Therapeutic Area Data Standards
User Guide for Vaccines
Version 1.1 (Provisional)
Notes to Readers
- This is the provisional version 1.1 of the Therapeutic Area Data Standards User Guide for Vaccines (TAUG-Vax).
- This document is based on the SDTM v1.4 and the SDTMIG v3.2.
Revision History
| Date | Version |
|---|---|
| 2018-04-09 | 1.1 Provisional |
© 2018 Clinical Data Interchange Standards Consortium, Inc. All rights reserved.
Contents
- Appendices
- Appendix A: Project Proposal
- Appendix B: Vaccines Team
- Appendix C: Glossary and Abbreviations
- Appendix D: Non-Standard Variables
- Appendix E: References
- Appendix F: Representations and Warranties, Limitations of Liability, and Disclaimers
1 Introduction
This Therapeutic Area Data Standards User Guide for Vaccines (TAUG-Vax) was developed under the Coalition for Accelerating Standards and Therapies (CFAST) initiative.
The purpose of this TAUG-VAX is to describe how to use CDISC standards to represent data pertaining to vaccines studies. This first version (v1.1) focuses on safety data for reactogenicity events collected during vaccines trials.
- Other standards related to reactogenicity (e.g., collection of data using the CDASH model and Statistical Assessments using the ADaM model) will be covered in subsequent versions of this guide.
- Other topics (e.g., unsolicited adverse events, potential immune-mediated medical conditions (PIMMC), immunogenicity assessment and clinical efficacy assessment) will also be covered in subsequent versions of this guide.
1.1 How to Read This Document
- First, read SDTM v1.4 and SDTMIG v3.2 to gain some familiarity with basic SDTM modeling. These standards are available from: http://www.cdisc.org/.
- Next, read Introduction to Therapeutic Area Standards (at http://wiki.cdisc.org/x/SSy8AQ) and/or take CDISC's free training module TA001 - Overview of Therapeutic Area User Guides (at https://secure.trainingcampus.net/uas/modules/trees/store/wcatalog.aspx?cat=1015&ci=4&vtype=2&pi=0) to be sure to know what to expect from a therapeutic area user guide such as this document.
- Read this guide all the way through (without skipping any sections) at least once.
- Finally, revisit any sections of particular interest.
All general caveats for TA standards given in the Introduction to Therapeutic Area Standards (http://wiki.cdisc.org/x/SSy8AQ) apply to this document.
1.2 Organization of This Document
This document is divided into the following sections:
- Section 1, Introduction, provides an overall introduction to the purpose and goals of the Therapeutic Area Data Standards User Guide for Vaccines.
- Section 2, Overview, provides some general information on vaccines trials and reactogenicity events, in particular.
- Section 3, Case Description, provides background information about the type of study that the examples are based on.
- Section 4, Trial Design, provides examples used to support the modeling of Reactogenicity Safety Assessments.
- Section 5, Vaccine Administration, provides examples used to support the modeling of Reactogenicity Safety Assessments.
- Section 6, Reactogenicity Safety Assessments, provides examples of modeling reactogenicity data using different data collection methods.
- Section 7, Relating Records, provides information on how domains used in these examples can be linked.
- Appendices provide additional background material and describe other supplemental material relevant to vaccines.
1.3 Known Issues
- Timing variables: The time when a reactogenicity event is evaluated within an assessment interval is generally not defined precisely. Instructions to subjects filling out a diary card might refer to calendar days, or might ask the subject to complete the diary card at the end of the day before retiring for the night. Other than the value of "END DAY X" used to represent the planned assessment time point (--TPT), it is difficult to represent the imprecise timing of the collection of data on reactogenicity events. The variables --EVLINT and --EVINTX are used to capture the evaluation interval. However, the values such as -P1D are not intended to imply that data are collected in strict 24-hour intervals based on the time of day of vaccination (e.g., 10:30 on one day to 10:30 on the next day). For point-in-time measurements (i.e., not taken over an interval) such as temperature and diameter of swelling or redness, the timing variables other than --DTC are not populated, as one cannot assume that those values remain constant over the entire interval.
- Representation of the evaluator: Generally, the person who provides the evaluation of an event or finding can be represented in the variable --EVAL. In some vaccine studies where the subject self-reports reactogenicity events using a diary card, the investigator's assessment of the reported reactogenicity event may complement or possibly conflict with that of the subject (e.g., event severity). In some cases, the conflicting portions of the investigator's assessment may replace the subject-reported data. Both the study subject and the investigator contribute to the evaluation of a reactogenicity event. This topic is under discussion. Currently there is no agreed-upon solution to attribute specific variables within a record to more than one evaluator, to represent multiple values from conflicting assessments for a specific variable, or to document why the investigator modified the subject data. Sponsors should discuss how this should be handled with the relevant regulatory authority prior to data submission.
- Representation of event end dates that continue beyond the planned observation interval: When reactogenicity events continue beyond the planned assessment period, it is important to follow the subject until the event resolves. When these are considered clinical events, another record can be added in FA along with the planned daily assessments. It is important to capture the date that the event resolves, but the best way to do this within a Findings structure is unclear. The variable --ENDTC is generally only used in a Findings structure to represent the end date of specimen collection, where --DTC represents the start of the specimen collection. For that reason, --ENDTC was not used in FA or VS. Instead, the summary CE record was expanded and CEENDTC was used to represent the end date of the event.
- Representation of the maximum number of vomiting episodes when vomiting continues beyond the planned observation interval: During the planned observation period a subject may be asked to report the total number of vomiting episodes experienced each day. In this scenario, the evaluation period of "one day" can be represented in ISO8601 format as "-P1D" using the variable --EVLINT. However, when vomiting continues beyond the planned assessment period, the subject may be asked to report the maximum number of daily episodes experienced over the entire continuation period. Because the follow-up period beyond the planned observation period is not pre-specified, the timing around the concept of "maximum daily episodes" is challenging to represent unambiguously. For this case, we decided to represent the concept of "maximum" in the NSV, COLSRT (Collected Summary Result Type) and to populate the variable FAORRESU with "/day", as the value in FAORRES represents the maximum number of daily episodes and not the total number of the episodes during the period. We acknowledge that this may be an awkward solution and should be addressed in an update to the SDTM/SDTMIG.
- Representation of daily maximum temperature, redness at administration site, and severity when only one record is recorded: If subjects assess their temperature, redness at administration site, or event severity multiple times throughout the day, the protocol may specify for subjects to record only the maximum measurement taken. The examples in this guide show the concept of "maximum" represented in the non-standard variable, COLSRT (Collected Summary Result Type). However, the discussion around this topic is ongoing and may be subject to change at a later date.
- Variables for occurrence and severity: The representation of the occurrence and severity of an event in SDTM is dependent on the type of data collected. If data are collected for the entirety of an event, the variables CEOCCUR and CESEV are used. If data are collected for a part of the event, the data are represented as findings about the event, using the FATESTs "Occurrence Indicator" and "Severity/Intensity". This modeling decision is based on recommendations from an SDS sub-team looking at best practices at representing occurrence data.
- EPOCH of TREATMENT: At the time of publication, the current published controlled terminology CDISC definition of TREATMENT is "a period in a clinical study during which subjects receive therapeutic treatment. Since vaccines studies are generally preventive, this does not fall under the current definition. The consensus is that the definition can be expanded to "a period in a clinical study during which subjects receive investigational product," which will cover vaccines studies. This amendment has undergone public review and will be published in Controlled Terminology Package 30. Based on this decision, this user guide uses the term TREATMENT in the data examples.
- CDASH and ADaM: The scope of this version of the TAUG did not include CDASH and ADaM components. CDASH and ADaM components will be considered for development in either a supplement or subsequent version of the user guide. SDTM examples showing multiple collection strategies are shown in this TAUG. It is recommended, however, that sponsors consult with the regulatory agencies regarding the data to be collected and the SDTM mapping strategy to be used.
- Vaccine examples in previously published TAUGs: Prior to the publication of this guide, vaccine reactogenicity data examples have been published in the Influenza, Tuberculosis, and Ebola TAUGs. When in conflict, the Vaccines TAUG should be considered the authoritative source for best practices on modeling vaccine reactogenicity examples. During a future update, the Influenza, Tuberculosis, and Ebola TAUGs will be updated to be consistent with the Vaccines TAUG.
- Use of --DTC variable for CE summary records: The --DTC variable is used to indicate the collection date/time of an observation. It can also be used as the anchor date for the Evaluation Interval variables (--EVLINT and --EVINTX). Generally, these two uses do not conflict with each other. However, when a sponsor is creating a summary CE record based on the daily records in FA, the variable CEEVINTX is populated with "SINCE VACCINATION" to reflect that the record is showing whether or not there was a reactogenicity event over the planned observation period. In this case, the variable CEDTC needs to reflect the last day of assessment in order to anchor the variable CEEVINTX. Thus, CEDTC in this scenario represents the last day of assessment and not the date that the CE record was transcribed.
- Representation of redness at the site of administration: A diary card that is completed by the study subject may use the term redness to refer in layman's terms to the concept of erythema. In the examples shown in this guide it is assumed that the CE domain is subject to MedDRA coding, so the term "Redness" is used to populate the --TERM variables while --DECOD is populated with the MedDRA term "Erythema". As described in Section 6.4.3 of the SDTMIG v3.2 Variables Unique to Findings About, FA examples that include assessments of redness use the term "Erythema" to populate FAOBJ so that this term matches the value in --DECOD in the dictionary-coded parent domain.
- Reactogenicity Events that become reportable as Adverse Events: Reactogenicity events are usually expected and relatively mild, and so are represented in the CE (clinical events) domain in SDTM. However, protocols usually include criteria specifying when unusually severe or long-lasting reactogenicity events should be reported as adverse events. It is not clear whether a reactogenicity event that becomes a reportable adverse event should be represented only in the AE domain, or in both the CE and AE domains. Sponsors should consult with regulatory authorities for direction on this point.
- Use of CESTDTC and CEENDTC in global CE reactogenicity records: Reactogenicity examples in this guide are based on the assumption that if a reactogenicity event occurred anytime during the planned observation period, the overall start and end dates of the reactogenicity event were collected on the eCRF in addition to the individual dates from the daily diary collection. Individual dates from the daily diary collection are represented in the FA domain using the variable FADTC and the overall start and end dates of the event are represented in the CE domain using the variables CESTDTC and CEENDTC respectively. Please note that if only the daily diary collection dates were collected, CESTDTC and CEENDTC would not be populated.
2 Overview
Vaccines are preparations containing antigenic substances capable of inducing a specific and active immunity against a disease or infection. Such immunity may then result in the reduced transmission of that disease or infection.
An important aspect of vaccine development is the assessment of the vaccine's reactogenicity. Reactogenicity refers to the property of a substance to produce an expected or common adverse reaction when introduced into the body. For the purpose of this guide, reactogenicity event refers to a specific expected or common reaction following vaccine administration. In vaccine studies, a reactogenicity event(s) is typically caused by an inflammatory response to the vaccine under study and may include reactions like fever or redness at the site of administration. Reactogenicity describes immediate short-term reactions to vaccines, not long-term sequelae.
The term reaction usually implies that the adverse event has a causal relationship with the vaccination, or at least there exists a distinct possibility (see Adverse Drug Reaction in ICH E2A). Note that the causal relationship of a reactogenicity event with a study drug can be assessed during a clinical trial. It is possible for a reactogenicity event to be eventually assessed as not causally related to a study drug.
Reactogenicity is assessed in studies by monitoring a pre-defined set of adverse events over a pre-defined observation period. Pre-defined means identified prior to the start of the trial to support reactogenicity assessments of one or more investigational products in the study protocol. The pre-defined observation period starts immediately after the administration of one or more investigational product(s) and lasts a pre-defined number of days. Typically, this is 3 to 7 days, but it can extend to 21 days or more (e.g., for live attenuated vaccines). This short, pre-defined observation period does not cover the long-term safety assessments of the vaccines. To be considered for reactogenicity assessment, the monitored events, signs, or symptoms should start during the pre-defined observation period, but may extend beyond it.
Reactogenicity events can be classified as either administration site or systemic events.
- Administration site events are those occurring at or around the vaccine's administration site. The term local is also commonly used to refer to events at or around the administration site. Another term, localized event, is used to denote adverse events that have a localized manifestation, but not necessarily at the administration site (e.g., rash that is considered a systemic event, whose manifestation can be localized). To avoid any possible confusion between the terms "local" in the sense of occurring at the administration site and "localized", the term "administration site" will be used instead of "local" in this guide.
- Systemic reactogenicity events are those affecting an entire system or body. These reactogenicity events can be non-localized (e.g., fatigue or fever) or they can have localized manifestations (e.g., rash), where the localization cannot be pre-specified or predicted.
Manifestations of reactogenicity typically assessed in vaccine trials include: pain, tenderness, itching, bruising, erythema/redness, induration, and swelling at the administration site; and fever, fatigue, malaise, myalgia, arthralgia, headache, and nausea for systemic events. Some manifestations are typically associated to a specific sub-population, for example, vomiting, abnormal crying, drowsiness, appetite loss, or irritability for infants and toddlers.
Severity of the reactogenicity event may be assessed by the subject, the investigator, or both. When the investigator assesses the severity, it is common to use a standard toxicity grading scale such as the FDA Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials[1].
Reactogenicity events are solicited and are typically collected on either diary cards (paper or electronic device) or a reactogenicity case report form. Please note that throughout the TAUG, the term "transcribed" is used to define data that are collected on the diary card and subsequently stored in the sponsor's database. Users should be aware that data can be collected on both paper and electronic diary cards; therefore, in this user guide the term "transcribed" is also used to define loaded data from electronic diary cards where applicable.
3 Case Description
A hypothetical trial was used as the basis for examples in this user guide. This was a parallel design, comparator-controlled vaccine trial involving two co-administered vaccines, with both vaccines administered on two different occasions. For the purposes of this user guide, "co-administered" is defined as the administration of two or more vaccines at the same occasion, with the administrations being separated only by a short period of time, typically a few minutes. The vaccines would generally be administered at different locations, as presented in this guide, but could be administered at the same location.
Data were collected at four visits:
- Visit 1: Screening and first administration of vaccines
- Visit 2: Second administration of vaccines
- Visit 3: Final assessments following the TREATMENT Epoch (e.g., blood sampling)
- Visit 4: At the end of a 90-day follow-up period
This example was chosen because it can be extrapolated easily to more vaccines and/or more vaccination occasions. It also can be easily simplified to a single vaccine and/or a single administration occasion. This document focuses on parallel design studies. Parallel designs are typically used in vaccines studies due to the long-term persistence of vaccine effects in the body.
Data on reactogenicity events were collected via a reactogenicity collection form (e.g., a subject diary) for a pre-defined observation period of 3 days starting on the day of each vaccine's administration. Examples show data for only two systemic events, "Vomiting" and "Fever," and one administration site event, "Redness at administration site," recorded for each administration site.
- For vomiting, the number of vomiting episodes experienced during the day was recorded on each day (0 in case of no vomiting).
- For fever, the temperature was recorded on each day, whether or not fever occurred. One value was reported per day. The maximum value observed during the day was reported.
- For redness at administration sites, the presence of redness was assessed and, if present, the maximum longest diameter of the redness observed during the day was reported (measured using a provided ruler).
If an event continued past the 3-day observation period and was considered a clinical event, follow-up information was captured on the reactogenicity collection form. Follow-up data consisted of the end date of the event and the maximum value of the measure (e.g., maximum number of daily vomiting episodes) observed over the period starting at the end of the third day and lasting until either the resolution of the event or the end of the study. If an event continued past the 3-day observation period and was considered to be an adverse event, information about the event as a whole was captured on the adverse events collection form.
Investigator-recorded data about reactogenicity events included action taken, resolution, causal relationship to vaccination, and start and end date of the event.
4 Trial Design
The examples in this section are based on the case description found in Section 3, Case Description.
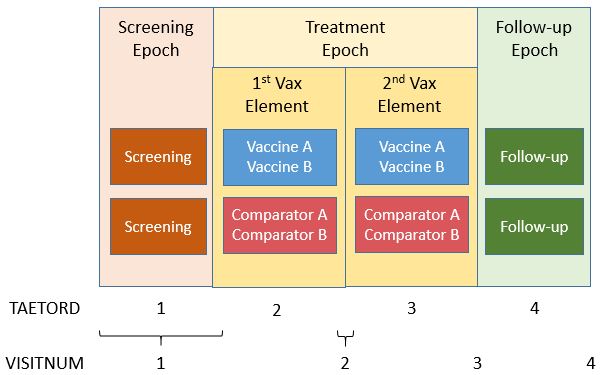
The example below shows a vaccine trial that has two study arms. During screening, subjects are randomized to receive either investigational Vaccine A and Vaccine B (ARMCD="VAXAB") or Comparator A and Comparator B (ARMCD="COMPAB"). In this example, both screening and vaccination activities are performed at the same visit (which is often the case in vaccines studies).
Subjects receive the co-administered vaccines at two different time points during the "TREATMENT" Epoch. This Epoch includes both the vaccination and the planned reactogenicity assessment period.
- The "TREATMENT" Epoch is divided into two Elements, each lasting 21 days.
- The "FOLLOW-UP" Epoch covers all activities related to the long-term safety follow-up and lasts 90 days.
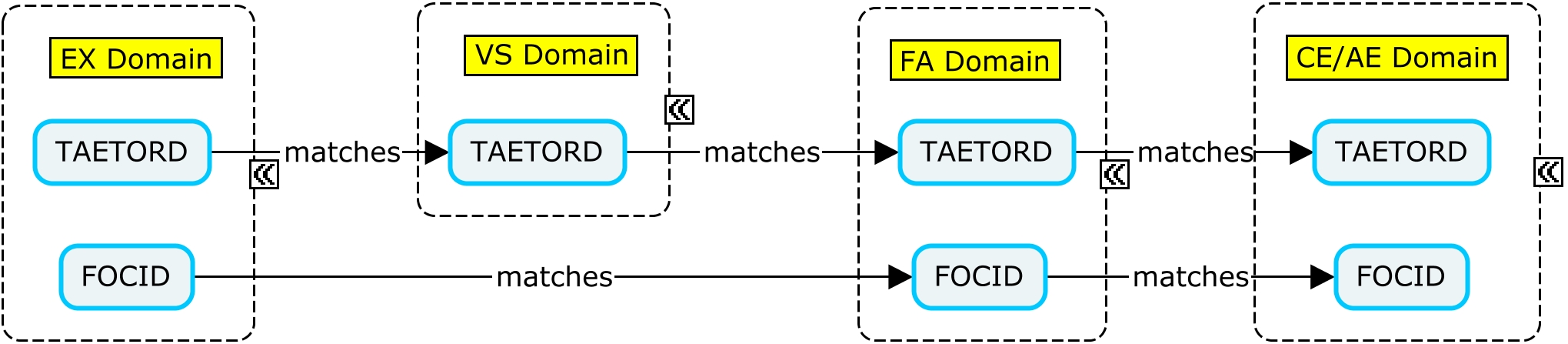
The variable TAETORD represents the planned order of the Element within an arm:
- TAETORD is equal to 1 for the screening Element.
- TAETORD is equal to 2 for the first vaccine administration Element.
- TAETORD is equal to 3 for the second vaccine administration Element.
- TAETORD is equal to 4 for the follow-up Element.
For the study design described above, the Trial Arms (TA), Trial Elements (TE), and Trial Visits (TV) example datasets are shown below. This trial could have been modeled differently, with each vaccination period considered a different Epoch. In either case, the Timing variable TAETORD can be used to distinguish between the first treatment Element, for which TAETORD="2" and the second, for which TAETORD="3". For this trial, the two vaccine administration Elements are the same. However, the vaccine administration Elements could be named differently (e.g., "Booster Vaccination") if the vaccine or combination of vaccines to be administered within the second Element differed from those to be administered within the first Element. For more information on trial design, refer to Section 7.2 of the SDTMIG v.3.2.
Figure - Trial Design
Example
The Trial Arms dataset shows the Elements and Epochs for the Vaccine A and B Arm (Rows 1-4) and the Comparator A and Comparator B (Rows 5-8).
ta.xpt
| Row | STUDYID | DOMAIN | ARMCD | ARM | TAETORD | ETCD | ELEMENT | TABRANCH | EPOCH |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | TA | VAXAB | Vaccine A Vaccine B | 1 | SCRN | Screening | Randomized to Vaccine A Vaccine B | SCREENING |
| 2 | ABC | TA | VAXAB | Vaccine A Vaccine B | 2 | VAXAB | Vaccine A and Vaccine B | TREATMENT | |
| 3 | ABC | TA | VAXAB | Vaccine A Vaccine B | 3 | VAXAB | Vaccine A and Vaccine B | TREATMENT | |
| 4 | ABC | TA | VAXAB | Vaccine A Vaccine B | 4 | FLLW | Follow-up | FOLLOW-UP | |
| 5 | ABC | TA | COMPAB | Comparator A Comparator B | 1 | SCRN | Screening | Randomized to Comparator A Comparator B | SCREENING |
| 6 | ABC | TA | COMPAB | Comparator A Comparator B | 2 | COMPAB | Comparator A and Comparator B | TREATMENT | |
| 7 | ABC | TA | COMPAB | Comparator A Comparator B | 3 | COMPAB | Comparator A and Comparator B | TREATMENT | |
| 8 | ABC | TA | COMPAB | Comparator A Comparator B | 4 | FLLW | Follow-up | FOLLOW-UP |
The Trial Elements dataset shows the start rule for each Element along with the end rule or Element duration.
te.xpt
| Row | STUDYID | DOMAIN | ETCD | ELEMENT | TESTRL | TEENRL | TEDUR |
|---|---|---|---|---|---|---|---|
| 1 | ABC | TE | SCRN | Screening | Informed consent | Time to complete eligibility, about an hour | |
| 2 | ABC | TE | VAXAB | Vaccine A and B | The first vaccination in the Element, with vaccine A and vaccine B | P21D | |
| 3 | ABC | TE | COMPAB | Comparator A and Comparator B | The first vaccination in the Element, with comparator vaccine A and comparator vaccine B | P21D | |
| 4 | ABC | TE | FLLW | Follow-up | 21 days after the start of the second occurrence of the vaccination Element | P90D |
The Trial Visits dataset is shown for this trial with explicitly scheduled start of visits.
tv.xpt
| Row | STUDYID | DOMAIN | VISITNUM | VISIT | TVSTRL | TVENRL |
|---|---|---|---|---|---|---|
| 1 | ABC | TV | 1 | VISIT 1 | Start of Screening Epoch | 30 minutes after completion of first set of vaccinations |
| 2 | ABC | TV | 2 | VISIT 2 | 21 days after start of first vaccination Element | 30 minutes after completion of second set of vaccinations |
| 3 | ABC | TV | 3 | VISIT 3 | 21 days after start of second vaccination Element | About 30 minutes after the start of the visit |
| 4 | ABC | TV | 4 | VISIT 4 | 90 days after end of the second vaccination Element | At trial exit |
5 Vaccine Administration
The example in this section is based on the case description found in Section 3, Case Description.
A vaccine trial may involve the administration of more than one vaccine at the same time point. In these cases, it is important to be able to distinguish between the administration sites when an assessment is performed. The variable FOCID (Focus of Study-Specific Interest) allows the assignment of identifiers to parts of the body to which treatments are applied and/or on which assessments are made. For vaccine studies, administration sites may be identified using FOCID, with values that distinguish between administration sites.
| Variable | Variable Label | Type | Description |
|---|---|---|---|
| FOCID | Focus of Study-Specific Interest | Char | Identification of a focus of study-specific interest on or within a subject or specimen as called out in the protocol for which a measurement, test, or examination was performed, such as a drug application site, e.g., "Injection site 1", "Biopsy site 1", "Treated site 1", or a more specific focus, e.g., "OD" (right eye) or "Upper left quadrant of the back". The value in this variable should have inherent semantic meaning. |
The Identifier variable FOCID was added to the SDTM in version 1.5. It is expected to be standard for use in human clinical trials in the next version of the SDTMIG; in this guide, however, it is represented as a non-standard variable. The examples in this guide show data for a subject who received two co-administered vaccines, with both vaccines administered on two different occasions. It is recommended that FOCID be populated for all administration site events, even in the case of a single administration. This provides a consistent approach, regardless of trial design, and supports future data aggregation. This guide uses the following naming convention:
| FOCID Value | Description of FOCID Value |
|---|---|
| SITE1A | Administration site at which vaccine/comparator A was applied at the first vaccination occasion |
| SITE1B | Administration site at which vaccine/comparator B was applied at the first vaccination occasion |
| SITE2A | Administration site at which vaccine/comparator A was applied at the second vaccination occasion |
| SITE2B | Administration site at which vaccine/comparator B was applied at the second vaccination occasion |
Example
This example shows vaccination data for a subject who received two vaccines, both at two different time points. On Study Day 1, the subject received vaccine A in her upper left arm (FOCID="SITE1A") and vaccine B in her upper right arm (FOCID="SITE1B"). The first set of vaccinations falls within the first Element of the "TREATMENT" Epoch and thus the variable TAETORD="2". On Study Day 22, the subject received a second round of vaccinations and again received vaccine A in her upper left arm (FOCID="SITE2A") and vaccine B in her upper right arm (FOCID="SITE2B"). The second set of vaccinations falls within the second Element of the "TREATMENT" Epoch and thus the variable TAETORD="3". The variables EXLOC, EXLAT, and EXDIR describe the FOCID location, laterality, and directionality. The total amount of product administered to the subject (EXTRT, EXDOSE, EXDOSEU), includes all active substances, adjuvants, and filler vehicles. Detailed composition is not provided in SDTM dataset but can be found by relating the total amount of product injected with the composition of the product detailed in the study protocol.
| Row 1: | Shows that the subject received vaccine A in her upper left arm on Study Day 1. |
|---|---|
| Row 2: | Shows that the subject received vaccine B in her upper right arm on Study Day 1. |
| Row 3: | Shows that the subject received vaccine A in her upper left arm on Study Day 22. |
| Row 4: | Shows that the subject received vaccine B in her upper right arm on Study Day 22. |
ex.xpt
| Row | STUDYID | DOMAIN | USUBJID | EXSEQ | EXTRT | EXCAT | EXDOSE | EXDOSU | EXDOSFRM | EXROUTE | EXLOC | EXLAT | EXDIR | VISITNUM | VISIT | TAETORD | EPOCH | EXDTC | EXSTDTC | EXENDTC | EXDY | FOCID | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | EX | ABC-1001 | 1 | VACCINE A | STUDY VACCINE | 0.5 | mL | INJECTION | INTRAMUSCULAR | ARM | LEFT | UPPER | 1 | VISIT 1 | 2 | TREATMENT | 2015-01-10 | 2015-01-10 | 2015-01-10 | 1 | SITE1A | |
| 2 | ABC | EX | ABC-1001 | 2 | VACCINE B | STUDY VACCINE | 0.5 | mL | INJECTION | INTRAMUSCULAR | ARM | RIGHT | UPPER | 1 | VISIT 1 | 2 | TREATMENT | 2015-01-10 | 2015-01-10 | 2015-01-10 | 1 | SITE1B | |
| 3 | ABC | EX | ABC-1001 | 3 | VACCINE A | STUDY VACCINE | 0.5 | mL | INJECTION | INTRAMUSCULAR | ARM | LEFT | UPPER | 2 | VISIT 2 | 3 | TREATMENT | 2015-01-31 | 2015-01-31 | 2015-01-31 | 22 | SITE2A | |
| 4 | ABC | EX | ABC-1001 | 4 | VACCINE B | STUDY VACCINE | 0.5 | mL | INJECTION | INTRAMUSCULAR | ARM | RIGHT | UPPER | 2 | VISIT 2 | 3 | TREATMENT | 2015-01-31 | 2015-01-31 | 2015-01-31 | 22 | SITE2B |
EX NSV Metadata
| Variable | Label | Type | Codelist | Role | Origin |
|---|---|---|---|---|---|
| FOCID | Focus of Study-Specific Interest | text | Non-Standard Identifier | CRF |
6 Reactogenicity Safety Assessments
The examples in this section are based on the case description found in Section 3, Case Description.
Systemic (e.g., fever, vomiting, and headache) and administration site (e.g., redness and pain) reactogenicity events are expected and monitored after vaccination. In this TAUG, the reactogenicity events are represented in the Clinical Events (CE) domain rather than the Adverse Events (AE) domain. However, a study protocol may specify conditions under which a reactogenicity event should also be reported as an adverse event. Those conditions may include seriousness (as defined for adverse events), duration, or other factors. Please note that it is important for sponsors to discuss with regulatory authorities which domain the reactogenicity data should be represented in. The collection and presentation of adverse events to support reactogenicity assessments have some requirements and specificities that set them apart from other adverse events collections and presentation. This will be further explained below.
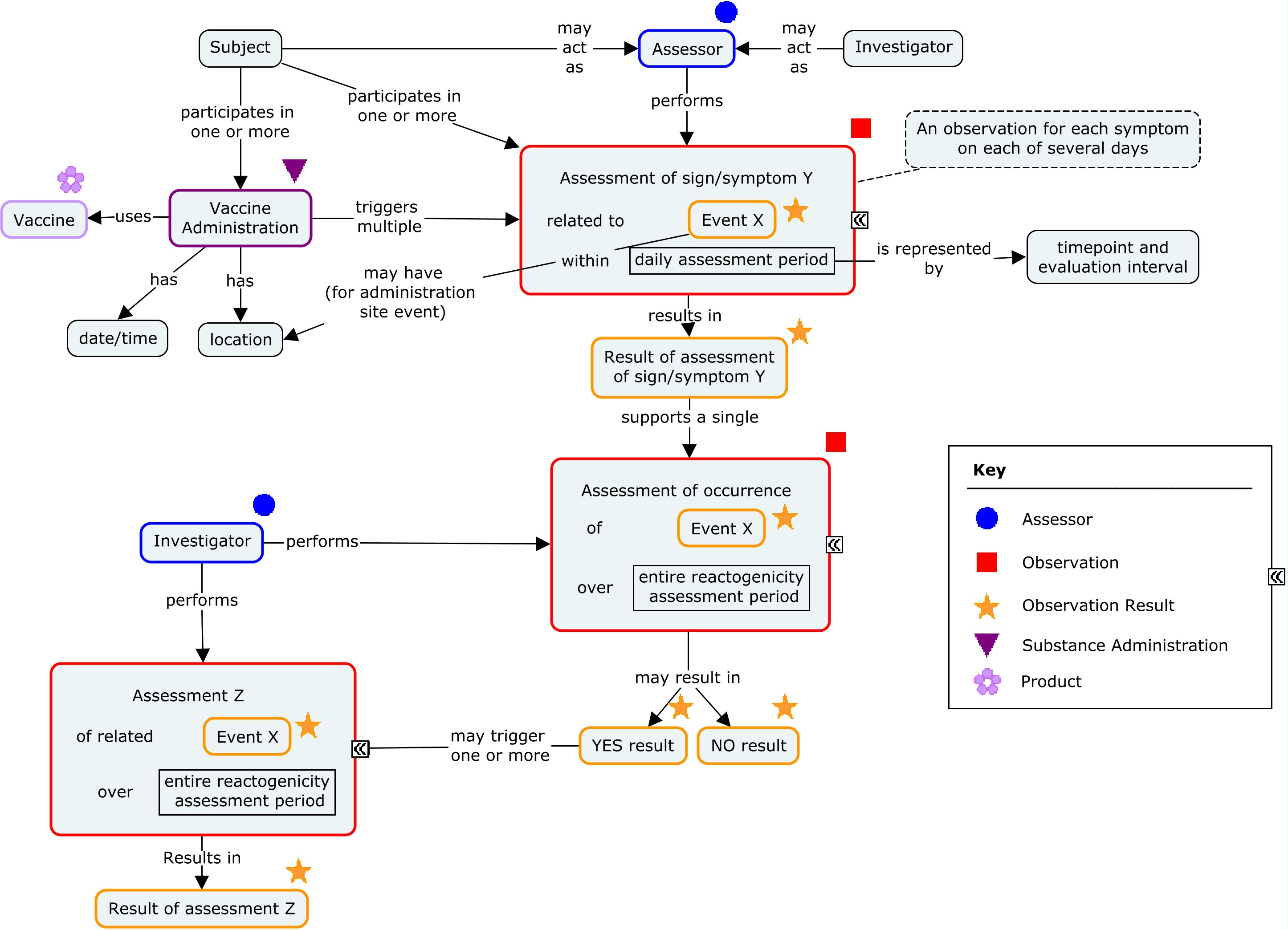
There are two generally used approaches to scheduling assessments of reactogenicity. One is to schedule daily assessments of reactogenicity for a pre-defined period following the vaccination (typically for 3-7 days). Such assessments are usually performed by the study subject and recorded in a diary or via an interactive voice response system (IVRS). An alternative approach is to schedule assessments by the investigator 3-7 days after the vaccination. These two approaches may also be combined. In all cases, reactogenicity events that are still present at the end of this initial assessment period will generally be followed until they end. The examples in this guide focus on daily assessments performed by the subject and reported on a daily diary card. These subject assessments are complemented by global assessments performed by the investigator at the end of the daily assessments period and reported in a case report form. The concept map below shows the components of a reactogenicity assessment.
Concept Map - Reactogenicity
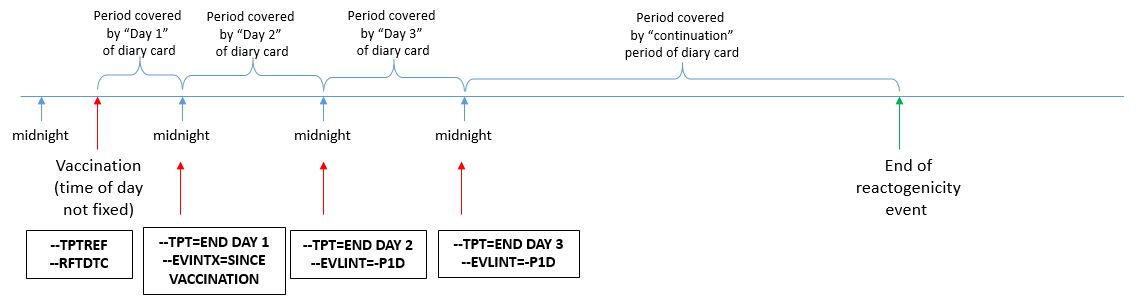
In the examples below, after each set of vaccinations, subjects monitor both systemic (fever and vomiting) and site administration (redness) reactogenicity events and record their findings on a daily diary form. Monitoring of symptoms starts immediately after vaccine administration. Monitoring continues through the end of the second calendar day after vaccination (day 3). The Planned Time Point variables (--TPT and --TPTNUM) provide a description of the time when the reactogenicity assessment should be completed (END DAY 1, END DAY 2, END DAY 3) relative to the vaccination as defined by the Time Point Reference variables (--TPTREF and --RFTDTC). The evaluation intervals during which reactogenicity events are monitored can be described using the variables Evaluation Interval (--EVLINT) and Evaluation Interval Text (--EVINTX) and are anchored by the --DTC variable. For all reactogenicity measurements taken on the day the vaccine was given (--TPT="END DAY 1"), the variable --EVINTX is populated with "SINCE VACCINATION". This is to make clear that the assessments do not apply to all of day 1 (i.e., not before the vaccine was given). For all assessments after day 1, the variable --EVLINT is populated with the appropriate ISO8601 evaluation interval .
In the scenario described in this guide, subjects were asked to report their maximum temperature and maximum redness over the course of a day. In this case, the concept of "maximum" is represented in the non-standard variable (COLSRT) and the use of the Planned Time Point, Time Point Reference, and Evaluation Interval variables are appropriate. However, for measurements taken at a single point in time (i.e., not a measurement over an interval), these variables may not be appropriate, as we cannot assume that these values remained constant over time.
It is important to note that in this scenario, subjects are not expected to complete the daily diary precisely at midnight, but to report the assessment(s) performed over the calendar day.
The figure below describes how the timing variables described above are used in the examples shown in this guide.
Figure - Assessment Timing
In the examples shown in this guide, all daily assessments from diary cards are represented in the FA and/or VS domains. If a global record is created to represent the event as a whole, that information is represented in the CE or AE domain (as defined by the protocol). Generally, the --DTC variable is used to indicate the collection date/time of an observation. It can also be used as the anchor date for the Evaluation Interval variables (--EVLINT and --EVINTX). Generally, these two uses do not conflict with each other. However, when a sponsor is creating a summary CE record based on the daily records in FA/VS, the variable CEEVINTX is populated with "SINCE VACCINATION" to reflect that the record is showing whether or not there was a reactogenicity event over the planned observation period. In this case, the variable CEDTC needs to reflect the last day of assessment in order to anchor the variable CEEVINTX. Thus, CEDTC in this scenario represents the last day of assessment and not the date that the CE record was transcribed.
A diary card that is completed by the study subject may use the term redness to refer in layman's terms to the concept of erythema. In the examples shown in this guide it is assumed that the CE domain is subject to MedDRA coding, so the term "Redness" is used to populate the --TERM variables, while --DECOD is populated with the MedDRA term "Erythema". As described in Section 6.4.3 of the SDTMIG v3.2 Variables Unique to Findings About, FA examples that include assessments of redness use the term "Erythema" to populate FAOBJ so that this term matches the value in --DECOD in the dictionary-coded parent domain.
In these examples, a reactogenicity event was considered to have occurred if:
- The subject's maximum temperature was greater than a specific value defined in the protocol (e.g., 98.6°F).
- The subject vomited at least once during the observation period.
- The subject experienced redness at the administration site.
Example
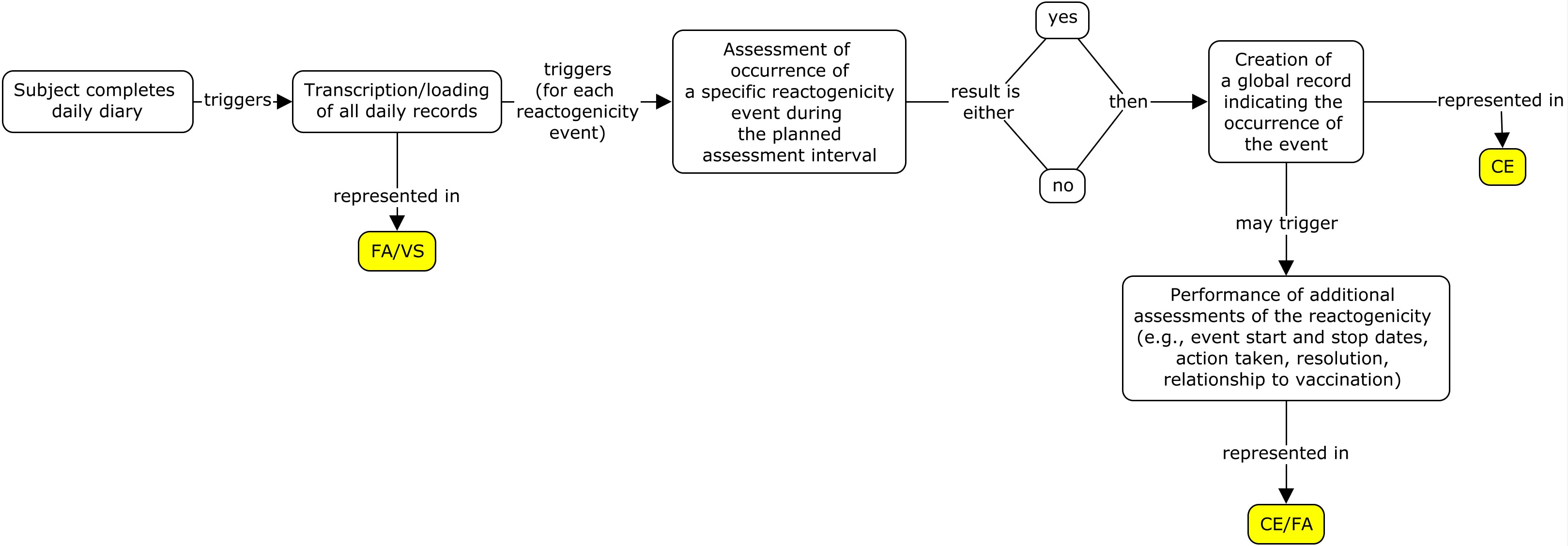
Different studies often use different strategies when transcribing data from paper diaries or loading data from electronic diaries. The following three examples show how to represent the same diary card data from one subject using three different strategies. Alternative strategies not described in this guide may be used by some sponsors. Before selecting a strategy, it is important to consult with the regulatory authority that the dataset is to be submitted to, as each may have a preference. The three strategies used in this guide are described below:
- All of the daily assessments are transcribed/loaded from the diary card and a global event record is created, whether or not a reactogenicity event occurred during the three-day assessment interval (flat model) .
- A global record is created for each reactogenicity event that is assessed; the daily assessment records for an event are transcribed/loaded only if the event occurred during the three-day assessment interval (nested model) .
- A global record is created for each type of reactogenicity category that is assessed (i.e., systemic and site administration events). If an event occurred in one of the categories, additional global records for all the events assessed in that category are created. Daily assessment records for an event are transcribed/loaded only if the event occurred during the three-day assessment interval (highly nested model) .
Please note that studies using electronic diary information tend to use strategy 1 since all of the diary information is easily loaded into the sponsor database and there is no manual data entry required for the subject diary. Paper diaries can use all three strategies, however strategy 2 and 3 may be used as a way to reduce the data entry burden from the paper diary to the diary eCRF page.
In these examples, the subject experienced all three events (fever, vomiting, and redness) at some point during the three-day evaluation period after the first set of vaccinations. She experienced vomiting on the first day, and fever and redness on both the first and second day. The subject took her temperature multiple times throughout the day but was asked to record only her maximum temperature on each day. After the second set of vaccinations, the subject did not experience any reactogenicity events during the three-day assessment interval.
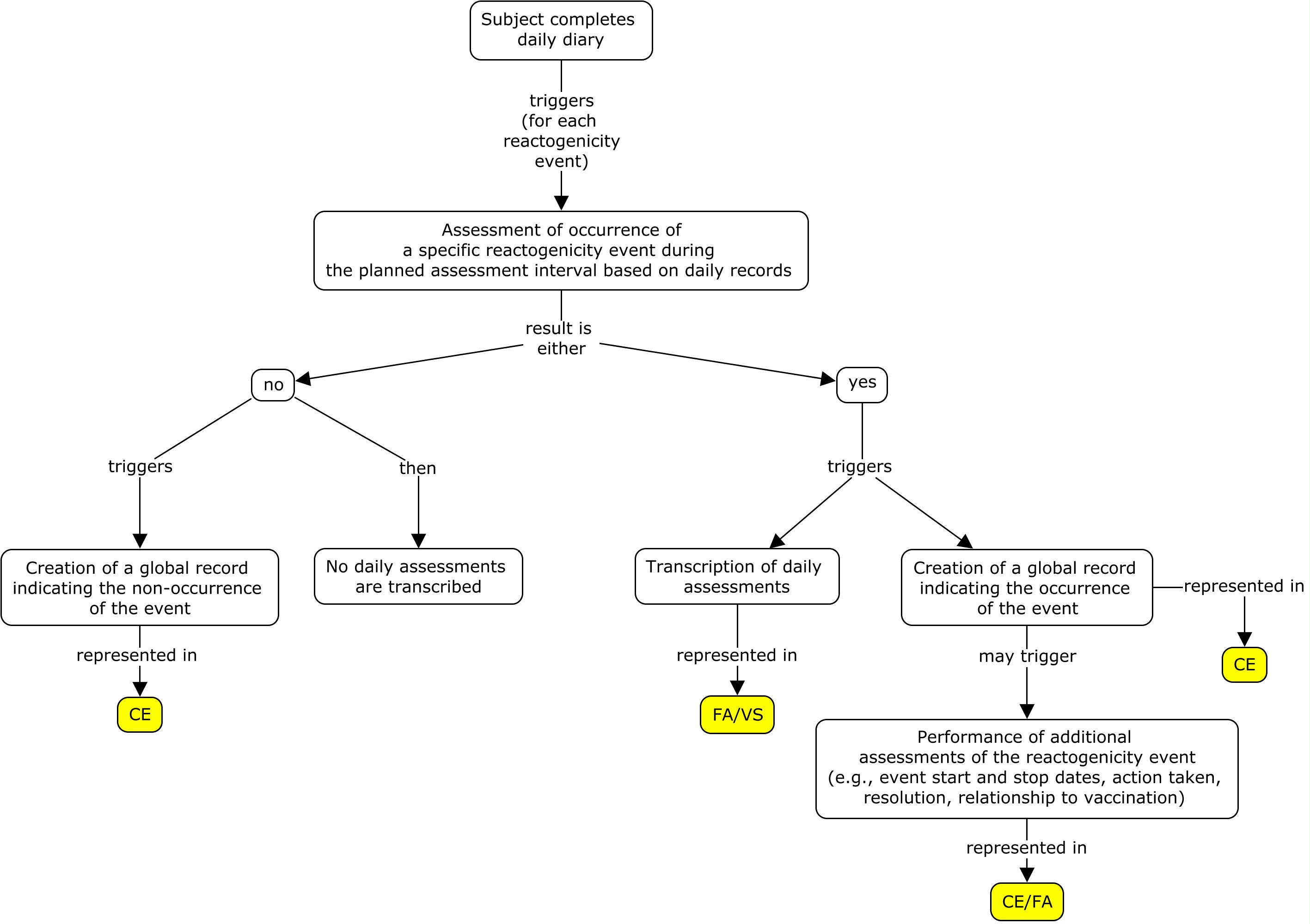
Example: Flat model
The example below shows daily assessments transcribed directly from the diary card. Based on the diary card, the sponsor created a global record in CE for each reactogenicity event assessed (fever, vomiting, and redness at administration site).
Daily assessments for redness and vomiting are represented using Findings About (FA). To represent the occurrence of redness, a value of "Occurrence Indicator" and "OCCUR" are used to populate FATEST and FATESTCD respectively, and the value in FAOBJ is the object of the assessment ("Erythema"). If redness occurred that day, the value in FAORRES was "Y"; otherwise, it was "N". Additionally, if redness occurred, the maximum longest diameter of the redness that was observed during the assessment period was collected. For vomiting, the number of vomiting episodes for the day was collected, where a value of 0 indicates vomiting did not occur. Temperature measurements are represented in the VS domain. In this example, if a reactogenicity event occurred anytime during the planned observation period, the overall start and end dates of the reactogenicity event were collected on the eCRF in addition to the individual dates from the daily diary collection. Individual dates from the daily diary collection are represented in the FA domain using the variable FADTC and the overall start and end dates of the event are represented in the CE domain using the variables CESTDTC and CEENDTC respectively. Please note that if only the daily diary collection dates were collected, CESTDTC and CEENDTC would not be populated.
Figure - Reactogenicity Flat Model
| Row 1: | Shows the temperature measurement on the first day of the first three-day observation period. The variable VSLNKGRP="2" and can be used to link the records for this observation period to the summary fever record in CE via CELNKGRP. |
|---|---|
| Rows 2-3: | Show the subject's temperature for the remaining two days of the first observation period. Since they belong to the same observation period as the record in row 1, the values in VSLNKGRP are the same. |
| Rows 4-6: | Show the subject's temperature for each day of the second three-day observation period. This time, the subject's temperature never rose above the protocol-defined value for determining fever. The variable VSLNKGRP="6" and can be used to link the records for this observation period to the summary fever record in CE via CELNKGRP. |
vs.xpt
| Row | STUDYID | DOMAIN | USUBJID | VSSEQ | VSLNKGRP | VSTESTCD | VSTEST | VSCAT | VSSCAT | VSORRES | VSORRESU | VSLOC | TAETORD | EPOCH | VSDTC | VSDY | VSTPT | VSTPTNUM | VSTPTREF | VSRFTDTC | VSEVLINT | VSEVINTX | VSCOLSRT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | VS | ABC-1001 | 1 | 2 | TEMP | Temperature | REACTOGENICITY | SYSTEMIC | 101 | F | AXILLA | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | MAXIMUM | ||
| 2 | ABC | VS | ABC-1001 | 2 | 2 | TEMP | Temperature | REACTOGENICITY | SYSTEMIC | 101 | F | AXILLA | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | MAXIMUM | ||
| 3 | ABC | VS | ABC-1001 | 3 | 2 | TEMP | Temperature | REACTOGENICITY | SYSTEMIC | 98.6 | F | AXILLA | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D | MAXIMUM | ||
| 4 | ABC | VS | ABC-1001 | 4 | 6 | TEMP | Temperature | REACTOGENICITY | SYSTEMIC | 98.6 | F | AXILLA | 3 | TREATMENT | 2015-01-31 | 22 | END DAY 1 | 1 | VACCINATION 2 | 2015-01-31 | SINCE VACCINATION | MAXIMUM | ||
| 5 | ABC | VS | ABC-1001 | 5 | 6 | TEMP | Temperature | REACTOGENICITY | SYSTEMIC | 98.6 | F | AXILLA | 3 | TREATMENT | 2015-02-01 | 23 | END DAY 2 | 2 | VACCINATION 2 | 2015-01-31 | -P1D | MAXIMUM | ||
| 6 | ABC | VS | ABC-1001 | 6 | 6 | TEMP | Temperature | REACTOGENICITY | SYSTEMIC | 98.6 | F | AXILLA | 3 | TREATMENT | 2015-02-02 | 24 | END DAY 3 | 3 | VACCINATION 2 | 2015-01-31 | -P1D | MAXIMUM |
VS NSV Metadata
| Variable | Label | Type | Codelist | Role | Origin |
|---|---|---|---|---|---|
| VSCOLSRT | Collected Summary Result Type | text | Non-Standard Variable Qualifier of --TESTCD and --TEST | CRF |
| Rows 1-3: | Represent the number of vomiting episodes over the day for each of the three days of the observation period following the Day 1 vaccine administration. |
|---|---|
| Rows 4, 6, 8: | Represent the results of questions about the occurrence of redness at the administration side with FOCID = "SITE1A" following the Day 1 vaccine administration. |
| Rows 5, 7: | Since there was redness on two of the days of the observation period, the longest diameter of the redness was collected. |
| Rows 9, 10, 11: | Represent the results of questions about the occurrence of redness at the administration side with FOCID = "SITE1B" following the Day 1 vaccine administration. Since redness did not occur, there are no records for the longest diameter of redness. |
| Rows 12-14: | Represent the results of questions about the number of vomiting episodes over the day on the three days of the observation period following the Day 22 vaccine administration. |
| Rows 15-20: | Represent the results of questions about the occurrence of redness at the administration sites with FOCID = "SITE2A" and FOCID = "SITE2B" following the Day 22 vaccine administration. Since redness did not occur, there are no records for the longest diameter of redness. |
face.xpt
| Row | STUDYID | DOMAIN | USUBJID | FASEQ | FALNKGRP | FATESTCD | FATEST | FAOBJ | FACAT | FASCAT | FAORRES | FAORRESU | TAETORD | EPOCH | FADTC | FADY | FATPT | FATPTNUM | FATPTREF | FARFTDTC | FAEVLINT | FAEVINTX | FOCID | FACOLSRT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | FA | ABC-1001 | 1 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 3 | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | |||||
| 2 | ABC | FA | ABC-1001 | 2 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 0 | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | |||||
| 3 | ABC | FA | ABC-1001 | 3 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 0 | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D | |||||
| 4 | ABC | FA | ABC-1001 | 4 | 3 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1A | ||||
| 5 | ABC | FA | ABC-1001 | 5 | 3 | LDIAM | Longest Diameter | Erythema | REACTOGENICITY | ADMINISTRATION SITE | 35 | mm | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1A | MAXIMUM | ||
| 6 | ABC | FA | ABC-1001 | 6 | 3 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1A | ||||
| 7 | ABC | FA | ABC-1001 | 7 | 3 | LDIAM | Longest Diameter | Erythema | REACTOGENICITY | ADMINISTRATION SITE | 25 | mm | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1A | MAXIMUM | ||
| 8 | ABC | FA | ABC-1001 | 8 | 3 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | N | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1A | ||||
| 9 | ABC | FA | ABC-1001 | 9 | 4 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | N | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1B | ||||
| 10 | ABC | FA | ABC-1001 | 10 | 4 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | N | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1B | ||||
| 11 | ABC | FA | ABC-1001 | 11 | 4 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | N | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1B | ||||
| 12 | ABC | FA | ABC-1001 | 12 | 5 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 0 | 3 | TREATMENT | 2015-01-31 | 22 | END DAY 1 | 1 | VACCINATION 2 | 2015-01-31 | SINCE VACCINATION | |||||
| 13 | ABC | FA | ABC-1001 | 13 | 5 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 0 | 3 | TREATMENT | 2015-02-01 | 23 | END DAY 2 | 2 | VACCINATION 2 | 2015-01-31 | -P1D | |||||
| 14 | ABC | FA | ABC-1001 | 14 | 5 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 0 | 3 | TREATMENT | 2015-02-02 | 24 | END DAY 3 | 3 | VACCINATION 2 | 2015-01-31 | -P1D | |||||
| 15 | ABC | FA | ABC-1001 | 15 | 7 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | N | 3 | TREATMENT | 2015-01-31 | 22 | END DAY 1 | 1 | VACCINATION 2 | 2015-01-31 | SINCE VACCINATION | SITE2A | ||||
| 16 | ABC | FA | ABC-1001 | 16 | 7 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | N | 3 | TREATMENT | 2015-02-01 | 23 | END DAY 2 | 2 | VACCINATION 2 | 2015-01-31 | -P1D | SITE2A | ||||
| 17 | ABC | FA | ABC-1001 | 17 | 7 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | N | 3 | TREATMENT | 2015-02-02 | 24 | END DAY 3 | 3 | VACCINATION 2 | 2015-01-31 | -P1D | SITE2A | ||||
| 18 | ABC | FA | ABC-1001 | 18 | 8 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | N | 3 | TREATMENT | 2015-01-31 | 22 | END DAY 1 | 1 | VACCINATION 2 | 2015-01-31 | SINCE VACCINATION | SITE2B | ||||
| 19 | ABC | FA | ABC-1001 | 19 | 8 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | N | 3 | TREATMENT | 2015-02-01 | 23 | END DAY 2 | 2 | VACCINATION 2 | 2015-01-31 | -P1D | SITE2B | ||||
| 20 | ABC | FA | ABC-1001 | 20 | 8 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | N | 3 | TREATMENT | 2015-02-02 | 24 | END DAY 3 | 3 | VACCINATION 2 | 2015-01-31 | -P1D | SITE2B |
FACE NSV Metadata
| Variable | Label | Type | Codelist | Role | Origin |
|---|---|---|---|---|---|
| FOCID | Focus of Study-Specific Interest | text | Non-Standard Identifier | CRF | |
| FACOLSRT | Collected Summary Result Type | text | Non-Standard Variable Qualifier of --TESTCD and --TEST | CRF |
As the subject experienced fever, vomiting, and redness (at SITE1A) after the first vaccination co-administration, the sponsor included an assessment of the event being causally related to the study drug (CEREL) and the outcome of the event (CEOUT) in the CE records.
ce.xpt
| Row | STUDYID | DOMAIN | USUBJID | CESEQ | CELNKGRP | CETERM | CEDECOD | CECAT | CESCAT | CEPRESP | CEOCCUR | CEREL | CEOUT | TAETORD | EPOCH | CEDTC | CESTDTC | CEENDTC | CEDY | CETPT | CETPTNUM | CETPTREF | CERFTDTC | CEEVINTX | FOCID | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | CE | ABC-1001 | 1 | 1 | Vomiting | Vomiting | REACTOGENICITY | SYSTEMIC | Y | Y | RELATED | RECOVERED/RESOLVED | 2 | TREATMENT | 2015-01-12 | 2015-01-10 | 2015-01-10 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | ||
| 2 | ABC | CE | ABC-1001 | 2 | 2 | Fever | Fever | REACTOGENICITY | SYSTEMIC | Y | Y | RELATED | RECOVERED/RESOLVED | 2 | TREATMENT | 2015-01-12 | 2015-01-10 | 2015-01-11 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | ||
| 3 | ABC | CE | ABC-1001 | 3 | 3 | Redness | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | Y | RELATED | RECOVERED/RESOLVED | 2 | TREATMENT | 2015-01-12 | 2015-01-10 | 2015-01-11 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1A | |
| 4 | ABC | CE | ABC-1001 | 4 | 4 | Redness | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | N | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1B | |||||
| 5 | ABC | CE | ABC-1001 | 5 | 5 | Vomiting | Vomiting | REACTOGENICITY | SYSTEMIC | Y | N | 3 | TREATMENT | 2015-02-02 | 24 | END DAY 3 | 3 | VACCINATION 2 | 2015-01-31 | SINCE VACCINATION | ||||||
| 6 | ABC | CE | ABC-1001 | 6 | 6 | Fever | Fever | REACTOGENICITY | SYSTEMIC | Y | N | 3 | TREATMENT | 2015-02-02 | 24 | END DAY 3 | 3 | VACCINATION 2 | 2015-01-31 | SINCE VACCINATION | ||||||
| 7 | ABC | CE | ABC-1001 | 7 | 7 | Redness | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | N | 3 | TREATMENT | 2015-02-02 | 24 | END DAY 3 | 3 | VACCINATION 2 | 2015-01-31 | SINCE VACCINATION | SITE2A | |||||
| 8 | ABC | CE | ABC-1001 | 8 | 8 | Redness | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | N | 3 | TREATMENT | 2015-02-02 | 24 | END DAY 3 | 3 | VACCINATION 2 | 2015-01-31 | SINCE VACCINATION | SITE2B |
CE NSV Metadata
| Variable | Label | Type | Codelist | Role | Origin |
|---|---|---|---|---|---|
| FOCID | Focus of Study-Specific Interest | text | Non-Standard Identifier | CRF |
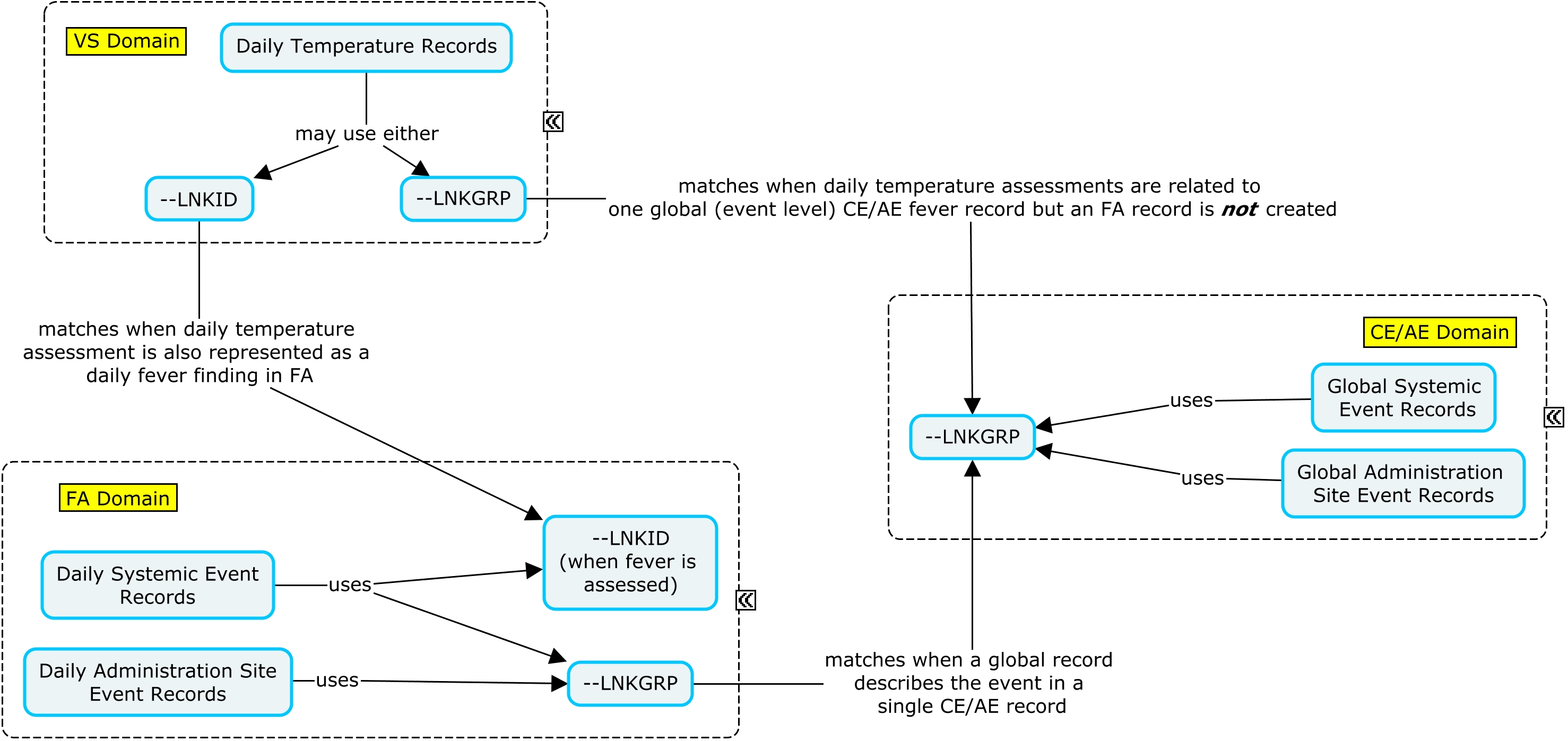
The RELREC dataset below shows that CE and FA can be linked via LNKGRP and that CE and VS can be linked via LNKGRP.
relrec.xpt
| Row | STUDYID | RDOMAIN | USUBJID | IDVAR | IDVARVAL | RELTYPE | RELID |
|---|---|---|---|---|---|---|---|
| 1 | ABC | CE | CELNKGRP | ONE | 1 | ||
| 2 | ABC | FACE | FALNKGRP | MANY | 1 | ||
| 3 | ABC | CE | CELNKGRP | ONE | 2 | ||
| 4 | ABC | VS | VSLNKGRP | MANY | 2 |
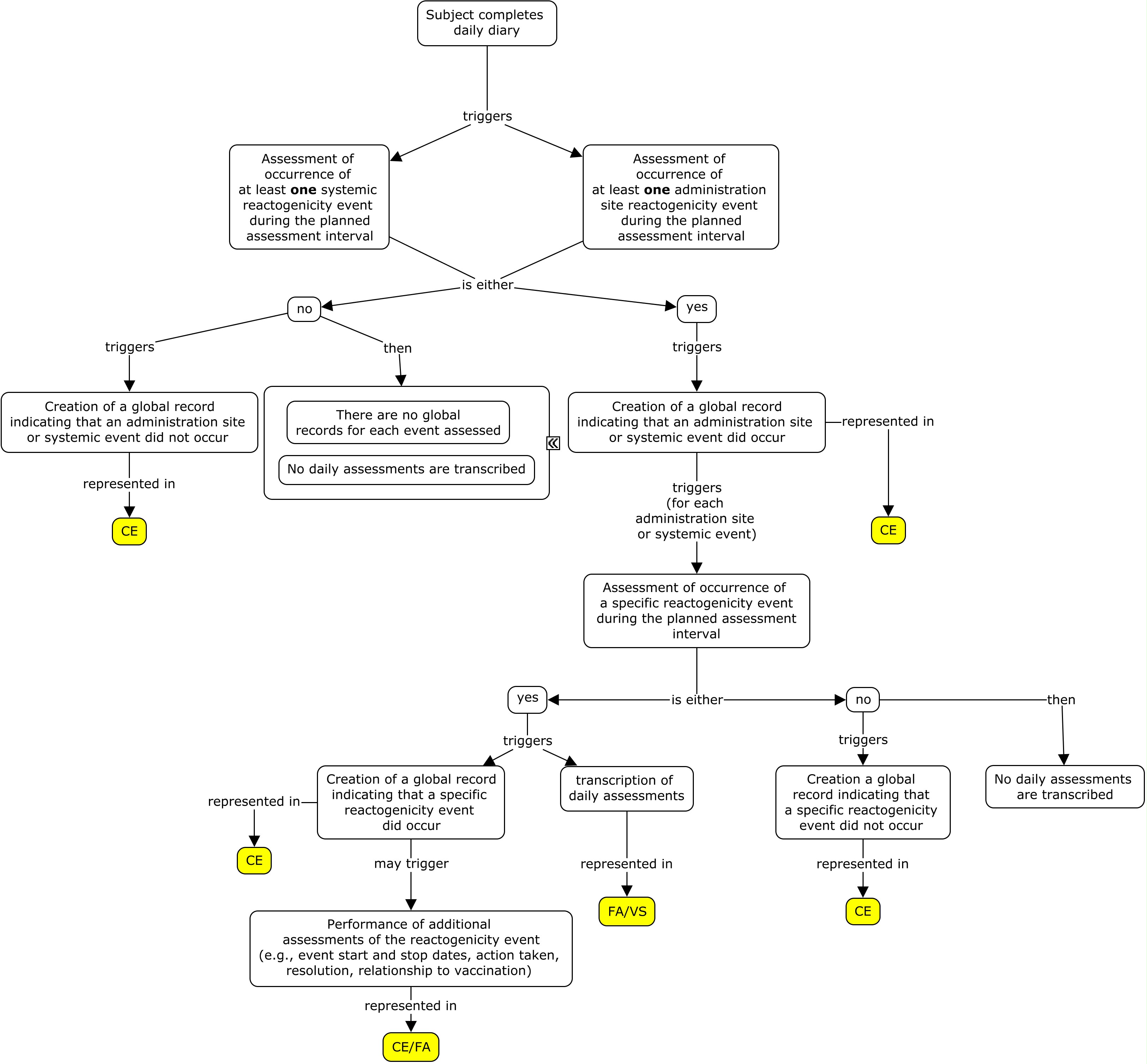
Example: Nested model
In the example below, an indicator question was asked regarding whether or not a specific reactogenicity event (fever, vomiting, or redness) had occurred during the three-day assessment period. If the specific event did not occur during the three-day assessment interval, a global occurrence record where CETERM is the event name and CEOCCUR="N" is represented in CE; in this case, the daily assessment records are not represented in FA. If the event did occur, a global occurrence record where CETERM is the event name and CEOCCUR="Y" is represented in CE; in this case, the daily records are also represented in FA or VS. When the subject experiences an event of fever, both a VS and an FA record are created where daily temperature measurements are represented in the VS domain and can be linked to the daily fever occurrence in FA via --LNKID. The variable --LNKGRP is used to relate the daily assessments in FA to the global record in CE. In this example, if a reactogenicity event occurred anytime during the planned observation period, the overall start and end dates of the reactogenicity event were collected on the eCRF in addition to the individual dates from the daily diary collection. Individual dates from the daily diary collection are represented in the FA domain using the variable FADTC and the overall start and end dates of the event are represented in the CE domain using the variables CESTDTC and CEENDTC respectively. Please note that if only the daily diary collection dates were collected, CESTDTC and CEENDTC would not be populated.
Figure - Reactogenicity Nested Model
| Rows 1-3: | Show that vomiting, fever, and redness at SITE1A occurred during the three-day observation period following the Day 1 vaccine administration. These global event records can be linked to the daily assessment records in FA via CELNKGRP and FALNKGRP. |
|---|---|
| Row 4: | Shows that redness did not occur at SITE1B during the three-day observation period following the Day 1 vaccine administration. CELNKGRP is not populated as the daily records are not created in FA for a non-event. |
| Rows 5-8: | Show that vomiting, fever, and redness did not occur during the three-day observation period following the Day 22 vaccine administration. CELNKGRP is not populated as the daily records are not created in FA for a non-event. |
ce.xpt
| Row | STUDYID | DOMAIN | USUBJID | CESEQ | CELNKGRP | CETERM | CEDECOD | CECAT | CESCAT | CEPRESP | CEOCCUR | CEREL | CEOUT | TAETORD | EPOCH | CEDTC | CESTDTC | CEENDTC | CEDY | CETPT | CETPTNUM | CETPTREF | CERFTDTC | CEEVINTX | FOCID | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | CE | ABC-1001 | 1 | 1 | Vomiting | Vomiting | REACTOGENICITY | SYSTEMIC | Y | Y | RELATED | RECOVERED/RESOLVED | 2 | TREATMENT | 2015-01-12 | 2015-01-10 | 2015-01-10 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | ||
| 2 | ABC | CE | ABC-1001 | 2 | 2 | Fever | Fever | REACTOGENICITY | SYSTEMIC | Y | Y | RELATED | RECOVERED/RESOLVED | 2 | TREATMENT | 2015-01-12 | 2015-01-10 | 2015-01-11 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | ||
| 3 | ABC | CE | ABC-1001 | 3 | 3 | Redness | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | Y | RELATED | RECOVERED/RESOLVED | 2 | TREATMENT | 2015-01-12 | 2015-01-10 | 2015-01-11 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1A | |
| 4 | ABC | CE | ABC-1001 | 4 | Redness | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | N | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1B | ||||||
| 5 | ABC | CE | ABC-1001 | 5 | Vomiting | Vomiting | REACTOGENICITY | SYSTEMIC | Y | N | 3 | TREATMENT | 2015-02-02 | 24 | END DAY 3 | 3 | VACCINATION 2 | 2015-01-31 | SINCE VACCINATION | |||||||
| 6 | ABC | CE | ABC-1001 | 6 | Fever | Fever | REACTOGENICITY | SYSTEMIC | Y | N | 3 | TREATMENT | 2015-02-02 | 24 | END DAY 3 | 3 | VACCINATION 2 | 2015-01-31 | SINCE VACCINATION | |||||||
| 7 | ABC | CE | ABC-1001 | 7 | Redness | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | N | 3 | TREATMENT | 2015-02-02 | 24 | END DAY 3 | 3 | VACCINATION 2 | 2015-01-31 | SINCE VACCINATION | SITE2A | ||||||
| 8 | ABC | CE | ABC-1001 | 8 | Redness | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | N | 3 | TREATMENT | 2015-02-02 | 24 | END DAY 3 | 3 | VACCINATION 2 | 2015-01-31 | SINCE VACCINATION | SITE2B |
CE NSV Metadata
| Variable | Label | Type | Codelist | Role | Origin |
|---|---|---|---|---|---|
| FOCID | Focus of Study-Specific Interest | text | Non-Standard Identifier | CRF |
The subject had a temperature of 101°F on the first two days of the three-day observation period (Rows 1 and 2). The variable VSLNKID has been populated with a value by which to link the records to the daily fever record in FA. The subject did not have a fever on the last day of observation.
vs.xpt
| Row | STUDYID | DOMAIN | USUBJID | VSSEQ | VSLNKID | VSTESTCD | VSTEST | VSCAT | VSSCAT | VSORRES | VSORRESU | VSLOC | TAETORD | EPOCH | VSDTC | VSDY | VSTPT | VSTPTNUM | VSTPTREF | VSRFTDTC | VSEVLINT | VSEVINTX | VSCOLSRT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | VS | ABC-1001 | 1 | 1 | TEMP | Temperature | REACTOGENICITY | SYSTEMIC | 101 | F | AXILLA | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | MAXIMUM | ||
| 2 | ABC | VS | ABC-1001 | 2 | 2 | TEMP | Temperature | REACTOGENICITY | SYSTEMIC | 101 | F | AXILLA | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | MAXIMUM | ||
| 3 | ABC | VS | ABC-1001 | 3 | 3 | TEMP | Temperature | REACTOGENICITY | SYSTEMIC | 98.6 | F | AXILLA | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D | MAXIMUM |
VS NSV Metadata
| Variable | Label | Type | Codelist | Role | Origin |
|---|---|---|---|---|---|
| VSCOLSRT | Collected Summary Result Type | text | Non-Standard Variable Qualifier of --TESTCD and --TEST | CRF |
| Rows 1-3: | Represent the number of vomiting episodes that occurred on each of the three days of the observation period following the Day 1 vaccine administration. |
|---|---|
| Rows 4-6: | Represent the results of questions about the occurrence of fever on the three days of the observation period following the Day 1 vaccine administration. FALNKID connects the daily fever records to the daily temperature records in VS via VSLNKID. |
| Rows 7, 9, 11: | Represent the results of questions about the occurrence of redness at the administration site at SITE1A following the Day 1 vaccine administration. |
| Rows 8, 10: | Since there was redness on two of the days of the observation period, the maximum longest diameter of the redness was collected. |
face.xpt
| Row | STUDYID | DOMAIN | USUBJID | FASEQ | FALNKID | FALNKGRP | FATESTCD | FATEST | FAOBJ | FACAT | FASCAT | FAORRES | FAORRESU | TAETORD | EPOCH | FADTC | FADY | FATPT | FATPTNUM | FATPTREF | FARFTDTC | FAEVLINT | FAEVINTX | FOCID | FACOLSRT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | FA | ABC-1001 | 1 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 3 | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | ||||||
| 2 | ABC | FA | ABC-1001 | 2 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 0 | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | ||||||
| 3 | ABC | FA | ABC-1001 | 3 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 0 | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D | ||||||
| 4 | ABC | FA | ABC-1001 | 4 | 1 | 2 | OCCUR | Occurrence Indicator | Fever | REACTOGENICITY | SYSTEMIC | Y | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | |||||
| 5 | ABC | FA | ABC-1001 | 5 | 2 | 2 | OCCUR | Occurrence Indicator | Fever | REACTOGENICITY | SYSTEMIC | Y | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | |||||
| 6 | ABC | FA | ABC-1001 | 6 | 3 | 2 | OCCUR | Occurrence Indicator | Fever | REACTOGENICITY | SYSTEMIC | N | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D | |||||
| 7 | ABC | FA | ABC-1001 | 7 | 3 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1A | |||||
| 8 | ABC | FA | ABC-1001 | 8 | 3 | LDIAM | Longest Diameter | Erythema | REACTOGENICITY | ADMINISTRATION SITE | 25 | mm | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1A | MAXIMUM | |||
| 9 | ABC | FA | ABC-1001 | 9 | 3 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1A | |||||
| 10 | ABC | FA | ABC-1001 | 10 | 3 | LDIAM | Longest Diameter | Erythema | REACTOGENICITY | ADMINISTRATION SITE | 10 | mm | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1A | MAXIMUM | |||
| 11 | ABC | FA | ABC-1001 | 11 | 3 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | N | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1A |
FACE NSV Metadata
| Variable | Label | Type | Codelist | Role | Origin |
|---|---|---|---|---|---|
| FOCID | Focus of Study-Specific Interest | text | Non-Standard Identifier | CRF | |
| FACOLSRT | Collected Summary Result Type | text | Non-Standard Variable Qualifierof --TESTCD and --TEST | CRF |
The RELREC dataset below shows that CE and FA can be linked via LNKGRP and that FA and VS can be linked via LNKID.
relrec.xpt
| Row | STUDYID | RDOMAIN | USUBJID | IDVAR | IDVARVAL | RELTYPE | RELID |
|---|---|---|---|---|---|---|---|
| 1 | ABC | CE | CELNKGRP | ONE | 1 | ||
| 2 | ABC | FACE | FALNKGRP | MANY | 1 | ||
| 3 | ABC | FACE | FALNKID | ONE | 2 | ||
| 4 | ABC | VS | VSLNKID | ONE | 2 |
Example: Highly nested model
In this example, two high-level indicator occurrence questions were asked: Any "Systemic Events" or any "Administration Site Events" during the three-day assessment interval? If CETERM="Systemic Event" and CEOCCUR="Y", an additional CE record is created for vomiting and fever. If CETERM="Administration Site Event" and CEOCCUR="Y", two additional CE records are created for redness, one for each administration site. The event category record can be connected to the individual reactogenicity clinical event records via CEGRPID. If the individual reactogenicity event occurred, then the daily records are also represented in FA. If the event did not occur, the daily records were not created. Daily temperature is linked to the daily fever record via --LNKID. The variable --LNKGRP is used to relate the daily measurements in FA to the global record in CE. If daily fever records are represented in FA, then the corresponding temperature records are also represented in VS. In this example, if a reactogenicity event occurred anytime during the planned observation period, the overall start and end dates of the reactogenicity event were collected on the eCRF in addition to the individual dates from the daily diary collection. Individual dates from the daily diary collection are represented in the FA domain using the variable FADTC and the overall start and end dates of the event are represented in the CE domain using the variables CESTDTC and CEENDTC respectively. Please note that if only the daily diary collection dates were collected, CESTDTC and CEENDTC would not be populated.
Figure - Reactogenicity Highly Nested Model
| Row 1: | Represents the high-level event of any "Systemic Events" during the three days of the observation period following the Day 1 vaccine administration. |
|---|---|
| Rows 2, 3: | Since there was a systemic event, the individual reactogenicity events of fever and vomiting are also represented. These records can be linked to the "Systemic event" event shown in the first row above by CEGRPID. CELNKGRP connects the global records in CE to the daily records in FA. |
| Row 4: | Represents the high-level event of any "Administration site event" during the three days of the observation period following the Day 1 vaccine administration. |
| Rows 5, 6: | Since there was an administration site event, the individual reactogenicity events of redness at both sites are also represented. These records can be linked to the "Administration site event" event shown in the fourth row above by CEGRPID. CELNKGRP connects the global records in CE to the daily records in FA. |
| Rows 7, 8: | Represents the high-level events of any "Systemic event" or "Administration site event" during the three days of the observation period following the Day 22 vaccine administration. Since the subject did not experience any reactogenicity events, no further records are represented in CE, FA, or VS. |
ce.xpt
| Row | STUDYID | DOMAIN | USUBJID | CESEQ | CELNKGRP | CEGRPID | CETERM | CEDECOD | CECAT | CESCAT | CEPRESP | CEOCCUR | CEREL | CEOUT | TAETORD | EPOCH | CEDTC | CESTDTC | CEENDTC | CEDY | CETPT | CETPTNUM | CETPTREF | CERFTDTC | CEEVINTX | FOCID | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | CE | ABC-1001 | 1 | 1 | Systemic event | REACTOGENICITY | Y | Y | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | |||||||||
| 2 | ABC | CE | ABC-1001 | 2 | 1 | 1 | Vomiting | Vomiting | REACTOGENICITY | SYSTEMIC | Y | Y | RELATED | RECOVERED/RESOLVED | 2 | TREATMENT | 2015-01-12 | 2015-01-10 | 2015-01-10 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | ||
| 3 | ABC | CE | ABC-1001 | 3 | 2 | 1 | Fever | Fever | REACTOGENICITY | SYSTEMIC | Y | Y | RELATED | RECOVERED/RESOLVED | 2 | TREATMENT | 2015-01-12 | 2015-01-10 | 2015-01-11 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | ||
| 4 | ABC | CE | ABC-1001 | 4 | 2 | Administration site event | REACTOGENICITY | Y | Y | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | |||||||||
| 5 | ABC | CE | ABC-1001 | 5 | 3 | 2 | Redness | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | Y | RELATED | RECOVERED/RESOLVED | 2 | TREATMENT | 2015-01-12 | 2015-01-10 | 2015-01-11 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1A | |
| 6 | ABC | CE | ABC-1001 | 6 | 2 | Redness | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | N | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1B | ||||||
| 7 | ABC | CE | ABC-1001 | 7 | Systemic event | REACTOGENICITY | Y | N | 3 | TREATMENT | 2015-02-02 | 24 | END DAY 3 | 3 | VACCINATION 2 | 2015-01-31 | SINCE VACCINATION | ||||||||||
| 8 | ABC | CE | ABC-1001 | 8 | Administration site event | REACTOGENICITY | Y | N | 3 | TREATMENT | 2015-02-02 | 24 | END DAY 3 | 3 | VACCINATION 2 | 2015-01-31 | SINCE VACCINATION |
CE NSV Metadata
| Variable | Label | Type | Codelist | Role | Origin |
|---|---|---|---|---|---|
| FOCID | Focus of Study-Specific Interest | text | Non-Standard Identifier | CRF |
The subject had a temperature of 101°F on the first two days of the three-day observation period (Rows 1 and 2). The variable VSLNKID has been populated with a value by which to link the records to the daily fever record in CE. The subject did not have a fever on the last day of observation.
vs.xpt
| Row | STUDYID | DOMAIN | USUBJID | VSSEQ | VSLNKID | VSTESTCD | VSTEST | VSCAT | VSSCAT | VSORRES | VSORRESU | VSLOC | TAETORD | EPOCH | VSDTC | VSDY | VSTPT | VSTPTNUM | VSTPTREF | VSRFTDTC | VSEVLINT | VSEVINTX | VSCOLSRT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | VS | ABC-1001 | 1 | 1 | TEMP | Temperature | REACTOGENICITY | SYSTEMIC | 101 | F | AXILLA | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | MAXIMUM | ||
| 2 | ABC | VS | ABC-1001 | 2 | 2 | TEMP | Temperature | REACTOGENICITY | SYSTEMIC | 101 | F | AXILLA | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | MAXIMUM | ||
| 3 | ABC | VS | ABC-1001 | 3 | 3 | TEMP | Temperature | REACTOGENICITY | SYSTEMIC | 98.6 | F | AXILLA | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D | MAXIMUM |
VS NSV Metadata
| Variable | Label | Type | Codelist | Role | Origin |
|---|---|---|---|---|---|
| VSCOLSRT | Collected Summary Result Type | text | Non-Standard Variable Qualifier of --TESTCD and --TEST | CRF |
| Rows 1-3: | Represent the results of questions about the number of vomiting episodes on the three days of the observation period following the Day 1 vaccine administration. |
|---|---|
| Rows 4-6: | Represent the results of questions about the occurrence of fever on the three days of the observation period following the Day 1 vaccine administration. FALNKID connects the daily fever records to the daily temperature records in VS. |
| Rows 7, 9, 11: | Represent the results of questions about the occurrence of redness at the administration side with FOCID = "SITE1A" following the Day 1 vaccine administration. |
| Rows 8, 10: | Since there was redness on two of the days of the observation period, the maximum longest diameter of the redness was collected. |
face.xpt
| Row | STUDYID | DOMAIN | USUBJID | FASEQ | FALNKID | FALNKGRP | FATESTCD | FATEST | FAOBJ | FACAT | FASCAT | FAORRES | FAORRESU | TAETORD | EPOCH | FADTC | FADY | FATPT | FATPTNUM | FATPTREF | FARFTDTC | FAEVLINT | FAEVINTX | FOCID | FACOLSRT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | FA | ABC-1001 | 1 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 3 | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | ||||||
| 2 | ABC | FA | ABC-1001 | 2 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 0 | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | ||||||
| 3 | ABC | FA | ABC-1001 | 3 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 0 | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D | ||||||
| 4 | ABC | FA | ABC-1001 | 4 | 1 | 2 | OCCUR | Occurrence Indicator | Fever | REACTOGENICITY | SYSTEMIC | Y | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | |||||
| 5 | ABC | FA | ABC-1001 | 5 | 2 | 2 | OCCUR | Occurrence Indicator | Fever | REACTOGENICITY | SYSTEMIC | Y | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | |||||
| 6 | ABC | FA | ABC-1001 | 6 | 3 | 2 | OCCUR | Occurrence Indicator | Fever | REACTOGENICITY | SYSTEMIC | N | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D | |||||
| 7 | ABC | FA | ABC-1001 | 7 | 3 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1A | |||||
| 8 | ABC | FA | ABC-1001 | 8 | 3 | LDIAM | Longest Diameter | Erythema | REACTOGENICITY | ADMINISTRATION SITE | 25 | mm | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1A | MAXIMUM | |||
| 9 | ABC | FA | ABC-1001 | 9 | 3 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1A | |||||
| 10 | ABC | FA | ABC-1001 | 10 | 3 | LDIAM | Longest Diameter | Erythema | REACTOGENICITY | ADMINISTRATION SITE | 10 | mm | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1A | MAXIMUM | |||
| 11 | ABC | FA | ABC-1001 | 11 | 3 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | N | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1A |
FACE NSV Metadata
| Variable | Label | Type | Codelist | Role | Origin |
|---|---|---|---|---|---|
| FOCID | Focus of Study-Specific Interest | text | Non-Standard Identifier | CRF | |
| FACOLSRT | Collected Summary Result Type | text | Non-Standard Variable Qualifier of --TESTCD and --TEST | CRF |
The RELREC dataset below shows that CE and FA can be linked via LNKGRP and that FA and VS can be linked via LNKID.
relrec.xpt
| Row | STUDYID | RDOMAIN | USUBJID | IDVAR | IDVARVAL | RELTYPE | RELID |
|---|---|---|---|---|---|---|---|
| 1 | ABC | CE | CELNKGRP | ONE | 1 | ||
| 2 | ABC | FACE | FALNKGRP | MANY | 1 | ||
| 3 | ABC | FACE | FALNKID | ONE | 2 | ||
| 4 | ABC | VS | VSLNKID | ONE | 2 |
Example
In this example, the subject experienced vomiting each day during the three-day assessment period (Rows 1-3) after the first set of vaccinations. The vomiting continued for two more days and ended on Study Day 5 (Row 4). The subject was told to record the date that the vomiting stopped and the maximum number of daily episodes that occurred since the observation period ended. When the reactogenicity event continues beyond the planned assessment interval and is still considered a clinical event as defined by the protocol (i.e., is not a protocol-defined adverse event), a new record can be created in FA. In the CE record that represents the vomiting event as a whole, the variable CEENDTC represents the day the event ended. In this example, if a reactogenicity event occurred anytime during the planned observation period, the overall start and end dates of the reactogenicity event were collected on the eCRF in addition to the individual dates from the daily diary collection. Individual dates from the daily diary collection are represented in the FA domain using the variable FADTC and the overall start and end dates of the event are represented in the CE domain using the variables CESTDTC and CEENDTC respectively. Please note that if only the daily diary collection dates were collected, CESTDTC and CEENDTC would not be populated.
| Rows 1-3: | Show the number of vomiting episodes that occurred on each of the three planned observation days. |
|---|---|
| Row 4: | Shows the maximum number of daily vomiting episodes that occurred during the event continuation period. The variable FADTC represents the date that the event ended and the variable FAEVINTX represents when the follow-up assessments began. The concept of "maximum" is represented in the NSV, COLSRT (Collected Summary Result Type). The variable FAORRESU is populated with "/day" because unlike the first three rows above, this row represents the maximum number of daily episodes and not the total number of the episodes during the period. |
face.xpt
| Row | STUDYID | DOMAIN | USUBJID | FASEQ | FALNKGRP | FATESTCD | FATEST | FAOBJ | FACAT | FASCAT | FAORRES | FAORRESU | TAETORD | EPOCH | FADTC | FADY | FATPT | FATPTNUM | FATPTREF | FARFTDTC | FAEVLINT | FAEVINTX | FACOLSRT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | FA | ABC-1002 | 1 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 2 | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | ||||
| 2 | ABC | FA | ABC-1002 | 2 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 1 | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | ||||
| 3 | ABC | FA | ABC-1002 | 3 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 1 | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D | ||||
| 4 | ABC | FA | ABC-1002 | 4 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 2 | /day | 2 | TREATMENT | 2015-01-14 | 5 | SINCE 3 DAYS AFTER VACCINATION | MAXIMUM |
FACE NSV Metadata
| Variable | Label | Type | Codelist | Role | Origin |
|---|---|---|---|---|---|
| FACOLSRT | Collected Summary Result Type | text | Non-Standard Variable Qualifier of --TESTCD and --TEST | CRF |
ce.xpt
| Row | STUDYID | DOMAIN | USUBJID | CESEQ | CELNKGRP | CETERM | CECAT | CESCAT | CEPRESP | CEOCCUR | CEREL | CEOUT | TAETORD | EPOCH | CEDTC | CESTDTC | CEENDTC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | CE | ABC-1002 | 1 | 1 | Vomiting | REACTOGENICITY | SYSTEMIC | Y | Y | Y | RECOVERED/ RESOLVED | 2 | TREATMENT | 2015-01-14 | 2015-01-10 | 2015-01-14 |
The RELREC dataset below shows that FA and CE can be linked via --LNKGRP.
relrec.xpt
| Row | STUDYID | RDOMAIN | USUBJID | IDVAR | IDVARVAL | RELTYPE | RELID |
|---|---|---|---|---|---|---|---|
| 1 | ABC | CE | CELNKGRP | ONE | 1 | ||
| 2 | ABC | FACE | FALNKGRP | MANY | 1 |
Example
In this example, the subject experienced vomiting each day during the three-day assessment period after the first set of vaccinations. The vomiting continued for two more days and ended on Study Day 5. According to the protocol, the entire event should also be reported as an adverse event (i.e, from the first day of occurrence until its resolution). The maximum number of daily vomiting episodes that occurred during the entire event is represented in FAAE. In this example, FA, CE, and AE are linked through FALNKGRP, CELNKGRP, and AELNKGRP.
face.xpt
| Row | STUDYID | DOMAIN | USUBJID | FASEQ | FALNKGRP | FATESTCD | FATEST | FAOBJ | FACAT | FASCAT | FAORRES | FAEVAL | TAETORD | EPOCH | FADTC | FADY | FATPT | FATPTNUM | FATPTREF | FARFTDTC | FAEVLINT | FAEVINTX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EFG | FA | EFG-1002 | 1 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 2 | STUDY SUBJECT | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | |
| 2 | EFG | FA | EFG-1002 | 2 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 1 | STUDY SUBJECT | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | |
| 3 | EFG | FA | EFG-1002 | 3 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 1 | STUDY SUBJECT | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D |
ce.xpt
| Row | STUDYID | DOMAIN | USUBJID | CESEQ | CELNKGRP | CETERM | CEDECOD | CECAT | CESCAT | CEPRESP | CEOCCUR | CEREL | CEOUT | TAETORD | EPOCH | CEDTC | CEDY | CETPT | CETPTNUM | CETPTREF | CERFTDTC | CEEVINTX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EFG | CE | EFG-1002 | 1 | 1 | Vomiting | Vomiting | REACTOGENICITY | SYSTEMIC | Y | Y | Y | NOT RECOVERED/NOT RESOLVED | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION |
The dataset example below shows that the vomiting was considered an adverse event once it continued beyond the planned observation period. In this example, the start of the AE is the date the event was first observed.
ae.xpt
| Row | STUDYID | DOMAIN | USUBJID | AESEQ | AELNKGRP | AETERM | AEDECOD | AECAT | AESCAT | AEPRESP | AEREL | AEOUT | TAETORD | EPOCH | AESTDTC | AEENDTC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EFG | AE | EFG-1002 | 1 | 1 | Vomiting | Vomiting | REACTOGENICITY | SYSTEMIC | Y | RELATED | RECOVERED/RESOLVED | 2 | TREATMENT | 2015-01-10 | 2015-01-14 |
The dataset example below shows the maximum number of daily vomiting episodes that occurred during the event . The concept of "maximum" is represented in the NSV, COLSRT (Collected Summary Result Type). The variable FAORRESU is populated with "/day" because unlike the FACE example above, this record in FAAE represents the maximum number of daily episodes and not the total number of the episodes during the event.
faae.xpt
| Row | STUDYID | DOMAIN | USUBJID | FASEQ | FALNKGRP | FATESTCD | FATEST | FAOBJ | FACAT | FASCAT | FAORRES | FAORRESU | TAETORD | EPOCH | FADTC | FACOLSRT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EFG | FA | EFG-1002 | 1 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 2 | /day | 2 | TREATMENT | 2015-01-14 | MAXIMUM |
FAAE NSV Metadata
| Variable | Label | Type | Codelist | Role | Origin |
|---|---|---|---|---|---|
| FACOLSRT | Collected Summary Result Type | text | Non-Standard Record Qualifier of --TESTCD and --TEST | CRF |
The RELREC dataset below shows that FA, CE, and AE can be linked via --LNKGRP.
relrec.xpt
| Row | STUDYID | RDOMAIN | USUBJID | IDVAR | IDVARVAL | RELTYPE | RELID |
|---|---|---|---|---|---|---|---|
| 1 | EFG | AE | AELNKGRP | ONE | 1 | ||
| 2 | EFG | FAAE | FALNKGRP | MANY | 1 | ||
| 3 | EFG | AE | AELNKGRP | ONE | 2 | ||
| 4 | EFG | CE | CELNKGRP | ONE | 2 | ||
| 5 | EFG | CE | CELNKGRP | ONE | 3 | ||
| 6 | EFG | FACE | FALNKGRP | MANY | 3 |
Example
The example below shows data from a subject who forgot to complete the last two days of the daily diary card for the assessment of vomiting. In some cases, it is not possible to recover missing information once detected. If measures like temperature or size of swelling are not taken in due time, subjects may not be able to provide that information retrospectively. With paper diaries, the transcription and detection of issues can even occur weeks after the vaccination, typically following the next visit when paper diary cards are collected; accurate recollection of daily assessments may not be possible by that time. Missing values are recorded because they require appropriate identification and processing (e.g., imputation) in reactogenicity analyses.
When information is missing, the variables FASTAT and FAREASND can be used to indicate that the daily assessment for vomiting was not done and the reason why. When the assessment was not done, FAORRES is null.
As a global CE record needs to be created, but it is not known whether or not the subject experienced vomiting on the last two days, the CESTAT and CEREASND variables are also populated. When it is not known whether or not the event has occurred, CEOCCUR is null. If vomiting had occurred on any of the days during the planned assessment period, a CE record where CEOCCUR="Y" would be created even if there were missing results on other days.
| Row 1: | Shows that the subject did not have vomiting episodes on Day 1 of the observation period following the Day 1 vaccination. |
|---|---|
| Rows 2, 3: | Show that subject has missing data for the vomiting assessment on Days 2 and 3 of the observation period following the Day 1 vaccination. |
face.xpt
| Row | STUDYID | DOMAIN | USUBJID | FASEQ | FALNKGRP | FATESTCD | FATEST | FAOBJ | FACAT | FASCAT | FAORRES | FASTAT | FAREASND | TAETORD | EPOCH | FADTC | FADY | FATPT | FATPTNUM | FATPTREF | FARFTDTC | FAEVLINT | FAEVINTX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | FA | ABC-1003 | 1 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | 0 | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | |||
| 2 | ABC | FA | ABC-1003 | 2 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | NOT DONE | SUBJECT FORGOT TO COMPLETE DIARY CARD | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | ||
| 3 | ABC | FA | ABC-1003 | 3 | 1 | EPSDNUM | Number of Episodes | Vomiting | REACTOGENICITY | SYSTEMIC | NOT DONE | SUBJECT FORGOT TO COMPLETE DIARY CARD | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D |
The dataset example below shows that a CE record was created (with CESTAT and CEREASND variables populated) when the daily assessments in FA were missing for Days 2 and 3 and the number of vomiting episodes was "0" for Day 1 of the evaluation period.
ce.xpt
| Row | STUDYID | DOMAIN | USUBJID | CESEQ | CELNKGRP | CETERM | CEDECOD | CECAT | CESCAT | CEPRESP | CEOCCUR | CESTAT | CEREASND | TAETORD | EPOCH | CEDTC | CEDY | CETPT | CETPTNUM | CETPTREF | CERFTDTC | CEEVINTX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | CE | ABC-1003 | 1 | 1 | Vomiting | Vomiting | REACTOGENICITY | SYSTEMIC | Y | NOT DONE | MISSING DAILY DIARY RECORDS | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION |
The RELREC dataset below shows that CE and FA can be linked via LNKGRP.
relrec.xpt
| Row | STUDYID | RDOMAIN | USUBJID | IDVAR | IDVARVAL | RELTYPE | RELID |
|---|---|---|---|---|---|---|---|
| 1 | ABC | CE | CELNKGRP | ONE | 1 | ||
| 2 | ABC | FACE | FALNKGRP | MANY | 1 |
Example
The example below shows daily reactogenicity assessments for redness for the first set of vaccinations at injection site "SITE1A". If present, the daily maximum longest diameter, daily maximum subject-reported severity, and investigator-determined severity were assessed. The daily records are shown in FA and the global event record is shown in CE. The variables FALNKGRP and CELNKGRP are used to connect the daily records to the global event records. In this example, the subject-reported severity is represented using a FATESTCD/FATEST of "SEV"/"Severity/Intensity" in FA and the variable CESEV in the CE domain. When the severity differs across the daily assessments, the maximum severity is shown in the CESEV variable in the summary CE record. This example also shows how the investigator-determined severity could be represented using a FATESTCD/FATEST of "TOXGR"/"Toxicity Grade" in FA and the CETOXGR variable in CE. When the toxicity grade differs across the daily assessments, the most severe toxicity grade is shown in the CETOXGR variable in the global event CE record. In the example below, the FDA Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials[1] was used. Sponsors should specify which scale and version is used in the Sponsor Comments column of the Define-XML document.
In this example, if a reactogenicity event occurred anytime during the planned observation period, the overall start and end dates of the reactogenicity event were collected on the eCRF in addition to the individual dates from the daily diary collection. Individual dates from the daily diary collection are represented in the FA domain using the variable FADTC and the overall start and end dates of the event are represented in the CE domain using the variables CESTDTC and CEENDTC respectively. Please note that if only the daily diary collection dates were collected, CESTDTC and CEENDTC would not be populated.| Rows 1-3: | Show that the subject had at least one occurrence of redness after vaccination on the first day and that, at the end of the first day, she reported that the severity was moderate and the maximum daily longest diameter was 35mm. |
|---|---|
| Row 4: | The investigator used the FDA Toxicity Grading Scale and determined that, based on the size, the severity on the first day was mild. |
| Rows 5-7: | Show that the subject had at least one occurrence of redness during the second day and that, at the end of the second day, she reported that the severity was mild and the maximum longest diameter was 25mm. |
| Row 8: | The investigator used the FDA Toxicity Grading Scale and determined that, based on the size, the severity on the second day was mild. |
| Row 9: | Shows that subject did not have an occurrence of redness at the administration site during the third day. |
face.xpt
| Row | STUDYID | DOMAIN | USUBJID | FASEQ | FALNKGRP | FATESTCD | FATEST | FAOBJ | FACAT | FASCAT | FAORRES | FAORRESU | FAEVAL | TAETORD | EPOCH | FADTC | FADY | FATPT | FATPTNUM | FATPTREF | FARFTDTC | FAEVLINT | FAEVINTX | FOCID | FACOLSRT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | FA | ABC-1001 | 1 | 1 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | STUDY SUBJECT | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1A | ||||
| 2 | ABC | FA | ABC-1001 | 2 | 1 | LDIAM | Longest Diameter | Erythema | REACTOGENICITY | ADMINISTRATION SITE | 35 | mm | STUDY SUBJECT | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1A | MAXIMUM | ||
| 3 | ABC | FA | ABC-1001 | 3 | 1 | SEV | Severity/Intensity | Erythema | REACTOGENICITY | ADMINISTRATION SITE | MODERATE | STUDY SUBJECT | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1A | MAXIMUM | |||
| 4 | ABC | FA | ABC-1001 | 4 | 1 | TOXGR | Toxicity Grade | Erythema | REACTOGENICITY | ADMINISTRATION SITE | MILD | INVESTIGATOR | 2 | TREATMENT | 2015-01-10 | 1 | END DAY 1 | 1 | VACCINATION 1 | 2015-01-10 | SINCE VACCINATION | SITE1A | MAXIMUM | |||
| 5 | ABC | FA | ABC-1001 | 5 | 1 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | STUDY SUBJECT | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1A | ||||
| 6 | ABC | FA | ABC-1001 | 6 | 1 | LDIAM | Longest Diameter | Erythema | REACTOGENICITY | ADMINISTRATION SITE | 25 | mm | STUDY SUBJECT | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1A | MAXIMUM | ||
| 7 | ABC | FA | ABC-1001 | 7 | 1 | SEV | Severity/Intensity | Erythema | REACTOGENICITY | ADMINISTRATION SITE | MILD | STUDY SUBJECT | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1A | MAXIMUM | |||
| 8 | ABC | FA | ABC-1001 | 8 | 1 | TOXGR | Toxicity Grade | Erythema | REACTOGENICITY | ADMINISTRATION SITE | MILD | INVESTIGATOR | 2 | TREATMENT | 2015-01-11 | 2 | END DAY 2 | 2 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1A | MAXIMUM | |||
| 9 | ABC | FA | ABC-1001 | 9 | 1 | OCCUR | Occurrence Indicator | Erythema | REACTOGENICITY | ADMINISTRATION SITE | N | STUDY SUBJECT | 2 | TREATMENT | 2015-01-12 | 3 | END DAY 3 | 3 | VACCINATION 1 | 2015-01-10 | -P1D | SITE1A |
FACE NSV Metadata
| Variable | Label | Type | Codelist | Role | Origin |
|---|---|---|---|---|---|
| FOCID | Focus of Study-Specific Interest | text | Non-Standard Identifier | CRF | |
| FACOLSRT | Collected Summary Result Type | text | Non-Standard Variable Qualifier of --TESTCD and --TEST | CRF |
A global CE record for the reactogenicity event was created showing the maximum reported severity and toxicity grade.
ce.xpt
| Row | STUDYID | DOMAIN | USUBJID | CESEQ | CELNKGRP | CETERM | CEDECOD | CECAT | CESCAT | CEPRESP | CEOCCUR | CESEV | CEREL | CEOUT | CETOXGR | TAETORD | EPOCH | CEDTC | CESTDTC | CEENDTC | CETPT | CETPTNUM | CEEVINTX | FOCID | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ABC | CE | ABC-1001 | 1 | 1 | Redness | Erythema | REACTOGENICITY | ADMINISTRATION SITE | Y | Y | MODERATE | Y | RECOVERED/RESOLVED | MILD | 2 | TREATMENT | 2015-01-12 | 2015-01-10 | 2015-01-11 | END DAY 3 | 3 | SINCE VACCINATION | SITE1A |
CE NSV Metadata
| Variable | Label | Type | Codelist | Role | Origin |
|---|---|---|---|---|---|
| FOCID | Focus of Study-Specific Interest | text | Non-Standard Identifier | CRF |
The RELREC dataset below shows that CE and FA can be linked via LNKGRP.
relrec.xpt
| Row | STUDYID | RDOMAIN | USUBJID | IDVAR | IDVARVAL | RELTYPE | RELID |
|---|---|---|---|---|---|---|---|
| 1 | ABC | CE | CELNKGRP | ONE | 1 | ||
| 2 | ABC | FACE | FALNKGRP | MANY | 1 |
In this example, if a reactogenicity event occurred anytime during the planned observation period, the overall start and end dates of the reactogenicity event were collected on the eCRF in addition to the individual dates from the daily diary collection. Individual dates from the daily diary collection are represented in the FA domain using the variable FADTC and the overall start and end dates of the event are represented in the CE domain using the variables CESTDTC and CEENDTC respectively. Please note that if only the daily diary collection dates were collected, CESTDTC and CEENDTC would not be populated.
7 Relating Records
For the examples shown in this guide, there are several important links that need to be made and defined across domains.
When several sets of vaccines are administered over several vaccination time points, it is important, when assessing reactogenicity events, to know which vaccine administration site and vaccination time point the event relates to. These two connections can be made by using the variables FOCID and TAETORD, respectively. These variables are not domain- or observation class-specific and do not use the two-letter domain code as a prefix, thus a RELREC dataset is not needed. The concept map below illustrates how these variables can be used to link administration site and vaccine timing information across domains using FOCID and TAETORD.
However, systemic reactogenicity events are not tied to a specific administration site and relate to all vaccines administered at the same vaccination time point. For that reason, FOCID is not populated for the assessment of systemic events.
Figure - Relating Records 1
Because reactogenicity assessment data can be spread out across several domains, it is important to create links to connect this information. The examples in this guide use the variable --LNKGRP to link the daily reactogenicity assessments that are represented in FA/VS to the global reactogenicity event record in CE. This guide also shows how to link the daily temperature records either to the daily fever finding in FA using --LNKID or to the global fever record in CE using --LNKGRP. The modeling strategy and thus the linking variable used depends on how daily diary cards are transcribed. The connections that use --LNKGRP and --LNKID require that these relationships be defined using a RELREC dataset. The concept map below shows how --LNKGRP and --LNKID can be used to link reactogenicity assessment data across domains.
Figure - Relating Records 2
Appendices
Appendix A: Project Proposal
Specialty Projects
Please note that the Vaccines Project was instigated as a CDISC specialty project and therefore did not have an officially approved project proposal. The project was adopted under the CFAST umbrella in order to provide consistency with other CFAST TAUGs and provide support and expertise to the Vaccines team from CDISC staff experienced in the development of TAUGs.
The information below was taken from the Vaccines Project Charter.
Team Mission
The mission of the Vaccines Team is to ensure a consistent use and development of the CDISC standards for use in vaccines studies, intended to support regulatory submissions, across all diseases/vaccine-preventable disease areas in all age groups.
Scope
Create a Vaccines User Guide, according to the CDISC Process, describing how to use CDASH, SDTM, ADaM standards and Controlled Terminology to support Vaccines studies intended to support regulatory submissions, across all diseases/vaccine-preventable disease areas in all age groups.
To achieve this goal, the team will:
- Define requirementsfor using CDISC standards on Vaccines studies and identify gaps and modifications required with current CDISC Standards and related material to meet these requirements.
- Develop guidelines complementing existing CDASH, SDTM, and ADaM Implementation Guides targeted at use of these standards in Vaccines studies.
- For Vaccines study needs not covered by existing standards, elaborate and submit proposals for extension/update of these standards to the related CDISC teams (Terminology, SDTM Governance Committee, CDASH, ADaM).
Interact with and provide feedback to other teams, especially the core CDISC and TA teams. All issues and suggestions for improvements identified by the Vaccines Team during the course of its work, and that fall within the scope of another team mission, will be forwarded to the appropriate team(s).
2016 Deliverables
- By Q3-2016: a User Guide on the use of CDISC Standards for Adverse events, including reactogenicity, in Vaccines trials
- Other objectives might be defined for delivery later in the year according to the 2014 Vaccines Survey results and suggestions to the team
When applicable during the course of its work:
- Proposals for extension of the CDISC data collection standards (CDASH), the corresponding data presentation standards (SDTM) and the related Controlled Terminology to cover vaccines needs not already covered in these standards
- Feedback to other teams with issues/suggestions
Appendix B: Vaccines Team
| Name | Institution/Organization |
|---|---|
| Cedric Davister, Team Lead | GlaxoSmithKline Vaccines |
| Paul Houston, Team Lead | CDISC |
| John Owen, Team Lead | CDISC |
| Frances M. Acton | Merck & Co. |
| Brenda Baldwin | FDA - CBER |
| Dorina Bratfalean | CDISC |
| Pamela Freese | Pfizer |
| John Ginis | Pfizer |
| Audrey Hagenbach | Sanofi-Pasteur |
| Geert Hannink | GlaxoSmithKline Vaccines |
| Abdul W. Khayat | Pfizer |
| Ragini Khedoe | GlaxoSmithKline Vaccines |
| Bess LeRoy | CDISC |
| Veronique Long | Sanofi-Pasteur |
| Dave O'Riordan | GlaxoSmithKline Vaccines |
| Deborah Paletta | Business & Decision Life Sciences |
| John Perez | Pfizer |
| Kirk Prutzman | FDA - CBER |
| Barbara Rath | Vienna Vaccine Safety Initiative (ViVI) |
| Ramana Setty | Sanofi-Pasteur |
| Lorraine Spencer | Takeda |
| Diane Wold | CDISC |
| Olive Yuan | Johnson & Johnson |
| Elodie Zaworski | Sanofi-Pasteur |
| Former Members | |
| Anne-Sophie Bekx | Business & Decision Life Sciences |
| Karin Botilde | GlaxoSmithKline Vaccines |
| Xavier Cornen | SanofiPasteur-MSD |
| Christian Hoppe | Vienna Vaccine Safety Initiative (ViVI) |
| Joan Lippincott | Merck & Co. |
| Magali Savoy | Sanofi-Pasteur |
| Lauren Shinaberry | Business & Decision Life Sciences |
Appendix C: Glossary and Abbreviations
| ADaMIG | ADaM Implementation Guide |
| CDISC | Clinical Data Interchange Standards Consortium |
| CFAST | Coalition for Accelerating Standards and Therapies |
| Collected | "Collected" refers to information that is recorded and/or transmitted to the sponsor. This includes data entered by the site on CRFs/eCRFs as well as vendor data such as core lab data. This term is a synonym for "captured". |
| Controlled Terminology | A finite set of values that represent the only allowed values for a data item. These values may be codes, text, or numeric. A code list is one type of controlled terminology. |
| CRF | Case report form (sometimes called a case record form). A printed, optical, or electronic document designed to record all required information to be reported to the sponsor for each trial subject. |
| Domain | A collection of observations with a topic-specific commonality about a subject |
| eCRF | Electronic case report form |
| Foundational Standards | Used to refer to the suite of CDISC standards that describe the clinical study protocol (Protocol), design (Study Design), data collection (CDASH), laboratory work (Lab), analysis (ADaM), and data tabulation (SDTM and SEND). See http://www.cdisc.org/ for more information on each of these clinical data standards. |
| NSV | Non-standard variable |
| SDS | Submission Data Standards. Also the name of the team that maintains the SDTM and SDTMIG. |
| SDTM | Study Data Tabulation Model |
| SDTMIG | SDTM Implementation Guide (for Human Clinical Trials) |
| Subject | A participant in a study |
Appendix D: Non-Standard Variables
The following table lists the non-standard variables used in this document, and gives their parent domain and variable-level metadata.
| Parent Domain | Variable | Label | SAS Data Type | XML Data Type | Codelist/ Controlled Terms | Role | Description | Comments |
|---|---|---|---|---|---|---|---|---|
| EX, CE, FACE | FOCID | Focus of Study-Specific Interest | Char | text | Non-Standard Identifier | Identification of a focus of study-specific interest on or within a subject or specimen as called out in the protocol, for which a measurement, test, or examination was performed, such as a drug application site. | FOCID was added to the SDTM in version 1.5. It is expected to be standard for use in human clinical trials in the next version of the SDTMIG. | |
| VS, FACE | --COLSRT | Collected Summary Result Type | Char | text | Non-Standard Variable Qualifier | Variable qualifier of --TESTCD and --TEST. Used to indicate the type of collected summary result (e.g., MAXIMUM, MINIMUM, MEAN, MEDIAN, NADIR). This includes source summary results collected on a CRF or provided by an external vendor (e.g., central lab). If the summary result is derived using individual source data records, this summary result should be represented in ADaM. If a sponsor has both a collected summary result and a derived summary result, the collected summary result should be represented in SDTM and the derived summary result should be represented in ADaM. |
Appendix E: References
Appendix E1: Works Cited
- U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research (FDA CBER). Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. September, 2007.
Appendix E2: Further Reading
On vaccines
- National Institutes of Health - National Cancer Institute (NIH NCI). Vaccine (code 923). In NCI Thesaurus v17.04d. Release date April 24, 2017. Accessed on June 7, 2017.
- Council of Europe - European Directorate for the Quality of Medicines & HealthCare (EDQM). Vaccine for Human Use. In European Pharmacopoeia 9th Edition 2017 (9.2). Accessed on June 7, 2017.
On adverse events and reactions
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Clinical Safety Data Management: Definitions and Standards for Expedited Reporting E2A. Step 4 Version. ICH Harmonised Tripartite Guideline. October 27, 1994.
- European Medicines Agency - Heads of Medicines Agencies (EMA HMA). Guideline on good pharmacovigilance practices (GVP): Module VI - Management and reporting of adverse reactions to medicinal products. EMA/873138/2011 Rev 1. September 8, 2014.
On collection of solicited signs and symptoms, on local and systemic adverse events
- World Health Organization (WHO). Guidelines on clinical evaluation of vaccines: regulatory expectations. WHO/BS/2016.2287. 2016
- European Medicines Agency - Committee for Human Medicinal Products (EMA CHMP). Note for Guidance on the Clinical Evaluation of Vaccines. CHMP/VWP/164653/2005. May 17, 2005.
On reactogenicity events
- National Institutes of Health - National Institute of Allergy and Infectious Diseases (NIH NIAID). DMID Interventional Protocol Template Version 5. March 25, 2011. https://www.niaid.nih.gov/sites/default/files/dmid-interventional-protocol-template-and-instructions.docx. Accessed June 1, 2017.
On adverse events following immunization
- Bonhoeffer J, Bentsi-Enchill A, Chen RT. Guidelines for collection, analysis and presentation of vaccine safety data in pre- and post-licensure clinical studies. Vaccine. 2009;27(16):2282-2288.
Appendix F: Representations and Warranties, Limitations of Liability, and Disclaimers
CDISC Patent Disclaimers
It is possible that implementation of and compliance with this standard may require use of subject matter covered by patent rights. By publication of this standard, no position is taken with respect to the existence or validity of any claim or of any patent rights in connection therewith. CDISC, including the CDISC Board of Directors, shall not be responsible for identifying patent claims for which a license may be required in order to implement this standard or for conducting inquiries into the legal validity or scope of those patents or patent claims that are brought to its attention.
Representations and Warranties
"CDISC grants open public use of this User Guide (or Final Standards) under CDISC's copyright."
Each Participant in the development of this standard shall be deemed to represent, warrant, and covenant, at the time of a Contribution by such Participant (or by its Representative), that to the best of its knowledge and ability: (a) it holds or has the right to grant all relevant licenses to any of its Contributions in all jurisdictions or territories in which it holds relevant intellectual property rights; (b) there are no limits to the Participant's ability to make the grants, acknowledgments, and agreements herein; and (c) the Contribution does not subject any Contribution, Draft Standard, Final Standard, or implementations thereof, in whole or in part, to licensing obligations with additional restrictions or requirements inconsistent with those set forth in this Policy, or that would require any such Contribution, Final Standard, or implementation, in whole or in part, to be either: (i) disclosed or distributed in source code form; (ii) licensed for the purpose of making derivative works (other than as set forth in Section 4.2 of the CDISC Intellectual Property Policy ("the Policy")); or (iii) distributed at no charge, except as set forth in Sections 3, 5.1, and 4.2 of the Policy. If a Participant has knowledge that a Contribution made by any Participant or any other party may subject any Contribution, Draft Standard, Final Standard, or implementation, in whole or in part, to one or more of the licensing obligations listed in Section 9.3, such Participant shall give prompt notice of the same to the CDISC President who shall promptly notify all Participants.
No Other Warranties/Disclaimers. ALL PARTICIPANTS ACKNOWLEDGE THAT, EXCEPT AS PROVIDED UNDER SECTION 9.3 OF THE CDISC INTELLECTUAL PROPERTY POLICY, ALL DRAFT STANDARDS AND FINAL STANDARDS, AND ALL CONTRIBUTIONS TO FINAL STANDARDS AND DRAFT STANDARDS, ARE PROVIDED "AS IS" WITH NO WARRANTIES WHATSOEVER, WHETHER EXPRESS, IMPLIED, STATUTORY, OR OTHERWISE, AND THE PARTICIPANTS, REPRESENTATIVES, THE CDISC PRESIDENT, THE CDISC BOARD OF DIRECTORS, AND CDISC EXPRESSLY DISCLAIM ANY WARRANTY OF MERCHANTABILITY, NONINFRINGEMENT, FITNESS FOR ANY PARTICULAR OR INTENDED PURPOSE, OR ANY OTHER WARRANTY OTHERWISE ARISING OUT OF ANY PROPOSAL, FINAL STANDARDS OR DRAFT STANDARDS, OR CONTRIBUTION.
Limitation of Liability
IN NO EVENT WILL CDISC OR ANY OF ITS CONSTITUENT PARTS (INCLUDING, BUT NOT LIMITED TO, THE CDISC BOARD OF DIRECTORS, THE CDISC PRESIDENT, CDISC STAFF, AND CDISC MEMBERS) BE LIABLE TO ANY OTHER PERSON OR ENTITY FOR ANY LOSS OF PROFITS, LOSS OF USE, DIRECT, INDIRECT, INCIDENTAL, CONSEQUENTIAL, OR SPECIAL DAMAGES, WHETHER UNDER CONTRACT, TORT, WARRANTY, OR OTHERWISE, ARISING IN ANY WAY OUT OF THIS POLICY OR ANY RELATED AGREEMENT, WHETHER OR NOT SUCH PARTY HAD ADVANCE NOTICE OF THE POSSIBILITY OF SUCH DAMAGES.
Note: The CDISC Intellectual Property Policy can be found at: cdisc_policy_003_intellectual_property_v201408.pdf.