATPT variable was used to indicate when the diary was completed (at the end of each diary day) and the FAEVLINT variable was used indicate that the subject was assessing the symptoms over that day.
• FAEVAL was used to represent that the GI symptoms were assessed by the subject.
• Note that sponsors should provide instructions to investigational sites/subjects on how to assess severity of symptoms (e.g., the maximum severity over the day, the most common severity over the day). In
this example, the sponsor provided instructions to record the worst (maximum) severity over the day. This is represented using the non-standard variable (NSV) FACOLSRT. face.xpt FACE NSV Metadata Nutrition studies often require collection of stool samples for analysis. Sample handling events may also be performed on these samples (e.g., freezing the sample). Evaluations (e.g., weight, color) may also be performed on these samples. Note, Some sponsors may choose to use a validated scale to rate attributes of a stool sample (e.g., Bristol Stool Form Scale). In this case, data would be represented following the QRS supplement. Please refer to Section 6, Questionnaires, Ratings, and Scales, of this TAUG for scales that have been identified by the Nutrition Team as important for nutrition studies. Samples are also sent to laboratories for additional analysis. These data are represented using the Laboratory Test Results (LB) domain. At the time of publication, there are no tests that require additional LB examples to be developed in the TAUG-Nutrition; however, the Lab Controlled Terminology team is working to determine whether additional tests may be needed. Example
This example shows stool sample biospecimen events (i.e., collection, drying, freezing). be.xpt BE NSV Metadata In this example, tests were performed on the stool sample (e.g., fresh/dry weight, color, consistency). Because these tests were performed to assess the state of the subject, the results are shown in the LB domain. The LBLOBXFL variable has been populated with "Y" to indicate that, for each of these tests, this is the subject's last record with a non-missing result prior to the first exposure to study treatment. For additional information on the usage of the --LOBXFL variable, see SDTMIG v3.3 Section 4.5.9, Baseline Values (available at: https://www.cdisc.org/standards/foundational/sdtmig). Certain Expected variables have been omitted in consideration of space and clarity. lb.xpt The relrec.xpt example reflects a many-to-many dataset-level relationship between Biospecimen Events (BE) and LB using --REFID. relrec.xpt Nutrition studies may use measures that assess functional status (i.e., physical activity), descriptions of stool samples, and feeding behaviors. Questionnaires, ratings, and scales (QRS) are maintained as stand-alone guides on the CDISC website
at http://www.cdisc.org/foundational/qrs. Table 6.1 lists the assessments that are being pursued as potential supplements as part of the development work for TAUG-Nutrition. Supplements may or may not be finalized at the time of publication of the TAUG-Nutrition, and depend on copyright approval where applicable. CDISC cannot produce supplements for copyrighted measures without the express permission of the copyright holder. Sponsors should refer to the link above if a measure of interest is not included below, as it may have been developed for another therapeutic area; new measures are implemented on an ongoing basis by the CDISC QRS Terminology and Standards Development subteams. See CDISC COP 017 ( http://www.cdisc.org/bylaws-and-policies, now part of COP 001) for details on implementing or requesting development of standards for SDTM-based submissions. Table 6.1 Identified QRS Measures of Interest to Nutrition The following table lists the non-standard variables used in this document, and gives their parent domain(s) and variable-level metadata. a - Parenthesis indicates CDISC/NCI codelist. CDISC Patent Disclaimers It is possible that implementation of and compliance with this standard may require use of subject matter covered by patent rights. By publication of this standard, no position is taken with respect to the existence or validity of any claim or of any patent rights in connection therewith. CDISC, including the CDISC Board of Directors, shall not be responsible for identifying patent claims for which a license may be required in order to implement this standard or for conducting inquiries into the legal validity or scope of those patents or patent claims that are brought to its attention. Representations and Warranties “CDISC grants open public use of this User Guide (or Final Standards) under CDISC’s copyright.” Each Participant in the development of this standard shall be deemed to represent, warrant, and covenant, at the time of a Contribution by such Participant (or by its Representative), that to the best of its knowledge and ability: (a) it holds or has the right to grant all relevant licenses to any of its Contributions in all jurisdictions or territories in which it holds relevant intellectual property rights; (b) there are no limits to the Participant’s ability to make the grants, acknowledgments, and agreements herein; and (c) the Contribution does not subject any Contribution, Draft Standard, Final Standard, or implementations thereof, in whole or in part, to licensing obligations with additional restrictions or requirements inconsistent with those set forth in this Policy, or that would require any such Contribution, Final Standard, or implementation, in whole or in part, to be either: (i) disclosed or distributed in source code form; (ii) licensed for the purpose of making derivative works (other than as set forth in Section 4.2 of the CDISC Intellectual Property Policy (“the Policy”)); or (iii) distributed at no charge, except as set forth in Sections 3, 5.1, and 4.2 of the Policy. If a Participant has knowledge that a Contribution made by any Participant or any other party may subject any Contribution, Draft Standard, Final Standard, or implementation, in whole or in part, to one or more of the licensing obligations listed in Section 9.3, such Participant shall give prompt notice of the same to the CDISC President who shall promptly notify all Participants. No Other Warranties/Disclaimers. ALL PARTICIPANTS ACKNOWLEDGE THAT, EXCEPT AS PROVIDED UNDER SECTION 9.3 OF THE CDISC INTELLECTUAL PROPERTY POLICY, ALL DRAFT STANDARDS AND FINAL STANDARDS, AND ALL CONTRIBUTIONS TO FINAL STANDARDS AND DRAFT STANDARDS, ARE PROVIDED “AS IS” WITH NO WARRANTIES WHATSOEVER, WHETHER EXPRESS, IMPLIED, STATUTORY, OR OTHERWISE, AND THE PARTICIPANTS, REPRESENTATIVES, THE CDISC PRESIDENT, THE CDISC BOARD OF DIRECTORS, AND CDISC EXPRESSLY DISCLAIM ANY WARRANTY OF MERCHANTABILITY, NONINFRINGEMENT, FITNESS FOR ANY PARTICULAR OR INTENDED PURPOSE, OR ANY OTHER WARRANTY OTHERWISE ARISING OUT OF ANY PROPOSAL, FINAL STANDARDS OR DRAFT STANDARDS, OR CONTRIBUTION. Limitation of Liability IN NO EVENT WILL CDISC OR ANY OF ITS CONSTITUENT PARTS (INCLUDING, BUT NOT LIMITED TO, THE CDISC BOARD OF DIRECTORS, THE CDISC PRESIDENT, CDISC STAFF, AND CDISC MEMBERS) BE LIABLE TO ANY OTHER PERSON OR ENTITY FOR ANY LOSS OF PROFITS, LOSS OF USE, DIRECT, INDIRECT, INCIDENTAL, CONSEQUENTIAL, OR SPECIAL DAMAGES, WHETHER UNDER CONTRACT, TORT, WARRANTY, OR OTHERWISE, ARISING IN ANY WAY OUT OF THIS POLICY OR ANY RELATED AGREEMENT, WHETHER OR NOT SUCH PARTY HAD ADVANCE NOTICE OF THE POSSIBILITY OF SUCH DAMAGES. Note: The CDISC Intellectual Property Policy can be found at: cdisc_policy_003_intellectual_property_v201408.pdf.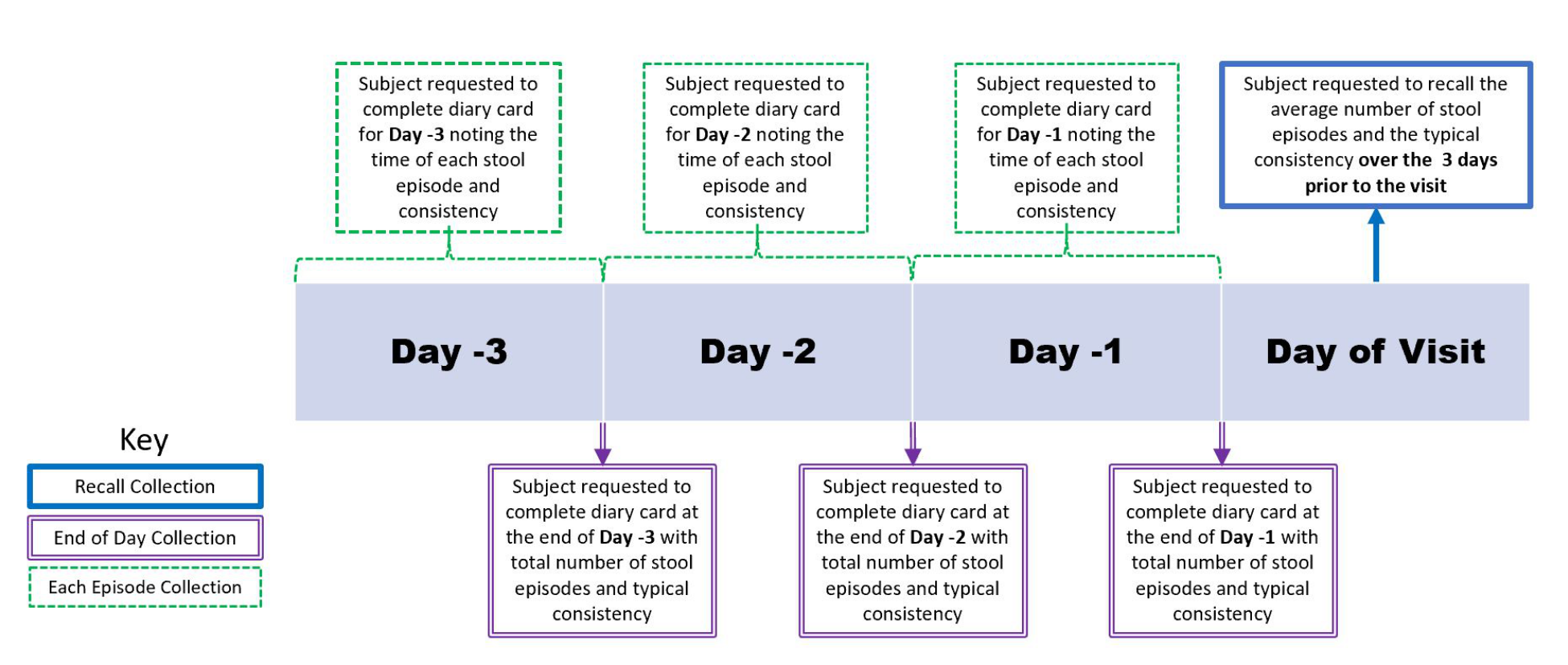
5.1.1 Bowel Movements - Subject Recall
Row STUDYID DOMAIN USUBJID FASEQ FALNKID FATESTCD FATEST FAOBJ FAORRES
FAORRESU FASTRESC FASTRESN FASTRESU FAEVAL
VISITNUM VISIT
VISITDY FADTC
FAEVINTX FACOLSRT FASOURCE 1 NUTR123 FA
NUTR123_001 1 BSL
EVENTFRQ Event Frequency BOWEL MOVEMENT
3
/day 3 3
/day STUDY SUBJECT 1
Baseline
-1 2017-01-05 PREVIOUS 3 CALENDAR DAYS AVERAGE RECALL
Variable Label Type Codelist Role Origin FACOLSRT Collected Summary Result Type text Non-standard Variable Qualifier CRF FASOURCE Source of Data text Non-standard Record Qualifier CRF
Row STUDYID DOMAIN USUBJID LBLNKID LBTESTCD LBTEST LBORRES LBSTRESC LBSPEC LBEVAL VISITNUM VISIT VISITDY
LBEVINTX
BCOLSRT LBSOURCE 1 NUTR123 LB NUTR123_001 BSL CONSIST Consistency LOOSE LOOSE STOOL STUDY SUBJECT 1 Baseline -1 2017-01-05 PREVIOUS 3 CALENDAR DAYS TYPICAL RECALL
Variable Label Type Codelist Role Origin LBCOLSRT Collected Summary Result Type text Non-standard Variable Qualifier CRF LBSOURCE Source of Data text Non-standard Record Qualifier CRF
Row STUDYID RDOMAIN USUBJID IDVAR IDVARVAL RELTYPE RELID 1 NUTR123 FA FALNKID ONE 1 2 NUTR123 LB LBLNKID ONE 1 5.1.2 Bowel Movements - End of Day
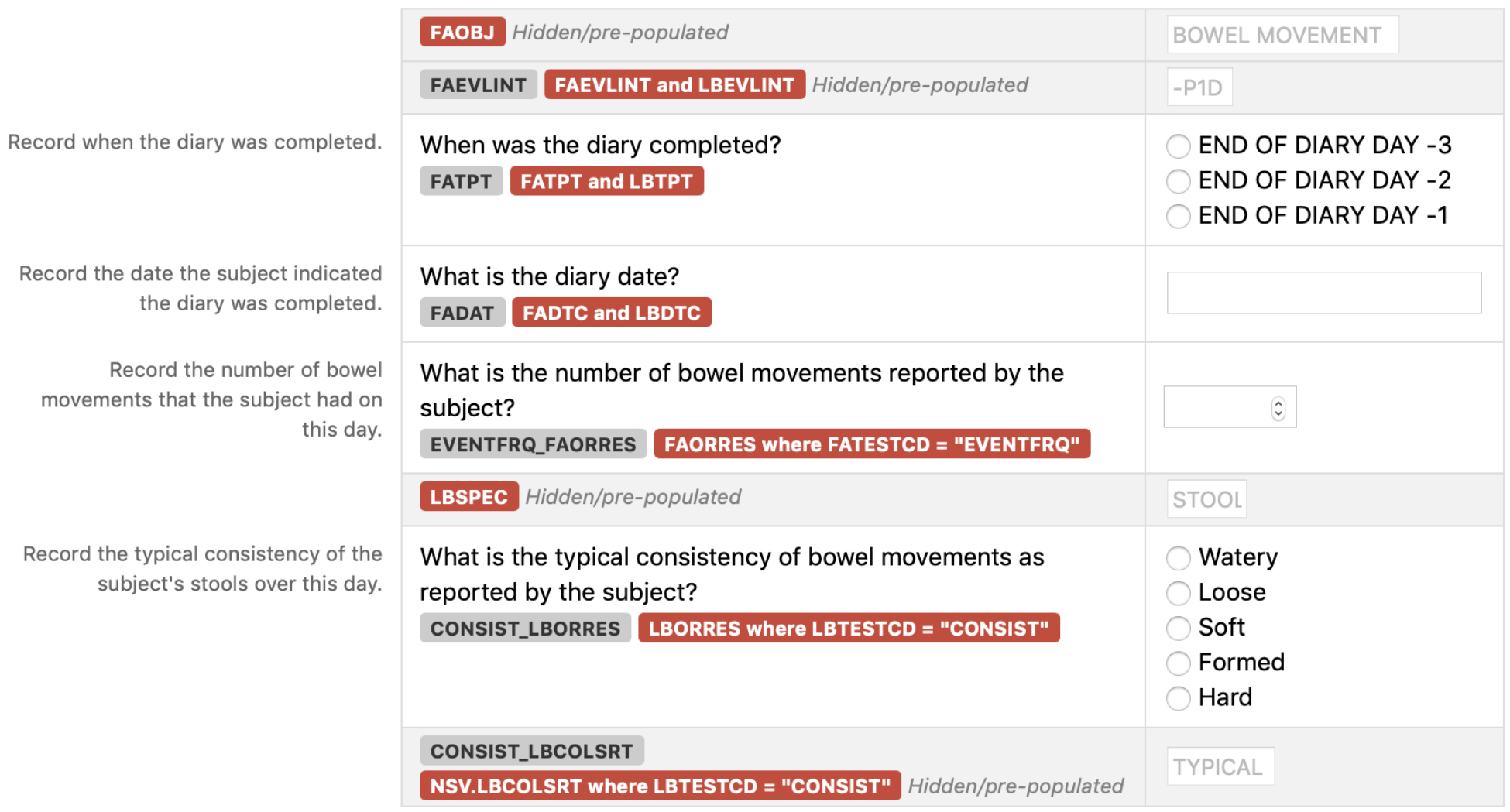
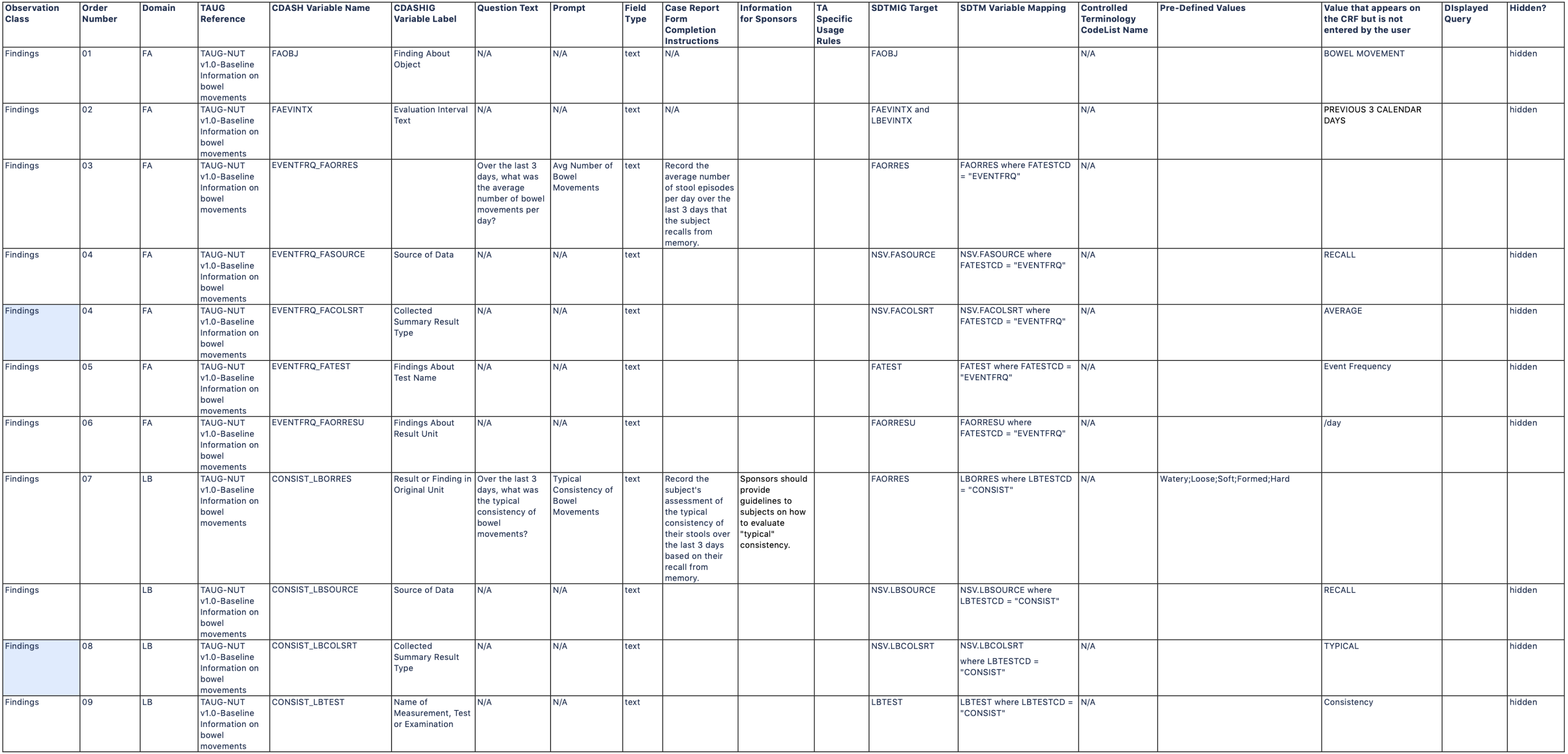
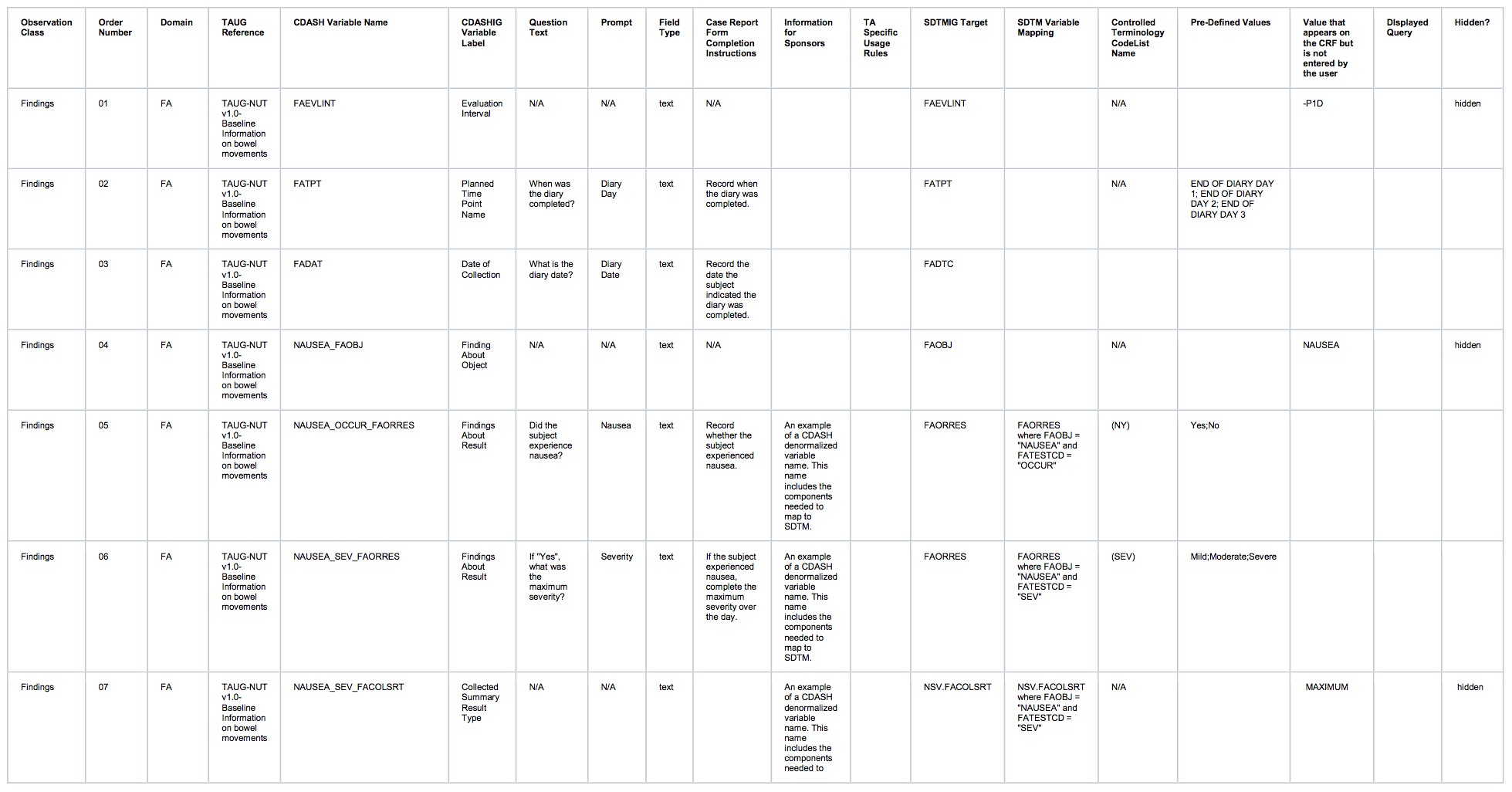
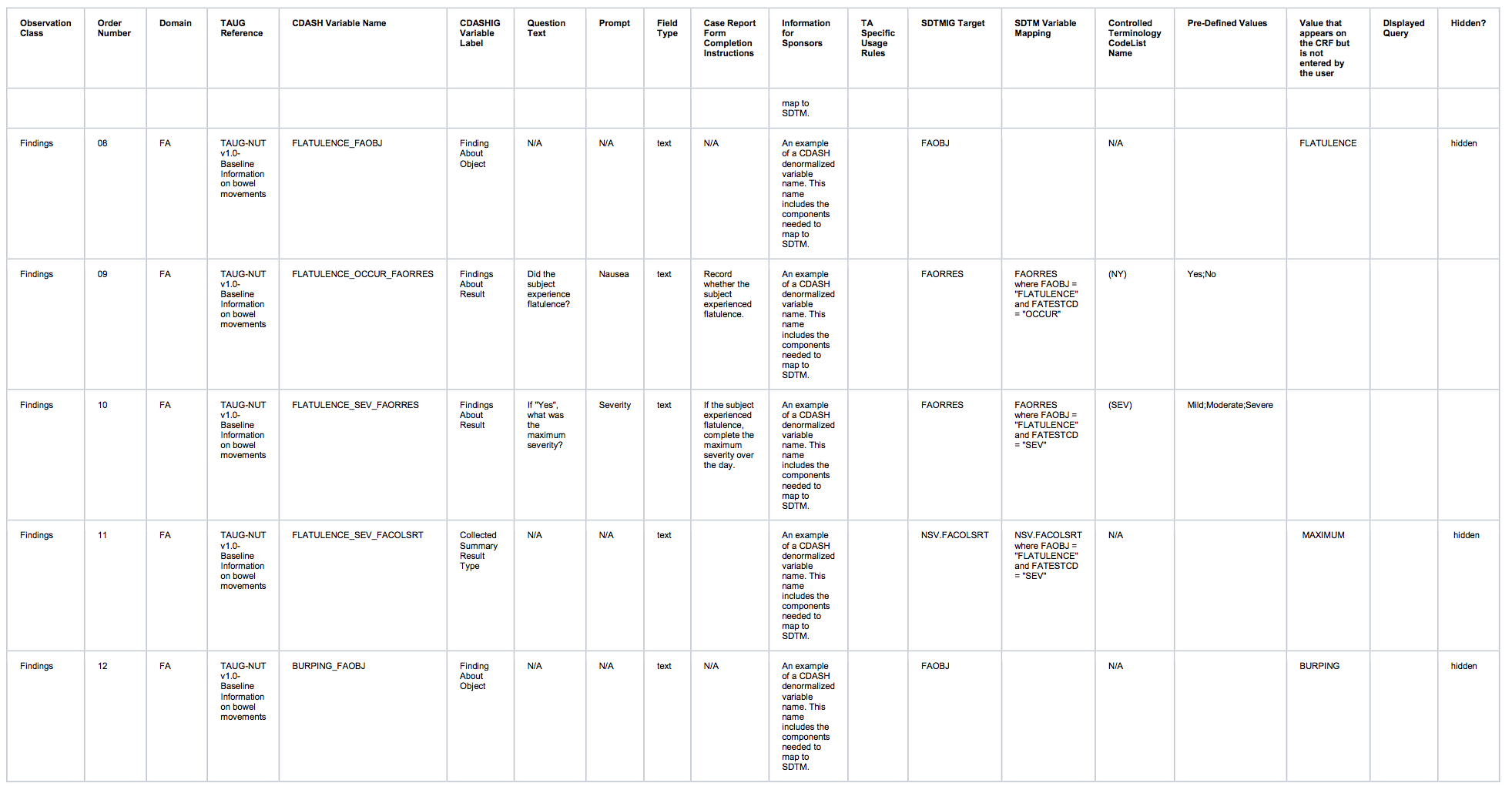
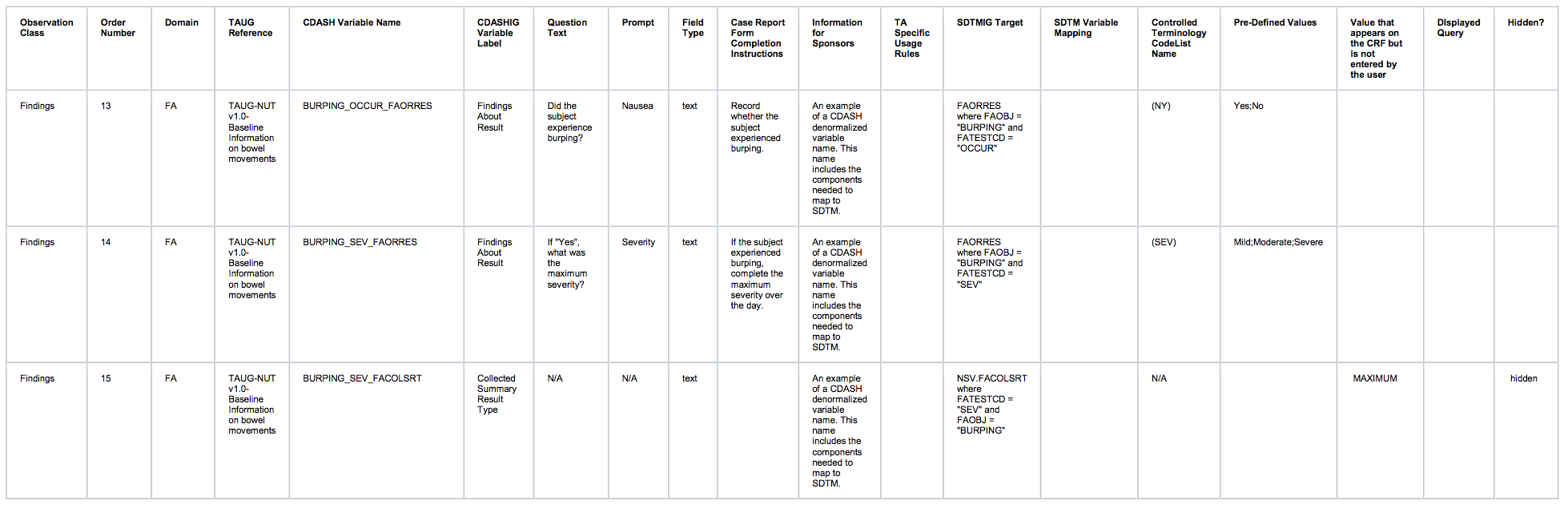
Observation Class Order Number Domain TAUG Reference CDASH Variable Name CDASHIGVariable Label Question Text Prompt Field Type Case Report FormCompletionInstructions Information for Sponsors TASpecificUsage Rules SDTMIG Target SDTM Variable Mapping ControlledTerminology CodeList Name Pre-Defined Values Value that appears on the CRF but is not entered by the user DIsplayed Query Hidden? Findings 01 FA TAUG-NUT v1.0-Baseline Information on bowel movements FAOBJ Finding About Object N/A N/A text N/A N/A FAOBJ N/A BOWEL MOVEMENT hidden Findings 02 FA TAUG-NUT v1.0-Baseline Information on bowel movements FAEVLINT Evaluation Interval N/A N/A text N/A N/A FAEVLINT and LBEVLINT N/A -P1D hidden
Findings 03 FA TAUG-NUT v1.0-Baseline Information on bowel movements FATPT PlannedTime PointName When was the diary completed? Diary Day text Record when the diary was completed. N/A FATPT and LBTPT N/A END OF DIARY DAY -3;ENDOF DIARY DAY -2;END OFDIARY DAY -1 Findings 04 FA TAUG-NUT v1.0-Baseline Information on bowel movements FADAT Date of Collection What is the diary date? Diary Date text Record the date the subject indicated the diary was completed. N/A FADTC and LBDTC Findings 05 FA TAUG-NUT v1.0-Baseline Information on bowel movements EVENTFRQ_FAORRES Findings About Result What is the number of bowel movements reported by the subject? Number of bowel movements integer Record the number of bowel movements that the subject had on this day. An example of a CDASH denormalized variable name. This name includes the components needed to map to SDTM FAORRES FAORRES where FATESTCD = "EVENTFRQ" N/A Findings 06 LB TAUG-NUT v1.0-Baseline Information on bowel movements LBSPEC Specimen Type N/A N/A text N/A N/A LBSPEC N/A STOOL hidden Findings 07 LB TAUG-NUT v1.0-Baseline Information on bowel movements CONSIST_LBORRES Findings About Result What is the typical consistency of bowel movements as reported by the subject? Typical bowel movement consistency text Record the typical consistency of the subject's stools over this day. An example of a CDASH denormalized variable name. This name includes the components needed to map to SDTM LBORRES LBORRES where LBTESTCD = "CONSIST" N/A Watery;Loose;Soft;Formed;Hard Findings 08 LB TAUG-NUT v1.0-Baseline Information on bowel movements CONSIST_LBCOLSRT CollectedSummaryResult Type N/A N/A text An example of a CDASH denormalized variable name. This name includes the components needed to map to SDTM NSV.LBCOLSRT NSV.LBCOLSRT where LBTESTCD = "CONSIST" N/A TYPICAL hidden
Row STUDYID DOMAIN USUBJID FASEQ FALNKID FASPID FATESTCD FATEST FAOBJ FAORRES FAORRESU FASTRESC FASTRESN FASTRESU FAEVAL VISITNUM FADTC FATPT FATPTNUM FAEVLINT 1 NUTR123 FA NUTR123_001 1 D-3 1 EVENTFRQ Event Frequency BOWEL MOVEMENT 2 /day 2 2 /day STUDY SUBJECT 2017-01-02 END OF DIARY DAY -3 -3 -P1D 2 NUTR123 FA NUTR123_001 2 D-2 2 EVENTFRQ Event Frequency BOWEL MOVEMENT 3 /day 3 3 /day STUDY SUBJECT 2017-01-03 END OF DIARY DAY -2 -2 -P1D 3 NUTR123 FA NUTR123_001 3 D-1 3 EVENTFRQ Event Frequency BOWEL MOVEMENT 2 /day 2 2 /day STUDY SUBJECT 2017-01-04 END OF DIARY DAY -1 -1 -P1D
Row STUDYID DOMAIN USUBJID LBSEQ LBLNKID LBSPID LBTESTCD LBTEST LBORRES LBSTRESC LBSPEC LBEVAL VISITNUM LBTPT LBTPTNUM LBEVLINT LBCOLSRT 1 NUTR123 LB NUTR123_001 1 D-3 1 CONSIST Consistency LOOSE LOOSE STOOL STUDY SUBJECT 2017-01-02 END OF DIARY DAY -3 -3 -P1D TYPICAL 2 NUTR123 LB NUTR123_001 2 D-2 2 CONSIST Consistency HARD HARD STOOL STUDY SUBJECT 2017-01-03 END OF DIARY DAY -2 -2 -P1D TYPICAL 3 NUTR123 LB NUTR123_001 3 D-1 3 CONSIST Consistency LOOSE LOOSE STOOL STUDY SUBJECT 2017-01-04 END OF DIARY DAY -1 -1 -P1D TYPICAL
Variable Label Type Codelist Role Origin LBCOLSRT Collected Summary Result Type text Non-standard Variable Qualifier CRF
Row STUDYID RDOMAIN USUBJID IDVAR IDVARVAL RELTYPE RELID 1 NUTR123 FA FALNKID ONE 1 2 NUTR123 LB LBLNKID
ONE 1 5.1.3 Bowel Movements - Each Episode
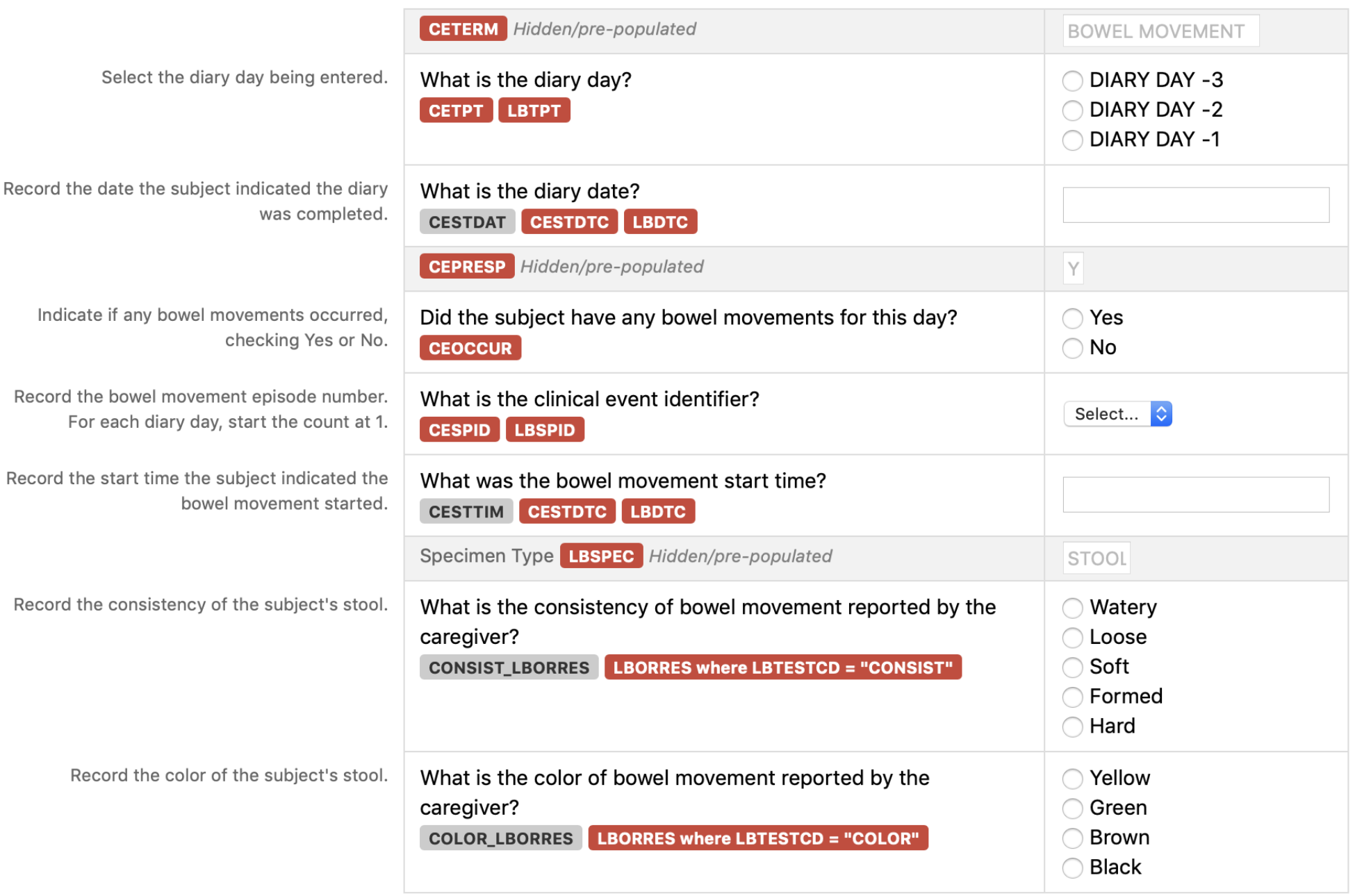
Observation Class Order Number Domain TAUG Reference CDASH Variable Name CDASHIGVariable Label Question Text Prompt Field Type Case Report FormCompletionInstructions Information for Sponsors TASpecific Usage Rules SDTMIG Target SDTM Variable Mapping ControlledTerminology CodeList Name Pre-Defined Values Value that appears on the CRF but is not entered by the user DIsplayed Query Hidden? Findings 01 CE TAUG-NUT v1.0-Baseline Information on bowel movements CETERM Clinical EventReportedTerm N/A N/A text N/A CETERM CETERM N/A BOWEL MOVEMENT Hidden Findings 02 CE TAUG-NUT v1.0-Baseline Information on bowel movements CETPT Planned Time Point Name What is the diary day? Diary Day text Select the diary day being entered. CETPT CETPT; LBTPT N/A DIARY DAY -3;DIARY DAY-2;DIARYDAY -1 Findings 03 FA TAUG-NUT v1.0-Baseline Information on bowel movements CESTDAT Clinical Event Start Date What is the diary date? Diary Date text Record the date the subject indicated the diary was completed. This date is combined with the Start Time of the bowel movement to populate CESTDTC and LBDTC. CESTDTC CESTDTC ; LBDTC Findings 04 FA TAUG-NUT v1.0-Baseline Information on bowel movements CEPRESP Clinical Event Pre-Specified N/A N/A text CEPRESP Y Hidden Findings 05 FA TAUG-NUT v1.0-Baseline Information on bowel movements CEOCCUR Clinical Event Occurrence Did the subject have any bowel movements for this day? Bowel Movements text Indicate if any bowel movements occurred, checking Yes or No. CEOCCUR Yes; No Findings 06 FA TAUG-NUT v1.0-Baseline Information on bowel movements CESPID Clinical EventSponsor-DefinedIdentifier What is the clinical event identifier? BowelMovementNumber text Record the bowel movement episode number. For each diary day, start the count at 1. CESPID CESPID; LBSPID N/A 1; 2; 3; 4; 5; 6; 7; 8; 9; 10 Findings 07 FA TAUG-NUT v1.0-Baseline Information on bowel movements CESTTIM Start Time of Clinical Event What was the bowel movement start time? Start Time of the bowel movement text Record the start time the subject indicated the bowel movement started. This time is combined with the diary date to populate CESTDTC and LBDTC. CESTDTC CESTDTC; LBDTC Findings 08 LB TAUG-NUT v1.0-Baseline Information on bowel movements LBSPEC Specimen Type N/A text N/A LBSPEC N/A STOOL Hidden Findings 09 LB TAUG-NUT v1.0-Baseline Information on bowel movements CONSIST_LBORRES What is the consistency of bowel movement reported by the caregiver? Bowel movement consistency text Record the consistency of the subject's stool. An example of a CDASH denormalized variable name. This name includes the components needed to map to SDTM. LBORRES LBORRES where LBTESTCD = "CONSIST" N/A Watery;Loose; Soft;Formed; Hard Findings 10 LB TAUG-NUT v1.0-Baseline Information on bowel movements COLOR_LBORRES What is the color of bowel movement reported by the caregiver? Bowel movement color text Record the color of the subject's stool. An example of a CDASH denormalized variable name. This name includes the components needed to map to SDTM. LBORRES LBORRES where LBTESTCD = "COLOR" N/A
Yellow;Green;Brown; Black
Rows 1-3: Show 3 bowel movement episodes on the first day of the diary. Rows 4-5: Show 2 bowel movement episodes on the second day of the diary. Rows 6-7: Show 2 bowel movement episodes on the third day of the diary. Row 8: Shows an example of where there are no bowel movements episodes for the first day of the diary (although this is quite unlikely). Row 9: Shows an example of where the caregiver forgot to record any bowel movement episodes for the second day of the diary. In this example, there was just a line struck through the diary with the note "Forgot to complete". As no other data was recorded by the caregiver, CESTDTC and CEOCCUR are blank. The CESTAT variable is used to show that the bowel movement assessments were not done on this day and the CEREASND variable is used to store the reason.
Row STUDYID DOMAIN USUBJID CESEQ CELNKID CESPID CETERM CEPRESP CEOCCUR CESTAT CEREASND CESTDTC CETPT CETPTNUM CEEVAL 1 NUTR123 CE NUTR123_001 1 D-3_1 1 BOWEL MOVEMENT Y Y 2017-01-02T09:45 DIARY DAY -3 -3 CAREGIVER 2 NUTR123 CE NUTR123_001 2 D-3_2 2 BOWEL MOVEMENT Y Y 2017-01-02T12:45 DIARY DAY -3 -3 CAREGIVER 3 NUTR123 CE NUTR123_001 3 D-3_3 3 BOWEL MOVEMENT Y Y 2017-01-02T19:45 DIARY DAY -3 -3 CAREGIVER 4 NUTR123 CE NUTR123_001 4 D-2_1 1 BOWEL MOVEMENT Y Y 2017-01-03T08:30 DIARY DAY -2 -2 CAREGIVER 5 NUTR123 CE NUTR123_001 5 D-2_2 2 BOWEL MOVEMENT Y Y 2017-01-03T20:00 DIARY DAY -2 -2 CAREGIVER 6 NUTR123 CE NUTR123_001 6 D-1_1 1 BOWEL MOVEMENT Y Y 2017-01-04T09:00 DIARY DAY -1 -1 CAREGIVER 7 NUTR123 CE NUTR123_001 7 D-1_2 2 BOWEL MOVEMENT Y Y 2017-01-04T21:00 DIARY DAY -1 -1 CAREGIVER 8 NUTR123 CE NUTR123_002 1 D-3_1 1 BOWEL MOVEMENT Y N DIARY DAY -3 -3 CAREGIVER 9 NUTR123 CE
NUTR123_002 2 D-2_1
1 BOWEL MOVEMENT Y
NOT DONE FORGOT TO COMPLETE
DIARY DAY -2 -2 CAREGIVER
Variable Label Type Role Codelist Origin CEEVAL Evaluator text Non-standard Record Qualifier (EVAL) CRF
Row STUDYID DOMAIN USUBJID LBSEQ LBLNKID LBSPID LBTESTCD LBTEST LBORRES LBSTRESC LBSPEC LBEVAL VISITNUM LBDTC LBTPT LBTPTNUM 1 NUTR123 LB NUTR123_001 1 D-3_1 1 CONSIST Consistency LOOSE LOOSE STOOL CAREGIVER 2017-01-02T09:45 DIARY DAY -3 -3 2 NUTR123 LB NUTR123_001 2 D-3_1 1 COLOR Color BROWN BROWN STOOL CAREGIVER 2017-01-02T09:45 DIARY DAY -3 -3 3 NUTR123 LB NUTR123_001 3 D-3_2 2 CONSIST Consistency HARD HARD STOOL CAREGIVER 2017-01-02T12:45 DIARY DAY -3 -3 4 NUTR123 LB NUTR123_001 4 D-3_2 2 COLOR Color BROWN BROWN STOOL CAREGIVER 2017-01-02T12:45 DIARY DAY -3 -3 5 NUTR123 LB NUTR123_001 5 D-3_3 3 CONSIST Consistency LOOSE LOOSE STOOL CAREGIVER 2017-01-02T19:45 DIARY DAY -3 -3 6 NUTR123 LB NUTR123_001 6 D-3_3 3 COLOR Color BROWN BROWN STOOL CAREGIVER 2017-01-02T19:45 DIARY DAY -3 -3 7 NUTR123 LB NUTR123_001 7 D-2_1 1 CONSIST Consistency HARD HARD STOOL CAREGIVER 2017-01-03T08:30 DIARY DAY -2 -2 8 NUTR123 LB NUTR123_001 8 D-2_1 1 COLOR Color BROWN BROWN STOOL CAREGIVER 2017-01-03T08:30 DIARY DAY -2 -2 9 NUTR123 LB NUTR123_001 9 D-2_2 2 CONSIST Consistency HARD HARD STOOL CAREGIVER 2017-01-03T20:00 DIARY DAY -2 -2 10 NUTR123 LB NUTR123_001 10 D-2_2 2 COLOR Color BROWN BROWN STOOL CAREGIVER 2017-01-03T20:00 DIARY DAY -2 -2 11 NUTR123 LB NUTR123_001 11 D-1_1 1 CONSIST Consistency HARD HARD STOOL CAREGIVER 2017-01-04T09:00 DIARY DAY -1 -1 12 NUTR123 LB NUTR123_001 12 D-1_1 1 COLOR Color BROWN BROWN STOOL CAREGIVER 2017-01-04T09:00 DIARY DAY -1 -1 13 NUTR123 LB NUTR123_001 13 D-1_2 2 CONSIST Consistency LOOSE LOOSE STOOL CAREGIVER 2017-01-04T21:00 DIARY DAY -1 -1 14 NUTR123 LB NUTR123_001 14 D-1_2 2 COLOR Color BROWN BROWN STOOL CAREGIVER 2017-01-04T21:00 DIARY DAY -1 -1
Row STUDYID DOMAIN USUBJID IDVAR IDVARVAL RELTYPE RELID 1 NUTR123 CE CELNKID ONE 1 2 NUTR123 LB LBLNKID
MANY 1 5.1.4 Symptom Assessments
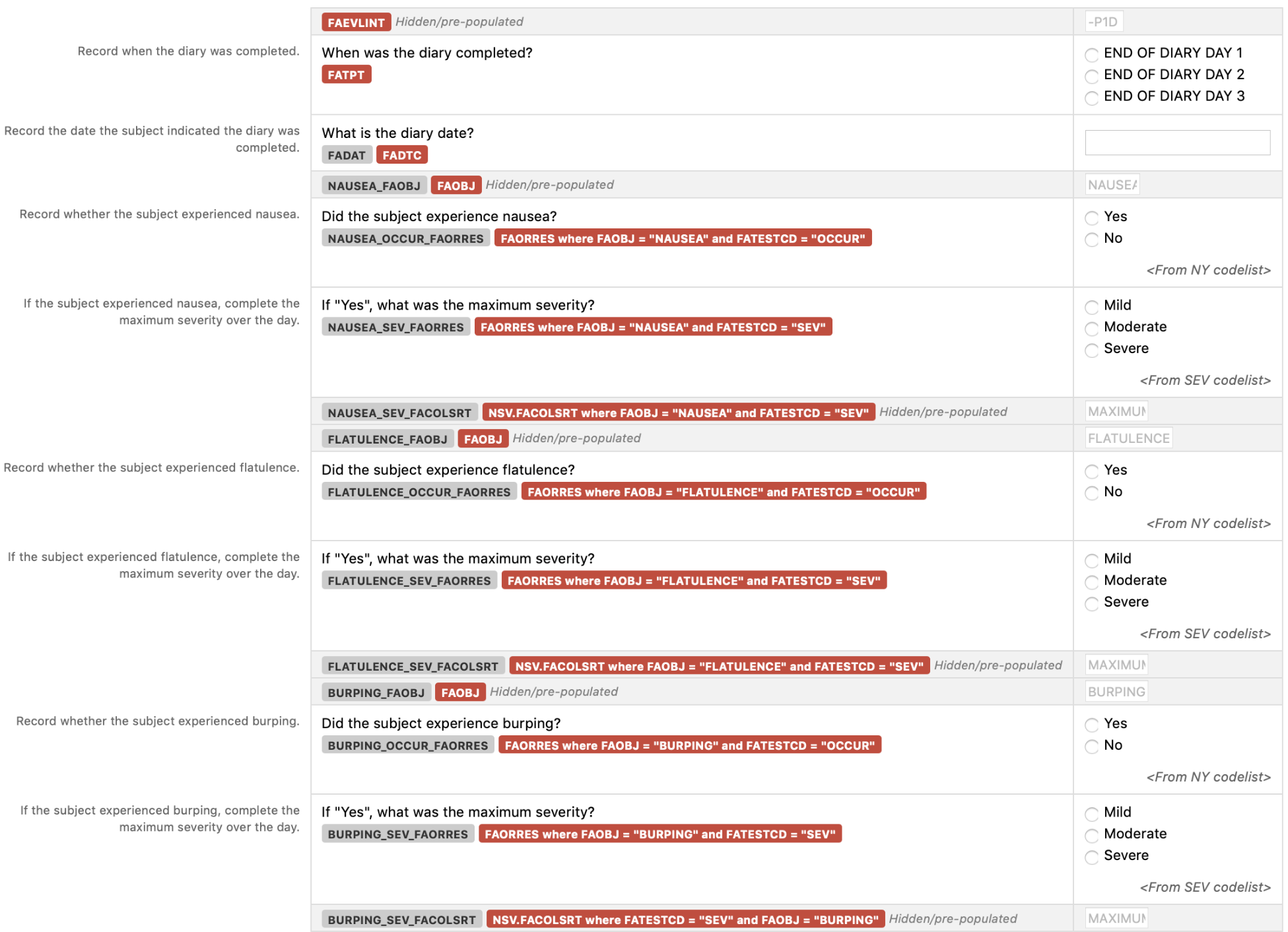
Rows 1-4: Show the diary results for diarrhea, flatulence, and burping for the first day of the diary (day 2 of the study). Rows 5-9: Show the diary results for diarrhea, flatulence, and burping for the second day of the diary (day 3 of the study).
Row STUDYID DOMAIN USUBJID FASEQ FASPID FATESTCD FATEST FAOBJ FACAT FAORRES FASTRESC FAEVAL VISITNUM FADTC FADY FATPT
FATPTNUM FAEVLINT FACOLSRT 1 NUTR123 FA NUTR123_001 1 1 OCCUR Occurrence Indicator DIARRHEA GI SYMPTOMS No N STUDY SUBJECT 2017-01-05 2 END OF DIARY DAY 1 1 -P1D 2 NUTR123 FA NUTR123_001 2 2 OCCUR Occurrence Indicator FLATULENCE GI SYMPTOMS No N STUDY SUBJECT 2017-01-05 2 END OF DIARY DAY 1 1 -P1D 3 NUTR123 FA NUTR123_001 3 3 OCCUR Occurrence Indicator BURPING GI SYMPTOMS Yes Y STUDY SUBJECT 2017-01-05 2 END OF DIARY DAY 1 1 -P1D
4 NUTR123 FA NUTR123_001 4 3 SEV Severity/Intensity BURPING GI SYMPTOMS Mild MILD STUDY SUBJECT 2017-01-05 2 END OF DIARY DAY 1 1 -P1D MAXIMUM 5 NUTR123 FA NUTR123_001 5 1 OCCUR Occurrence Indicator DIARRHEA GI SYMPTOMS No N STUDY SUBJECT 2017-01-06 3 END OF DIARY DAY 2 2 -P1D 6 NUTR123 FA NUTR123_001 6 2 OCCUR Occurrence Indicator FLATULENCE GI SYMPTOMS Yes Y STUDY SUBJECT 2017-01-06 3 END OF DIARY DAY 2 2 -P1D 7 NUTR123 FA NUTR123_001 7 2 SEV Severity/Intensity FLATULENCE GI SYMPTOMS Moderate MODERATE STUDY SUBJECT 2017-01-06 3 END OF DIARY DAY 2 2 -P1D MAXIMUM 8 NUTR123 FA NUTR123_001 8 3 OCCUR Occurrence Indicator BURPING GI SYMPTOMS Yes Y STUDY SUBJECT 2017-01-06 3 END OF DIARY DAY 2 2 -P1D 9 NUTR123 FA NUTR123_001 9 3 SEV Severity/Intensity BURPING GI SYMPTOMS Severe SEVERE STUDY SUBJECT 2017-01-06 3 END OF DIARY DAY 2 2 -P1D MAXIMUM
Variable Label Type Codelist Role Origin FACOLSRT Collected Summary Result Type text Non-standard Variable Qualifier CRF 5.2 Stool Sample Collection and Characteristics
Rows 1, 3: Show the date and time of the stool sample collection. Row 2: Shows the start and end date and time of drying the stool sample. Row 4: Shows the start date and time of freezing the stool sample. Because the samples remained in the freezer for long-term storage, the end date and time are not represented. The container number was represented in BEREFID to identify the sample.
Row STUDYID DOMAIN USUBJID BESEQ BEREFID BETERM BEDECOD VISITNUM VISIT VISITDY BEDTC BESTDTC BEENDTC BESTDY BEENDY BESPEC 1 NUTR123 BE NUTR123_001 1 ST123 Collected COLLECTING 1 BASELINE -1 2017-01-03T13:05 2017-01-03T13:05 -1 STOOL 2 NUTR123 BE NUTR123_001 2 ST123 Dried DRYING 1 BASELINE -1 2017-01-03T13:05 2017-01-03T14:45 2017-01-03T19:45 -1 -1 STOOL 3 NUTR123 BE NUTR123_002 1 ST124 Collected COLLECTING 1 BASELINE -1 2017-01-23T15:00 2017-01-23T15:00 -1 STOOL 4 NUTR123 BE NUTR123_002 2 ST124 Frozen FREEZING 1 BASELINE -1 2017-01-23T15:00 2017-01-23T17:00 -1 STOOL
Variable Label Type Role Codelist Origin BESPEC Specimen Type text Non-standard Record Qualifier SPECTYPE CRF
Row STUDYID DOMAIN USUBJID LBSEQ LBREFID LBTESTCD LBTEST LBCAT LBORRES LBORRESU LBSTRESC LBSTRESN LBSTRESU LBSPEC LBSPCCND LBLOBXFL VISITNUM VISIT VISITDY LBDTC 1 NUTR123 LB NUTR123_001 1 ST123 SPWEIGHT Specimen Weight SPECIMEN ASSESSMENT 95 g 95 95 g STOOL FRESH Y -1 Baseline -1 2017-01- 03T13:05 2 NUTR123 LB NUTR123_001 2 ST123 COLOR Color SPECIMEN ASSESSMENT Brown BROWN STOOL FRESH Y -1 Baseline -1 2017-01- 03T13:05 3 NUTR123 LB NUTR123_001 3 ST123 CONSIST Consistency SPECIMEN ASSESSMENT Soft SOFT STOOL FRESH Y -1 Baseline -1 2017-01- 03T13:05 4 NUTR123 LB NUTR123_001 4 ST123 SPWEIGHT Specimen Weight SPECIMEN ASSESSMENT 20 g 20 20 g STOOL DRIED Y -1 Baseline -1 2017-01- 03T13:05
Row STUDYID RDOMAIN USUBJID IDVAR IDVARVAL RELTYPE RELID 1 NUTR123 BE BEREFID MANY 1 2 NUTR123 LB LBREFID MANY 1 6 Questionnaires, Ratings, and Scales
Full Name and Abbreviation Copyright Permission Status Supplement Status Amsterdam Stool Scale Requested King's Stool Chart Requested
Baby Eating Behaviour Questionnaire - Concurrent (BEBQ CONCURRENT) Public domain Supplement in progress Baby Eating Behaviour Questionnaire - Retrospective (BEBQ RETROSPECTIVE) Public domain Supplement in progress Bristol Stool Form Scale (BSFS) No response received
International Physical Activity Questionnaire - Short Last 7 Days Telephone Format (IPAQ SF PHONE VERSION) Public domain Supplement in progress International Physical Activity Questionnaire - Long Last 7 Days Telephone Format (IPAQ LF PHONE VERSION) Public domain Supplement in progress International Physical Activity Questionnaire - Short Last 7 Days Self- Administered Format (IPAQ SF SELF-ADMINISTERED VERSION) Public domain Supplement in progress International Physical Activity Questionnaire - Long Last 7 Days Self- Administered Format (IPAQ LF SELF-ADMINISTERED VERSION) Public domain Supplement in progress 7 Appendices
Appendix A: Nutrition Team
Name Institution/Organization John Owen CDISC - Team lead Simon Lebeau Ividata Stats/Danone Research - Team Lead Stephane Auger Danone Research Emilie Ba Nestlé Geraldine Baggs Abbott Nutrition Dana Booth CDISC Fabien Foltzer Nestlé LuAnn Hitzemann Abbott Nutrition Hanneke Lankheet Danone Nutricia Research Jenni Reimari Dupont Johan Schilt Unilever Crystal Stollings Abbott Nutrition Elsbeth Verdonk Danone Nutricia Research Diane Wold CDISC Sue Zhang Abbott Nutrition Appendix B: Glossary and Abbreviations
aCRF Annotated case report form ADaM Analysis Data Model CDASH Clinical Data Acquisition Standards Harmonization Project CDASHIG Clinical Data Acquisition Standards Harmonization Project Implementation Guide CDISC Clinical Data Interchange Standards Consortium Collected Collected refers to information that is recorded and/or transmitted to the sponsor. This includes data entered by the site on CRFs/eCRFs as well as vendor data such as core lab data. This term is a synonym for captured. Controlled terminology A finite set of values that represent the only allowed values for a data item. These values may be codes, text, or numeric. A codelist is one type of controlled terminology. CRF Case report form (sometimes called a case record form). A printed, optical, or electronic document designed to record all required information to be reported to the sponsor for each trial subject. Domain A collection of observations with a topic-specific commonality about a subject. eCRF Electronic case report form EDC Electronic data collection GGG Global Governance Group GI Gastrointestinal NCI National Cancer Institute NSV Non-standard variable Patient A recipient of medical treatment SDS Submission Data Standards. Also the name of the team that maintains the SDTM and SDTMIG. SDTM Study Data Tabulation Model SDTMIG SDTM Implementation Guide for Human Clinical Trials SDTMIG-PGx SDTM Implementation Guide for Pharmacogenomics/Genetics Subject A participant in a study TAUG Therapeutic area user guide TAUG-Nutrition Therapeutic Area Data Standards User Guide for Nutritional Research Appendix C: Non-Standard Variables
Parent Domain Variable Label SAS Data Type Define-XML Data Type Codelist/ Controlled Termsa Role Description Notes FA, LB --COLSRT Collected Summary Result Type Char text Non-standard Variable Qualifier Variable qualifier of --TESTCD and --TEST. Used to indicate the type of collected summary result. (e.g., MAXIMUM, MINIMUM, MEAN, MEDIAN, NADIR). This includes source summary results collected on a CRF or provided by an external vendor (e.g., central lab). If the summary result is derived using individual source data records, this summary result should be represented in ADaM. If a sponsor has both a collected summary result and a derived summary result, the collected summary result should be represented in SDTM and the derived summary result should be represented in ADaM. FA, LB --SOURCE Source of Data Char text Non-standard Record Qualifier The original source of the data. Such as RECALL, MEDICAL RECORD, CENTRAL LAB. This should not be confused with EVAL which describes the role of the person who provided the evaluation. BE --SPEC Specimen Type Char text (SPECTYPE) Non-standard Record Qualifier Defines the type of specimen that undergoes the event. EX --NADEVI Number of Administrations in Eval. Int. Num integer Non-standard Record Qualifier The number of times the treatment was administered within a specific evaluation interval. CE --EVAL Evaluator Char text (EVAL) Non-standard Record Qualifier Role of the person who provided the evaluation. Appendix D: References
Appendix E: Representations and Warranties, Limitations of Liability, and Disclaimers