
Clinical Data Acquisition Standards Harmonization Implementation Guide for Human Clinical Trials
Version 2.1 (Final)
|
Notes to Readers
|
Revision History
| Date | Version |
|---|---|
| 2019-11-01 | 2.1 Final |
| 2017-09-20 | 2.0 Final |
| 2012-04-12 | 1.0 Final |
See Appendix D for Representations and Warranties, Limitations of Liability, and Disclaimers.
Contents
1.1 Purpose
2 How to Use the CDASH Standard
2.1 The Three Components of the CDASH Standard
2.1.1 CDASH Model
2.1.2 CDASHIG
2.1.3 CDASHIG Metadata Table
3 General Assumptions for Implementing CDASH
3.1 How CDASH and SDTM Work Together
3.2 Core Designations for Basic Data Collection Fields
3.3 Form-level CRF Instructions
3.3.1 General Design Considerations for Completion Instructions
3.3.2 General Content Considerations for Completion Instructions
3.4 How to Create New Data Collection Fields When No CDASHIG Field Has Been Defined
3.5 Explanation of Table Headers in the CDASH Model and CDASHIG Metadata Table
3.5.1 CDASH Model
3.5.2 CDASHIG Metadata Table
3.6 Collection, Conversion, and Imputation of Dates
3.6.1 Collection of Dates
3.6.2 Conversion of Dates for Submission
3.6.3 Imputation of Dates
3.7 Mapping Relative Times from Collection to Submissions
3.7.1 Study Reference Period
4 Best Practice Recommendations
4.1 Best Practices for Creating Data Collection Instruments
4.3 Organizational Best Practices to Support Data Collection
5 Conformance to the CDASH Standard
8.1 Interventions Class Domains
8.1.1 General CDASH Assumptions for Interventions Domains
8.1.2 CM - Prior and Concomitant Medications
8.1.3 EC - Exposure as Collected and EX - Exposure
8.1.4 PR - Procedures
8.1.5 SU - Substance Use
8.2.1 General CDASH Assumptions for Events Domains
8.2.2 AE - Adverse Events
8.2.3 CE - Clinical Events
8.2.4 DS - Disposition
8.2.5 DV - Protocol Deviations
8.2.6 HO - Healthcare Encounters
8.2.7 MH - Medical History
8.3.1 General CDASH Assumptions for Findings Domains
8.3.2 DA - Drug Accountability
8.3.3 DD - Death Details
8.3.4 EG - ECG Test Results
8.3.5 IE - Inclusion/Exclusion Criteria Not Met
8.3.6 LB - Laboratory Test Results
8.3.7 MB - Microbiology Specimen
8.3.8 MS - Microbiology Susceptibility
8.3.9 MI - Microscopic Findings
8.3.10 PC - Pharmacokinetics Sampling
8.3.11 PE - Physical Examination
8.3.12 QRS - Questionnaires, Ratings, and Scales
8.3.13 RP - Reproductive System Findings
8.3.14 RS - Disease Response and Clin Classification
8.3.15 SC - Subject Characteristics
8.3.16 TU - Tumor/Lesion Identification Domain
8.3.17 TR - Tumor/Lesion Results
8.3.18 VS - Vital Signs
8.3.19 FA - Findings About Events or Interventions
8.3.19.1 SR - Skin Response
1 Orientation
This implementation guide has been developed to assist in the following activities associated with the collection and compilation of data in a clinical trial.
|
There is arguably no more important document than the instrument that is used to acquire the data from the clinical trial, with the exception of the protocol, which specifies the conduct of that trial. The quality of the data collected relies first and foremost on the quality of that instrument. No matter how much time and effort go into conducting the trial, if the correct data points were not collected, a meaningful analysis may not be possible. It follows, therefore, that the design, development and quality assurance of such an instrument must be given the utmost attention. — Good Clinical Data Management Practices, Version 4, October 2005, Society for Clinical Data Management |
1.1 Purpose
The Clinical Data Acquisition Standards Harmonization (CDASH) Model, the CDASH Implementation Guide (CDASHIG), and the CDASHIG Metadata Table define basic standards for the collection of clinical trial data and how to implement the standard for specific case report forms (CRFs). CDASH establishes a standard way to collect data in a similar way across studies and sponsors, so that data collection formats and structures provide clear traceability of submission data into the Study Data Tabulation Model (SDTM), delivering more transparency to regulators and others who conduct data review. The CDASH standard directly supports the production of clinical data collection instruments. Through this support, the standard also contributes to:
- Consistency and detail in representations of research protocol concepts
- Streamlined processes within medical research
- Development of a corporate library of standardized CRFs
- Use of metadata repositories
- Reporting and regulatory submission
- Data warehouse population
- Data archiving
- Post-marketing studies/safety surveillance
There is growing recognition around the globe that industry standards promote data interchange, which is essential to effective partnering and information exchange between and among clinicians and researchers. Clinical care can more easily reap benefits through medical research findings, and more clinicians will be interested in conducting research if the research process can be integrated into their clinical care workflow. CDISC encourages the adoption of its global standards for clinical research, which should continue to be harmonized with healthcare standards, to provide a means for interoperability among healthcare and research systems such that medical research can support informed healthcare decisions and improve patient safety.
This document is intended to be used by persons involved in the planning, collection, management, and analysis of clinical trials and clinical data, including clinical investigators, medical monitors, clinical research associates (monitors), clinical research study coordinators, clinical data standards subject matter experts (SMEs), clinical data managers, clinical data and statistical programmers, biostatisticians, drug safety monitors, CRF designers, and others tasked with the responsibility to collect, clean, and ensure the integrity of clinical trial data. Although much of the language in this standard addresses development of (e)CRFs, the CDASH standard can also be leveraged for other data sources. The principles and the metadata presented here can be applied to eSource (also known as non-CRF) data such as vendors' electronic data transfer standards, ePRO data structures, and direct data acquisition from electronic healthcare record (EHR) systems.
1.2 Organization of this Document
This document has been organized into the following sections:
- Section 1, Orientation
- Section 2, How to Use the CDASH Standard
- Section 3, General Assumptions for Implementing CDASH
- Section 4, Best Practice Recommendations
- Section 5, Conformance to the CDASH Standard
- Section 6, Other Recommendations
- Section 7, Special-purpose Domains
- Section 8, General Observation Classes
- Appendices
1.2.1 General Notes
Throughout this document, a deliberate decision was made to use a variety of synonyms for various terms in order to reflect the fact that sponsors also use a variety of terms.
- Paper CRFs vs. electronic CRFs: The term CRF used throughout this document refers to both paper and electronic formats, unless otherwise specified.
- Fields vs. variables: Data collection fields refers to terms that are commonly on the CRF. Data collection variables refers to what is in a clinical database.
- Study treatment: The phrase study treatment has been used instead of investigational/medicinal product, study drug, test article, vaccine, study product, medical device, and so on, in order to include all types of study designs and products.
- Mechanisms for data collection: Different data collection mechanisms can be used to control how data are collected (e.g., tick boxes, check boxes, radio buttons, drop-down lists). For the purposes of this document, these terms are used interchangeably.
2 How to Use the CDASH Standard
2.1 The Three Components of the CDASH Standard
CDASH is composed of the CDASH Model and the CDASH Implementation Guide (CDASHIG), with its associated CDASHIG Metadata Table. A domain is a collection of data points related by a common topic, such as adverse events or demographics. CDASHIG domains are aligned with Study Data Tabulation Model Implementation Guide (SDTMIG) domains for beginning-to-end traceability.
2.1.1 CDASH Model
CDASH Model v1.1 provides a general framework for creating fields to collect information on CRFs and includes the model metadata, which shows the standard variables in the model. CDASHIG provides information on the implementation of the CDASH Model and includes the CDASHIG Metadata Table, which details additional specifications for data collection variables within each domain.
CDASH Model v1.1 provides root naming conventions for CDASHIG variables that are intended to facilitate mapping to SDTMIG variables. The variables defined in the CDASH Model follow the same "--XXXX" naming convention as the SDTM. The 2 dashes are replaced by the domain code when applied to create the CDASHIG variable. For example, --DOSFRQ is the CDASH Model variable name to for Dosing Frequency per Interval in the Interventions Class. When a domain abbreviation is applied (e.g., "CM"), CMDOSFRQ is the CDASHIG variable for the frequency of the concomitant medication use. The CDASH Model includes metadata for variables used in each of the SDTM general observation classes, Timing variables, Identifier variables, variables for Special Purpose domains, and domain-specific variables. See Section 3.5.1, CDASH Model, for specific information on this content.
2.1.2 CDASHIG
The CDASHIG provides general information on the implementation of CDASH standards. The CDASH standards include the CDASH Model and the CDASHIG, which includes the supporting CDASHIG Metadata Table. The informative content of the CDASHIG and the normative content metadata table comprise the CDASHIG and must be referenced together.
2.1.3 CDASHIG Metadata Table
The CDASHIG Metadata Table includes only those variables commonly implemented by a significant number of the organizations/companies that provided information/examples (e.g., Medical History, Adverse Events). Implementers can add appropriate variables to their CDASHIG domain using the associated General Observation class within the CDASH Model. The CDASHIG Domain Metadata illustrates the use of Question Text and Prompts employed by many sponsors. Implementers should reference the CDASH Model to see all available options for Question Text and Prompts for parameters and verb tenses that may be substituted.
2.2 CDASHIG Metadata Table Attributes
The CDASHIG Metadata Table attributes provide building blocks for the development of a case report form (CRF) and the underlying database or other data collection structure.
2.2.1 CRF and Data Management System Design Metadata
Certain metadata attributes are essential to CDASH conformance. Combined with the variable naming conventions discussed in Section 5.1, Conformance Rules, these metadata attributes will assist the designer of the CRF(s) and the underlying database structure to remain in conformance with the standard:
- Question Text (full sentence/question forms to prompt for data) OR Prompts (short phrases, often suitable as column headers, to prompt for data)
- CDISC Controlled Terminology lists and subsets of list values when applicable
- DRAFT CDASH Definition (to assist in understanding the purpose of each variable, which ensures proper usage and simplifies subsequent pooled data analyses)
- CDASHIG Core designations and implementation notes (which, when used together, can assist a designer in determining the complete set of data to be collected on a form)
2.2.2 SDTMIG Programming Metadata
Columns in the CDASHIG Metadata Table that will assist in developing programs to generate SDTM domain datasets from CDASHIG-compliant data include:
- Domain
- CDASHIG Variable
- Data Type
- SDTMIG Target
- Mapping Instructions
- Controlled Terminology Codelist Name
- Subset Controlled Terminology/CDASH Codelist Name
- Implementation Notes
2.2.3 Additional Metadata
Clear and consistent completion instructions for sites help to ensure collection of quality, reliable data, a critical factor in the development of high-quality pooled/submission data. The CDASHIG Metadata Table includes the Case Report Form Completion Instructions column to assist authors in creating this study-level documentation for instructing sites how to complete CRF fields.
2.3 CRF Development Overview
The key steps to developing CRFs using CDASH are as follows:
1. Each organization may maintain a corporate library of standardized CRFs. Determine the requirements for data domains from these (if applicable) or from the protocol data collection requirements for the study.
2. Review the domains published in the CDASHIG to determine which of the data collection domains and fields are already specified in the published domains.
3. As much as possible, the standard domains should be used to collect data in a manner that will be effective for data collection. Develop the data collection tools using these published, standard domains first.
4. During the development of CDASH-conformant collection instruments (e.g., CRFs, eCOA screens), the SDTMIG domain to which the collected data is to be mapped must be determined. The choice of the SDTMIG domain to use does NOT depend upon the mode of transmission, the methodology used to generate the data, the medium used to store the data, the person who recorded the data, nor the subject whom the data describes. The SDTMIG domain to be used impacts what CDASH variable names, question texts, prompts, controlled terminology, and so on, to use. CDASH suggests a format to be presented to the person entering the data, but it does not dictate any data structure in which to store the collected data (often referred to as a data management operational database).
Example 1: A study has meal consumption diary data captured via a subject-completed PRO. Another study also captures meal consumption data, but the subject takes a photo of the food prior to and after the meal, and sends the photos to a third party, which determines food consumption. Even though captured in a different way, the data from both studies will map into the SDTMIG ML (Meal Data) domain.
Example 2: A study where subjects have their blood samples sent to a central lab, which analyzes the samples and sends results to the sponsor via electronic data transfer. In a second study, the samples are analyzed locally and results are captured on a CRF. The laboratory results from both studies are stored in the SDTMIG LB (Laboratory Test Results) domain. CDASH recommends that dates be collected in an unambiguous format and suggests using the DD-MON- YYYY format. This defines the format to be presented to the person entering the data, but it does not define the electronic format in which to store the data. One system may store each date as a character field, another may store them as numeric values (e.g., an SAS date), and yet another as 3 separate fields formatted as day, month, and year. Each of these is a legitimate way to store the data collected.
5. Using the root variables and other CDASH metadata in the CDASH Model, add any additional variables that are needed to meet the requirements of data collection. Follow CDISC Variable Naming Fragment (see Appendix B, Glossary and Abbreviations) conventions, and CDASH root variable naming conventions where they exist (e.g., --DAT for dates, --TIM for times, --YN for prompts as described in the CDASH Model). Example: Replace "--" with the 2-character domain code that matches the other variables in the same domain. For example, to add the --LOC variable to a Medical History CRF, the domain code is "MH", so the variable would become "MHLOC" in that domain.
6. The Question Text and Prompt columns in the CDASH Model metadata provide different variations in the recommended text for asking the question on a CRF. For each question, the sponsor may elect to either use the Question Text or the Prompt on the CRF. Some text is presented using brackets [ ], parentheses ( ), and/or incorporating forward slashes. These different formats are used to indicate how the Question Text or Prompt may be modified by the sponsor.
a. The text inside the brackets provides an option on the verb tense of the question, or text that can be replaced with protocol-specific verbiage.
b. The text inside the parentheses provides options (e.g., singular/plural) or text that may be eliminated.
c. Text separated with a forward slash provides optional words that the sponsor may choose.
Example: The CDASH variable --PERF, from the CDASH Model, has the following Question Text and Prompt.
Question Text:
[Were any/Was the] [--TEST/ topic] [measurement(s)/test(s)/examination(s)/specimen(s)/sample(s)] [performed/collected]?
Prompt:
[--TEST/Topic] [Measurement(s)/Test(s)/Examination(s)/Specimen(s)/Sample(s)] [Performed/Collected]?
The sponsor wants to add a question to a CRF that asks whether a lab specimen was collected using a Yes/No response.
The sponsor selects the CDASH variable --PERF and adds the appropriate domain code. LBPERF Use either the Prompt or the full Question Text on the CRF.
Question Text: Was the laboratory specimen collected?
i. In the first set of brackets, the text option "Was the" is selected as the study required only 1 lab test to be performed. [Were any/Was the]
ii. In the second set of brackets, the text used is "laboratory" which is the topic of interest. [-- TEST/Topic (laboratory)]
iii. In the third set of brackets, the text option "specimen" without the optional "s" is selected. [measurement(s)/test(s)/examination(s)/specimen(s)/sample(s)]
iv. In the fourth set of brackets, the text option "collected" is selected. [performed/collected]
Prompt: Laboratory Specimen Collected
i. In the first set of brackets, the text used is the topic of interest (i.e., laboratory). [-- TEST/Topic (Laboratory)]
ii. In the second set of brackets, the text option "specimen" without the optional "s" is selected. [Measurement(s)/Test(s)/Examination(s)/Specimen(s)/Sample(s)]
iii. In the third set of brackets, the text option "collected" is selected. [Performed/Collected]
7. Create custom domains based on one of the General Observation Classes in the CDASH Model. See Section 3.4, How to Create New Data Collection Fields When No CDASHIG Field Has Been Defined, for more information.
The CDASHIG Metadata Table attributes provide building blocks for the development of a CRF and the underlying database or other data collection structure.
3 General Assumptions for Implementing CDASH
3.1 How CDASH and SDTM Work Together
1. The Study Data Tabulation Model (SDTM) and the SDTM Implementation Guide (SDTMIG) provide a standard for the submission of data. CDASH is earlier in the data flow and defines a basic set of data collection fields that are expected to be present on the majority of case report forms (CRFs). SDTM and CDASH are clearly related. The use of CDASH data collection fields and variables is intended to facilitate mapping to the SDTM structure. When submitted data can be collected as represented in an SDTM dataset, with no transformations or derivations, the SDTMIG variable names are presented in the CDASHIG Metadata Table and should be used to collect the data. In cases where the collected data must be transformed or reformatted prior to inclusion in an SDTM dataset, or where a corresponding SDTMIG variable does not exist, CDASH has created standardized data collection variable names.
2. CDASHIG Version 2.1 content is based on SDTMIG Version 3.2.
3. All SDTMIG “Required” variables have been addressed either directly through data collection or by determining what needs to be collected to derive the SDTMIG variable. In some cases, SDTMIG variable values can be obtained from data sources other than the CRF or are populated during the preparation of the submission datasets (e.g., --SEQ values).
4. CDASHIG domains contain variables that may be used in the creation of the RELREC submission dataset. RELREC is an SDTM dataset that describes relationships between records for a subject within or across domains, and relationships of records across datasets. The specific set of identifiers necessary to properly identify each type of relationship is collected in each dataset to support merging the data. Collected data may be across records within a domain, records in separate domains, and/or in sponsor-defined variables. For example, the CDASHIG variable CMAENO, having a question text of "What [is/was] the identifier for the adverse event(s) related to this (concomitant) [medication/therapy]?", may be used to collect data that identifies a relationship between records in the CM dataset and records in the AE dataset.
5. The CDASH standard includes some data collection fields that are not in the SDTMIG (e.g., “Were any adverse events experienced?”, “Were any medication(s) taken?”, "Was the medication taken prior to study start?", “Was the medication ongoing?”). These fields support site-friendly data collection and help with data cleaning/data monitoring by providing verification that other fields on the CRF are deliberately left blank. For use of a field with this intent in an electronic data capture (EDC) system, a CDASH variable name is provided in the CDASHIG Metadata Table (e.g., AEYN, CMYN, CMPRIOR, CMONGO). When the CDASHIG field is confirming that data collection is not expected in other records (e.g., AEYN, CMYN), the corresponding SDTMIG Variable Name column indicates “N/A” and the Mapping Instruction column indicates that “this field is NOT SUBMITTED”. When the CDASHIG field is confirming that data collection is not expected in another date field (e.g., CMPRIOR, CMONGO), the SDTMIG Variable Name lists the applicable SDTM timing variables and Mapping Instructions.
6. The CDASHIG Findings domain (e.g., Drug Accountability (DA), ECG Test Results (EG), Vital Signs (VS)) tables are presented in a structure that is similar to the SDTMIG, which is to list the variable names and some examples of the tests. Implementers are expected to include protocol-specific tests in a CRF presentation layout, using the appropriate values from the relevant CDISC Controlled Terminology codelists. For example, VSTEST values are used to name the test on the CRF, and the corresponding test code is determined from the VSTESTCD codelist. Implementers may use synonyms when the xxTEST values are long or not commonly recognized (e.g., ALT in place of Alanine Aminotransferase). Implementers should use the CDASHIG recommendations to identify the types of data to collect while referring to the SDTMIG and CDISC Controlled Terminology for additional metadata (e.g., labels, data type, controlled terminology).
7. The CDASH standard has intentionally not reproduced other sections of the SDTM standard and implementers are asked to refer to the SDTM and SDTMIG for additional information (both available on the CDISC website, http://www.cdisc.org/sdtm.
8. The CDASHIG data collection fields included in the CDASHIG Metadata Table are the most commonly used and should be easily identified by most implementers. Additional data collection fields may be necessary to capture therapeutic area-specific data points as well as other data specified in the clinical study protocol or for local regulatory requirements. Reference the CDASH Model and relevant CDISC therapeutic area user guide(s) for additional information.
9. Use the CDASH recommendations to develop company standards, taking into consideration the stage of clinical development and the individual therapeutic area requirements. To gain the greatest benefit from using the CDASH standard, CRFs should not be developed on a trial-by-trial basis within the implementer organization, but rather be brought into a study from a library of approved CRFs based on the CDASH Model and Implementation Guide, whenever practicable.
10. The CDASHIG is divided into sections of similar types of data and the CDASHIG Metadata Table is arranged in alphabetical order (by domain abbreviation) within the respective general observation class. CRF layout was not within the original scope of the CDASH project; however, to assist with standardization of CRF layout, data collection fields are presented within the CDASHIG Metadata Table in a logical order, and annotated example CRFs have been provided (if available). In addition, implementers are referred to Section 4.1, Best Practices for Creating Data Collection Instruments, for a discussion on best practices for ordering fields on a CRF.
3.2 Core Designations for Basic Data Collection Fields
The CDASH Team initially considered utilizing the SDTMIG Core Designations of Required, Expected, and Permissible to capitalize on prior understanding of these descriptive designations as well as to enable a consistent categorization across CDASH and SDTM standards. However, when the CDASHIG Metadata Table was constructed, it quickly became apparent that CDASHIG core designations would often differ from SDTMIG core designations due to the inherent differences in the manner in which data are collected (to ensure the most accurate data) and the structure in which data are reported and submitted. For example, a variable categorized as Required in the SDTMIG may not be required in the CDASHIG if it can be derived in the SDTM datasets (rather than be a field captured explicitly on a CRF). Also, the SDTMIG core designation of “Required” imposes a rule that the variable cannot be null. CDASHIG core designations are not intended to impose any rules that require a field to be populated with data. They are only intended to designate which fields should be present on the CRF.
In order to facilitate classification of the different types of data collection fields, the following categories were used:
- Highly Recommended (HR): A data collection field that should always be on the CRF (e.g., the data are needed to meet a regulatory requirement or are required to create a meaningful dataset).
- Recommended/Conditional (R/C): A data collection field that should be on a CRF based on certain conditions (e.g., complete date of birth is preferred, but may not be allowed in some regions; AE time should be captured only if there is another data point with which to compare it). For any R/C fields, the "condition" is described in the Implementation Notes column of the CDASHIG Metadata Table.
- Optional (O): A data collection field that is available for use.
3.3 Form-level CRF Instructions
3.3.1 General Design Considerations for Completion Instructions
Whenever possible, details related to the completion of a single field should be placed with the field itself on the CRF. If this is not possible due to the medium and/or system being used to create CRFs, then it is permissible to include the field-level instructions at the top of the form, in what is generally considered the form-level instruction area. In some cases, such as when the form-level instructions are very lengthy or include graphics or flowcharts, a separate CRF completion instruction guideline may be required.
3.3.2 General Content Considerations for Completion Instructions
When creating form-level instructions for a CRF, the following points should be considered:
- The instructions should include clear references to the time period for which data are to be reported for the study, or to specific time windows that are allowed.
- The instructions should provide references to protocol sections for the specifics of and/or limitations on the data to be reported.
- The instructions should include any special instructions for additional reporting or actions required beyond what is collected on the CRF.
- The instructions should include considerations on how data collected on one CRF might have an impact on data that are reported on a different CRF.
- The instructions should refer to any other forms that are related to the CRF being completed.
3.4 How to Create New Data Collection Fields When No CDASHIG Field Has Been Defined
Adding new sponsor-defined collection fields is often constrained by business rules, as well as by clinical data standards subject matter experts (SMEs), clinical data management processes, and electronic data capture (EDC) systems. The naming conventions and other variable creation recommendations in CDASHIG are designed to allow collection of data regardless of subsequent inclusion in SDTM, as well as to consistently facilitate transforming the collected data into submission datasets.
Prior to adding any new fields to a sponsor's study CRF, the CDASH Model should be reviewed to see if there is a root field that will work for the data collection need.
New data collection fields (not already defined in the CDASH Model) will fall under one of following categories.
- Fields used for data cleaning purposes only and not submitted in SDTM datasets (e.g., --YN): The field --YN with Question Text "Were there any [interventions/events/findings]?" can be added to a domain for this purpose. Replace the 2 dashes (--) with the 2-character domain code, and create the Question Text or Prompt using generic Question Text or Prompt from the CDASH Model as a base. Always create custom data cleaning/operational variables using consistent naming conventions.
- Fields with a direct mapping to an SDTMIG variable: If a value can be collected exactly as it will be reported in the SDTM dataset (i.e., same value, same datatype, same meaning, same controlled terminology), the SDTMIG variable name should be used as the data collection variable name in the operational database to streamline the mapping process. Extensions may be appended if needed to create a unique variable name in the collection database. Any collection variable whose meaning is the same as an SDTMIG variable should be a copy of the SDTMIG variable, and the meaning should not be modified for data collection.
- Fields without a direct one-to-one mapping to SDTM datasets:
- If a study requires a field that is not identical to an SDTMIG field, for example the collected data type is different from the data type in the corresponding SDTMIG variable, or the SDTMIG variable is derived from collected data, the operational database should use a variable with a different name from the SDTMIG variable into which it will be mapped.
- Example 1: A study collects Findings data in a denormalized format and then maps the data to the normalized SDTM structure. The --TESTCD values can be used as the CDASHIG variable names, and the corresponding --TEST value can be used as the prompt on the CRF (See Section 8.3.1, General CDASH Assumptions for Findings Domains, for more information).
- Example 2: Dates and times are collected in a local format, familiar to the CRF users, and then reported in the SDTM-specified ISO 8601 format. In the operational database, the CDASH variables --DAT and --TIM (if collected) map into the single SDTM variable (--DTC).
- Example 3: If the mapping to SDTM is similar, but not direct, "C" can be included before the root variable name to indicate a "collected" version of the variable to which that data will map. For example, if an injection is to be administered to a subject’s LEFT THIGH, RIGHT THIGH, LEFT ARM, or RIGHT ARM, the sponsor may create the variable EXCLOC. The SDTM mapping would split these into EXLOC and EXLAT, which would avoid having to split the collection of the data into 2 fields on the CRF.
- An STDM variable that is not defined in the SDTM version being used by the sponsor can be included as a non-standard variable or a supplemental qualifier.
- If a study requires a field that is not defined in CDASH and SDTM with the same meaning or intent (e.g., would map to SDTM SUPP--), a unique name should be assigned based on sponsor business rules using CDASH naming fragments (e.g., --DAT, --TIM) as appropriate and CDISC Variable Naming Fragments where possible. See SDTMIG v3.2 Appendix D.
3.5 Explanation of Table Headers in the CDASH Model and CDASHIG Metadata Table
3.5.1 CDASH Model
This section provides an explanation of the columns used in the CDASH Model.
- Observation Class: This column contains the SDTM Class for the domain.
- Domain: This column contains the 2-letter domain code.
- Order Number: The values in this column are used to help sequence the variables as they appear in the pmetadata table. There are no implied meaning, significance or conformance expectations. The values increase by one for each variable within a unique grouping of Observation Class plus Domain.
- CDASH Variable: This column provides the CDASH root variable names (e.g., --ONGO, --DAT).
- CDASH Variable Label: This column contains a suggested root variable label that that may be used for the CDASHIG variable.
- DRAFT CDASH Definition: This column provides a draft definition of the root variable. This text may or may not mirror any text in the SDTM. Currently, there is a new CDASH/SDTM team creating variable definitions. Once these definitions are finalized, the CDASH definitions will be updated to harmonize with them.
- Question Text: This column in the CDASH Model contains the recommended question text for the data collection field. Question Text is a complete sentence. Some text is presented inside brackets [ ] or parentheses (). The text inside brackets should be replaced with protocol-specified verbiage; text inside parentheses is optional. Text separated with a forward slash indicates optional wording from which the sponsor may choose.
- Prompt: This column in the CDASH Model contains the recommended prompt text for the data collection field. The Prompt is a short version of the question. Some text is presented inside brackets [ ] or parentheses (). The text inside brackets should be replaced with protocol-specified verbiage; text inside parentheses is optional. Text separated with a forward slash indicates optional wording from which the sponsor may choose.
- Data Type: This column contains the simple data type of the CDASH variable (i.e., Char, Num, Date, Time).
- SDTM Target: This column provides the suggested mapping to the SDTM root variable. When no direct mapping to an SDTM root variable is available, the column contains "N/A". When the column contains "SUPP--.QNAM", it means that the value represented in the CDASH variable shall be mapped to an SDTM Supplemental Qualifier. Note: CDASH variables noted as not having a direct map to SDTM variables (i.e., non-standard variables) may have SDTM variable equivalents in future versions.
- Mapping Instructions: This column contains information on the suggested mapping of the root variable to the SDTM variable.
- Controlled Terminology Codelist Name: This column contains the Controlled Terminology (CT) codelist name, e.g., "LOC" that is associated with the field. Certain variables (e.g., dates) use ISO formats as CT; however, in CDASH these variables are generally not collected using the ISO CT. These variables are converted to the ISO format when the SDTM-based submission datasets are created.
- Implementation Notes: This column contains further information, such as rationale and implementation instructions, on how to implement the CRF data collection fields and how to map CDASH variables to SDTM variables.
Note: When multiple options are contained in a single cell, the options are separated by a semicolon.
3.5.2 CDASHIG Metadata Table
This section provides an explanation of the columns used in the CDASHIG Metadata Table.
- Observation Class: This column contains the SDTM Class for the domain.
- Domain: This column contains the 2-letter domain code.
- Data Collection Scenario: This column in the CDASHIG Metadata Table identifies the different data collection options in CDASH for the same domain and is best used for filtering the table. The information in this column provides the context for the CDASHIG Core Designations (e.g., denoting which fields should be present on the CRF). When only 1 data collection scenario is provided for the domain, the column contains "N/A".
- Implementation Options: When this column contains "Horizontal-Generic", a sampling of the CDASHIG metadata is provided as a template for the metadata of the CRF in a denormalized structure.
- Order Number: The values in this column are used to help sequence the variables as they appear in the metadata table and provide a suggested order of CDASHIG variables to be displayed on a CRF. There are no implied meaning, significance or conformance expectations. The values increase by one for each variable within a unique grouping of Observation Class plus Domain plus Implementation Options.
- CDASHIG Variable: This column provides the CDASHIG variable names (e.g., CMONGO, AEDAT).
- CDASHIG Variable Label: This column provides the CDASHIG variable label.
- DRAFT CDASHIG Definition: This column provides a draft definition of the CDASHIG variable. This text may or may not mirror any text in the SDTMIG. Currently, there is a new CDASH/SDTM team creating variable definitions. Once these definitions are finalized, the CDASH definitions will be updated to harmonize with them.
- Question Text: This column in the CDASHIG Metadata Table provides the suggested text for the specific domain. Implementers should refer to the CDASH Model to create alternative question text that may be used that meets the CDASH conformance rules. Question Text is a complete sentence. Some text is presented inside brackets [ ] or parentheses (). Text inside brackets should be replaced with protocol- specified verbiage; text inside parentheses is optional. Text separated with a forward slash indicates optional wording from which the sponsor may choose.
- Prompt: This column in the CDASHIG Metadata Table provides the suggested text for the specific domain. Implementers should refer to the CDASH Model to create alternative prompt text that may be used that meets the CDASH conformance rules The Prompt is a short version of the question. Some text is presented inside brackets [ ] or parentheses (). Text inside brackets should be replaced with protocol- specified verbiage. [NULL] in this column indicates that populating a prompt is not required on a CRF screen/page if not needed.
- Data Type: This column contains the simple data type of the CDASH variable (i.e., Char, Num, Date, Time).
- CDASHIG Core: This column contains the CDASHIG core designations for basic data collection fields (i.e., Highly Recommended (HR), Recommended/Conditional (R/C), Optional (O)). See Section 3.2, Core Designations for Basic Data Collection Fields.
- Case Report Form Completion Instructions: This column contains recommended example instructions for the clinical site on how to enter collected information on the CRF.
- SDTMIG Target: This column provides the suggested mapping to the SDTMIG variable name. It may help facilitate the creation of the SDTMIG variables needed for submission. When no direct mapping to an SDTMIG variable is available, the column contains "N/A". When the column contains "SUPP--.QNAM", it means that that the value represented in the CDASH field shall be mapped to an SDTM Supplemental Qualifier.
- Note: CDASHIG variables noted as not having a direct map to SDTMIG variables (i.e., non-standard variables) may have SDTM variable equivalents in future versions.
- Mapping Instructions: This column contains information on the suggested mapping of the CDASHIG variable to the SDTMIG variable. Mapping instructions in the CDASHIG Metadata Table provide more complete guidance than those present in the CDASH Model. When domain-level metadata are not available, consult the CDASH Model for SDTM Mapping Instructions.
- Controlled Terminology Codelist Name: This column contains the Controlled Terminology (CT) codelist name, e.g., "LOC", that is associated with the field. The SDTMIG indicates that certain variables (e.g., dates) use ISO formats as CT. However, in CDASH these variables are generally not collected using the ISO CT; these variables are converted to the ISO format when the SDTM-based submission datasets are created.
- Subset Controlled Terminology/CDASH Codelist Name: This column contains the CDISC Controlled Terminology or CDASH Subset Codelist name that may be used for that specific variable (e.g., EXDOSFRM).
- Implementation Notes: This column contains further information, such as rationale and implementation instructions, on how to implement the CRF data collection fields and how to map CDASHIG variables to SDTMIG variables.
Note: When multiple options are contained in a single cell, the options are separated by a semicolon.
3.6 Collection, Conversion, and Imputation of Dates
3.6.1 Collection of Dates
Collect dates in such a way to allow sites to record only the precision they know. The system should also store only the collected precision. Any incomplete dates must remain incomplete with no imputation and no “zero-filling” of missing components.
Data collection and database processes should allow for the possibility of partial dates and times, because a partial date may be the most precise information that can be collected for some data. For an example of when it may be necessary or appropriate to collect partial dates, see Section 7.3, DM - Demographics. In some countries, collection of a complete date of birth is restricted under privacy rules, so only a year, or year and month of birth, might be collected. Other examples of commonly collected partial dates occur in the CM and MH domains, where the subject may not remember the complete date of when they started to take a medication or when a significant medical history condition began.
If a full date is collected, the CDASH variable --DAT or all 3 date components (i.e., --DATYY, --DATMO, -- DATDD) should be included on the collection tool. If a partial date can be collected in a single field, the CDASH --
DAT should be used. If a partial date must be collected as separate database fields to collect year, month, and day, refer to the CDASH Model for examples of standard naming fragments (--YY, --MO, --DD, --TIM). The capabilities of individual software systems (e.g., EDC) will determine which variable names are needed. CDASH uses separate data collection fields for dates and times. If times are collected, it is expected that they will be used with the appropriate collected date to derive the related SDTM date variable in ISO8601 format.
3.6.2 Conversion of Dates for Submission
See SDTMIG v3.2 Sections 4.1.4.1 and 4.1.4.2 for detailed information about converting dates and times from the collection format to the submission format using ISO 8601. A specific example of mapping birth date is shown here.
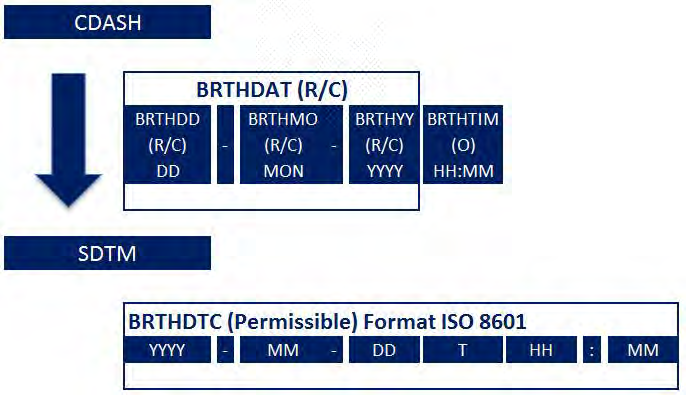
The SDTM date format allows this partial date to be submitted so the reviewer can see what was collected.
3.6.3 Imputation of Dates
If missing parts of the date are imputed for analysis purposes, the imputed dates will be generated in the Analysis Data Model (ADaM) but not in the SDTM submission data sets.
3.7 Mapping Relative Times from Collection to Submissions
Relative timing variables are sets of variables that provide information about how the timing of the record relates to either the study reference period or another fixed point in time. CDASH relative timing variables are collected for those observations where a date either is not collected or is not available. The CDASH set of variables serve as an indicator (or flag) that the observation's "start" was "prior" to the study reference period or prior to another fixed point in time OR that the observation's "end" was "after" or "ongoing" as of the study reference period or another fixed point in time. The CDASH variables of --PRIOR and --ONGO serve this purpose. How these CDASH flags are translated to SDTM (according to controlled terminology) depends on whether the comparison is against the protocol-defined study reference period or against another fixed point in time that may serve as the "reference" for the timing of the record. To emphasize, collection of these CDASH relative timing variables is always dependent on the actual date either being prospectively "not collected" or not available. For more information, see Section 8.1.1, General CDASH Assumptions for Interventions Domains and Section 8.2.1, General CDASH Assumptions for Events Domains.
For all SDTM submissions, there is a defined timeframe called the study reference period. According to the SDTMIG v3.2, the start and end dates of the study reference period are submitted in the variables RFSTDTC and RFENDTC. The defined period may be protocol-specific or set by company policy, standard operating procedures, or other documented procedures. The study reference period might be defined as being from the date/time of informed consent through the date/time of subject's completion of the study, or it might be from the date/time of first dose to the date/time of last dose. Regardless of how the study reference period is defined, the dates (and optionally times) of the start and end of that period must be collected.
If there is a need to collect information about whether an observation of interest occurred prior to a reference point or milestone other than the beginning of the study reference period, or was ongoing or continuing at some reference point or milestone in the study other than the end of the defined study reference period, the date/time of that reference point or milestone should also be collected. If this date/time has been collected, reasonable comparisons can be made to that date/time with “prior”, “coincident”, “continuing”, or “ongoing” questions.
The following steps should be taken to ensure observations of interest that occur over time can be related to the study reference period or to a fixed point in time/milestone in a meaningful way. Figure 1 provides a representation of an intervention as it relates to the study reference period, and Figure 2 and Figure 3 provide a representation of comparisons related to other fixed points in time or a milestone.
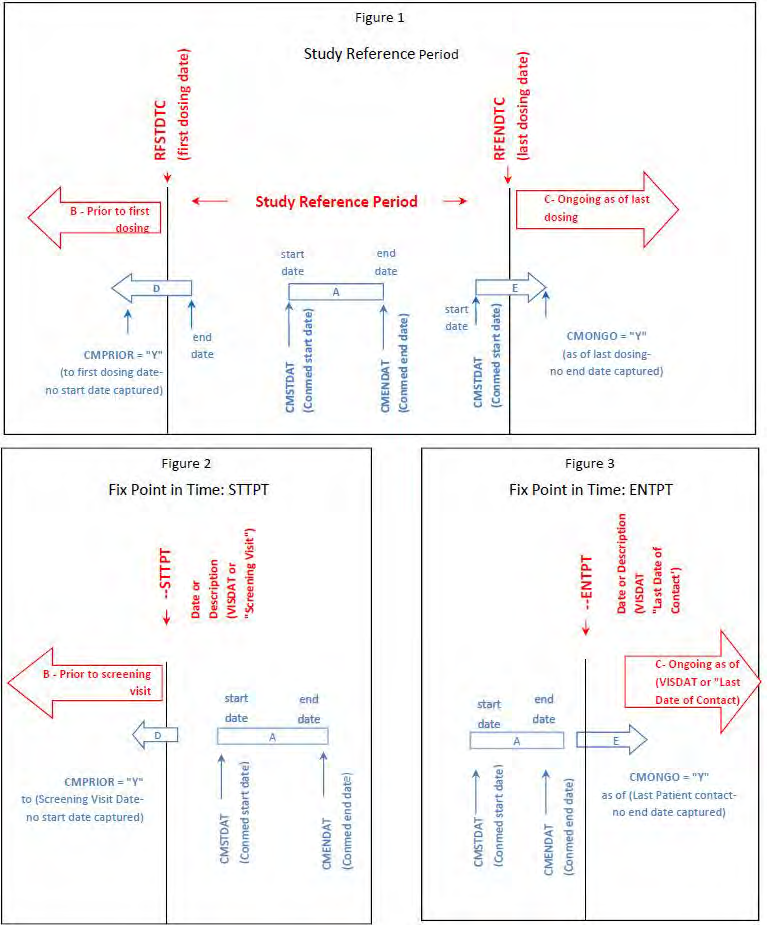
3.7.1 Study Reference Period
1. Define the “on study” period (B-C). Once the overall “on study” period has been defined (B-C), collect the dates (/times) of the start of the study reference period (e.g., date of informed consent, date of first dose) and end of the study reference period (e.g., date of last contact, date of last dose), as part of the clinical data with their respective domains (e.g., Disposition (DS), Exposure (EX)). These dates will map into the RFSTDTC (B; start of Study Reference Period) and RFENDTC (C; end of Study Reference Period) variables in the SDTMIG Demographics (DM) dataset.
2. Collected comparisons (D, E) using CDASHIG variables (e.g., “prior”, “ongoing”) of when something started or ended in relation to the “on study” reference period (i.e., RFSTDTC-RFENDTC: B-C). These CDASH variables are used to populate the SDTMIG variables--STRF and --ENRF variables when the SDTM-based datasets are created.
Note: These relative timing variables are only populated in the SDTM -based datasets when a date is not collected.
3.7.2 Fixed Point in Time/Milestone
1. Define the fixed point in time or milestone (B or C). The fixed point in time or milestone can be a date or a description. This will map into the SDTMIG variables --STTPT or --ENTPT when the SDTM-based datasets are created.
2. Collected comparisons (D or E) using CDASHIG variables (e.g., “prior”, “ongoing”) of when something started or ended in relation to the fixed point in time or milestone (B or C). These CDASH variables are used to populate the SDTMIG variables--STRTPT or --ENRTPT when the SDTM-based datasets are created.
Note: These relative timing variables are only populated in the SDTM -based datasets when a date is not collected.
For information about mapping what is collected in “prior”, “ongoing”, and “continuing” fields into the appropriate SDTMIG variables, see SDTMIG v3.2 Section 4.1.4.7.
3.8 CDISC Controlled Terminology
Submission data standards are required by some global regulators, and controlled terminology (CT) is part of the requirement. Using CT from the start during data collection builds in traceability and transparency, and reduces the problems associated with trying to convert legacy codelists and variables to the submission standards. CT can be used in the following ways during data collection:
1. To collect data using a standardized list of values (e.g., Mild, Moderate, Severe)
2. To ask a specific question on the CRF (e.g., Temperature)
3. To create a variable name in the database (e.g., TEMP for the collection of vital sign data when a unique variable name must be created for each vital sign result)
Terminology applicable to CDASH data collection fields is either in production or under development by the CDISC Terminology Team. Production terminology is published by the National Cancer Institute‘s Enterprise Vocabulary Services (NCI EVS) and can be accessed at http://www.cancer.gov/cancertopics/terminologyresources/CDISC. The examples in this document use CDISC Controlled Terminology where possible, but some values that seem to be CT may still be under development at the time of publication, or even especially plausible "best-guess" placeholder values. Do not rely on any source other than the CDISC value set in the NCI Thesaurus for CT.
In some cases it is more appropriate to use a subset of a published SDTM terminology list, rather than the entire list. To begin with an established subset of the SDTM terminology, go to https://www.cancer.gov/research/resources/terminology/cdisc and reference the CDASH terminology. These CDASH codelists have been subsetted from the complete SDTM terminology lists and are available to implementers as a way to quickly set up codelists for data collection. However, most implementers will also need to review the SDTM terminology to determine which other values are needed for their particular implementation. The CDASH terminology subset names are provided in the CDASHIG Metadata Table for easy reference.
Some codelists, such as Laboratory Test Codes (LBTESTCD), are extensible. This means that values that are not already represented in that list (either as a CDISC Submission Value, a synonym, or an NCI preferred term) may be added as needed. Other codelists, such as AE Action Taken with Study Treatment, are non-extensible and must be used without adding any terms to the list. Where no CDISC Controlled Terminology exists, implementers should develop sponsor defined terminology to ensure consistency and transparency. If gaps are identified, sponsors should submit requests to add values to controlled terminology by using the Term Suggestion form (available at http://ncitermform.nci.nih.gov/ncitermform/?version=cdisc).
In cases where a CDASH/CDASHIG variable has associated CT, the codelist is referenced in the Controlled Terminology column in the CDASH Model and CDASHIG Metadata Table in this format: (codelist name).
4 Best Practice Recommendations
CDASH best practices describe operational recommendations to support data collection, suggested case report form (CRF) development workflow, and methods for creating data collection instruments. Adherence to Section 4.1, Best Practices for Creating Data Collection Instruments, is an important part of conformance to the CDASH standard. The best practices in this section provide an overview of key data collection methodologies to expedite the clinical data flow from CDASH to the Study Data Tabulation Model (SDTM). For additional guidance, see the Society for Clinical Data Management's (SCDM) Good Clinical Data Management Practices (GCDMP) standard at https://scdm.org/gcdmp/.
4.1 Best Practices for Creating Data Collection Instruments
| Num | Best Practice Recommendation | Rationale |
|---|---|---|
1 | When a binary response is expected, "Yes/No" responses are preferred over "Check all that apply", because a missing response could lead to a misinterpretation of critical data. For example, if AEs are determined to be serious based only upon checking the applicable serious criteria (e.g., Hospitalization, Congenital Anomaly), failure to check a criterion would potentially delay identification of an SAE. If an assessment has composite responses (e.g., presence or absence of 2 or more symptoms), "Yes/No" questions for each component response (e.g., symptom) are preferred to "Check all that apply" questions. One exception to this recommendation might be assessments where the majority of options would be answered "No". An example would be the collection of ECG abnormality data where approximately 45 abnormalities may be listed, but only a few will apply. Another exception is when a validated instrument contains checkboxes. In this case, they should remain checkboxes in the CRF or eCRF. Another exception to this recommendation is when there are controlled terminologies governing the values being collected. For example, if collecting RACE using the "Check all that apply" option, the RACE values defined by controlled terminology should be collected as individual check boxes, and not as a "Yes/No" response. In cases where the sponsor chooses to use "Check all that apply", additional quality checks should be considered (e.g., SDV) to ensure the data collected in the CRF are correct and complete. | "Yes/No" questions provide a definite answer. The absence of a response is ambiguous as it can mean "No", "None", or that the response is missing. In situations where there is no other dependent or related field by which to gauge the completeness of the field in question, a "Yes/No" response ensures that the data are complete. For example, when an AE End Date is blank, a "Yes" response to the question "Is the AE ongoing?" ensures that the data are complete. When the end date is provided, it is not necessary to answer the question "No". |
2 | The database should contain an indication that a planned exam/assessment was not performed. The mechanism for this may be different from system to system or from paper to EDC. For example, the data collection instrument/CRF could contain a field that allows the site to record an indication that a Vital Sign assessment was not performed (e.g., VSPERF="N" or TEMP_VSSTAT="NOT DONE") A "Yes/No – assessment completed" question is preferred over a "Check if not done" box, unless the "Check if not done" field can be compared to a completed data field using a validation check to confirm that one or the other has data.
| This will provide a definitive indicator that a data field has missing data and has not been overlooked. This will prevent unnecessary data queries to clarify whether an assessment has been performed. The use of the "Yes/No" format helps to eliminate ambiguity about whether an assessment has been completed. |
3 | Data cleaning prompts should be used to confirm that blank CRFs are intentionally blank. Usually this will be a "Yes/No" question (e.g., AEYN) but it may be a "Check if blank" box if a validation check can be used to confirm that either the "Check if blank" box is checked, or that there are data recorded in the CRF. | "Yes/No" questions provide a definite answer. The absence of a response is ambiguous as it can mean "No", "None", or that the response is missing. This will provide a definitive indicator that a CRF is blank on purpose and has not been overlooked. This will prevent unnecessary data queries. |
4 | The same data (i.e., the same information at the same time) should not be collected more than once. |
Collecting the same data more than once:
|
5 | A "Check if ongoing" question is recommended to confirm ongoing against an end date. This is a special-use case of "Yes/No", where the data entry field may be presented as a single possible response of "Yes" in conjunction with an End Date variable. If the box is checked, the operational variable may contain "Yes". If the box is not checked and the End Date is populated, the value of the variable may be set to "No". For some EDC systems, it may be better to display the possible responses to the "Check if ongoing" question as radio buttons. Conditional logic can then be used to solicit the collection of the end date only if the answer to the "Ongoing" question is "N" (No). |
For the use case of "Check if ongoing", for the data to be considered "clean", 1 of the 2 responses must be present and the other response must be blank. So, the presence of the end date provides confirmation that the event is not ongoing. |
6 | CRFs should use a consistent order of responses (e.g., "Yes/No") from question to question, for questions with response boxes or other standardized lists of values. Exceptions to this would be cases where a validated instrument (e.g., a standardized assessment questionnaire) is used. |
A consistent order of response boxes promotes ease of use of the CRF to help reduce data entry errors and to avoid introducing bias or leading the investigator to a desired response. |
7 | CRF questions and completion instructions should be unambiguous, and should not "lead" the site to answer the question in a particular way. |
Data should be collected in a way that does not introduce bias or errors into the study data. Questions should be clear and unambiguous. This includes making sure that the options for answering the question are complete, such as providing options for "Other" and "None" when applicable. |
8 |
CRF questions should be as self-explanatory as possible, thereby reducing the need for separate instructions. If required, short instructions may be placed on the CRF page, especially if the Prompt is not specific enough. More detailed instructions may be presented in a CRF completion guideline. All instructions should be concise. Instructions should be standardized as much as possible. |
Putting short instructions and prompts on the CRF increases the probability that they will be read and followed, and can reduce the number of queries and the overall data cleaning costs. Having standard instructions supports all sites using the same conventions for completing the fields. Providing short instructions and prompts on the CRF and moving long instructions to a separate instruction booklet, facing page, or checklist will decrease the number of CRF pages, with the following benefits:
|
9 | Collection of dates should use an unambiguous format, such as DD-MON-YYYY, where each part of the date is a unique format: "DD" is the day as a 2-digit numeric value; "MON" is the month as a 3-character letter abbreviation in English, or similar character abbreviation or representation n the local language; and "YYYY" is the year as a 4-digit numeric value. For EDC, the user may be able to select a date from a calendar, and this would also meet the recommendation for an unambiguous date. If the recommended approach is not adaptable to the local language, a similarly unambiguous format should be used. The method for capturing date values should allow the collection of partial dates, and should use a consistent method or convention for collecting the known date parts to facilitate standard mapping to SDTM. Reference the CDASH Model for standard date variable names. |
Using this data collection format (i.e., DD-MON-YYYY) will provide unambiguous dates. For example, the date "06/08/02" is ambiguous because it can be interpreted as "June 8, 2002" or "August 6, 2002". If subject-completed CRF pages are translated into a local language, the CDASH recommended date format for collection may make translation of the documents easier. Dates are collected in this format, but reformatted and submitted in ISO 8601 format. See the SDTMIG and Section 3.6, Collection, Conversion, and Imputation of Dates, for more information about the ISO 8601 format. |
10 |
To eliminate ambiguity, times should be collected with the use of a 24-hour clock, using the hh:mm:ss format for recording times. Use only as many of the hh:mm:ss elements as are needed for a particular field. Sites should be cautioned not to "zero- fill" time components if these are not known (for example 21:00:00 means "exactly 9 pm", but if the site did not know how many seconds after 9 PM, they should not record the seconds). Subject-completed times may be recorded using a 12-hour clock and an "am" or "pm" designation. The time should then be transformed to a 24-hour clock in the database. |
SDTM-based datasets use ISO 8601 date/time formats. Collecting times using a 24-hour clock eliminates both ambiguity and the need to convert values from 12-hour to 24- hour clock time. |
11 | Manually calculated fields should not typically be recorded within the CRF when the raw data on which the calculation is based are recorded in the CRF. An exception is when a treatment and/or study conduct decision should be made based on those calculations. In such cases it may be useful for the calculated field to be recorded within the CRF. It may also be useful to provide the site a step-by-step worksheet to calculate this data. |
Data items that can be calculated from other data captured within the CRF are more accurately reported if they are calculated programmatically using validated algorithms. The noted exception may be in cases where it is important to show how the investigator determined a protocol-defined endpoint from collected raw data. |
12 | Questions with free-text responses should be limited to cases of specific safety or therapeutic need in reporting or analysis, such as Adverse Events, Concomitant Medications, or Medical History—generally in cases where the data will be subsequently coded. Questions should be specific and clear rather than open- ended. Instead of free-text comment fields, a thorough review of the CRF by the protocol development team should be performed to maximize the use of predefined lists of responses. Refer Section 7.2, CO - Comments domain for additional recommendations. |
The collection and processing of free text requires significant resources for data entry: It requires CDM resources to review the text for safety information and for inconsistencies with other recorded data and is of limited use when analyzing and reporting clinical data. Another risk is that sites may enter data into free-text fields that should be recorded elsewhere. |
13 | Subject-specific data should be collected and recorded by the site and should not be prepopulated in the CRF/eCRF. |
The CRF is a tool to collect subject-level data. However, prepopulation of some identifying (e.g., investigator name, site identification, protocol number) or timing (e.g., Visit Name) information prevents errors and reduces data entry time. Fields on the CRF or in the database that are known to be the same for all subjects may be pre-populated (e.g., measurements for which there is only 1 possible unit, such as Respiratory Rate or Blood Pressure). The units can be displayed on the CRF and populated in the database. |
14 | The anatomical location of a measurement, position of subject, or method of measurement should be collected only if the protocol specifies the allowable options, or if the parameter is relevant to the consistency or meaning of the resulting data. |
When a parameter, such as location, position, or method, is specified in a protocol and is part of the analysis, the CRF may include the common options for these parameters to ensure the site can report what actually happened and protocol deviations can be identified. If the parameter is pre- populated on the CRF and other options are not available, then the site should be directed to not record data that was not collected per protocol specifications. Taking measurements in multiple anatomical locations may affect the value of the measurement and/or the ability to analyze the data in a meaningful way (e.g., when data obtained from different locations may bias or skew the analysis). In this case, collecting the location may be necessary to ensure consistent readings. For example, temperature obtained from the ear, mouth, or skin may yield different results. If there is no such rationale for collecting location, position, method, or any other value, it would be considered unnecessary data. See Section 4.3, Organizational Best Practices to Support Data Collection, Num 1. |
15 | Sites should record verbatim terms for non-solicited adverse events, concomitant medications, or medical history reported terms. Sites should not be asked to select a preferred term from a coding dictionary as a mechanism for recording data. |
When the site records information about spontaneously reported adverse events or medical history, recording responses verbatim ensures that no information is omitted. Sites are not expected to be coding experts and are probably not familiar with the coding dictionaries used in clinical research. Having sites record adverse events from a standardized list is the same as having them code these events. Having multiple sites "coding" data will result in inconsistencies in the coding across sites. See Section 6, Other Recommendations, for more information about collecting data for coding purposes. |
16 | An SDTMIG variable name should only be used as a data collection/operational variable name if the collected value will directly populate the SDTMIG variable with no transformation (other than changing case). Otherwise, create a "collected" version of the variable and write a standard mapping to the SDTMIG variable. |
This practice provides clearer traceability from data collection to submission, and facilitates a more automated process of transforming collected data to the standardized data tabulations for submission. |
4.2 CRF Design Best Practices
The following recommendations are general principles that may be implemented during CRF form design and/or database set-up in different ways, depending on the systems used.
Providing the clinical site with a consistent and clinically logical order of these fields will reduce data entry time and result in more reliable data. The CRF should be quick and easy for site personnel to complete.
Clinical Operations staff should review the CRF for compatibility with common site workflow and site procedures.
| Num | Best Practice Recommendation |
|---|---|
1 | Place fields that routinely appear on multiple forms at the top of the form. For example, if the collection date and time are both asked, they should appear first and second, respectively, on each form where they are used. |
2 | Fields should be placed on the form in the order that they are expected to be collected during the clinical assessment. It is acceptable to include fields from different domains on the same form if consistent with the clinical flow. |
3 | Group related fields for a single clinical encounter together, although multiple time points or visits may appear together on one form. For example, if heart rate and temperature are taken every hour for 4 hours on study day 1, the form can collect the data for hour 1 (e.g., heart rate result and unit, temperature result and unit), followed by the data for hour 2, hour 3, and hour 4. In this scenario, there would be labels indicating each time point within study day 1. |
4 | Group related fields together. Test results and their associated units should always appear next to each other. For example, the results of “TEMP” should be followed by its allowable units of “F” and "C". In some cases, the result might have only 1 applicable unit. For example, the only applicable unit for "PULSE" is "beats/min". The unit should be displayed on the CRF and databased. |
5 |
Data fields that are dependent on other data fields should be placed in the CRF in such a way that this dependence is obvious. For example, if there is a question in a paper CRF where “Other, specify” is an option, the text box used to collect what is being specified should be placed in proximity to the “Other” question to indicate that it is a subpart of the “Other” question. Example: An EDC system that requires a specific response in order to render 1 or more additional, related questions. |
6 | Lists of values that have a logical order should be provided on the CRF in that logical order. For example, the values of “Low”, “Medium”, and “High” are logically placed in this order. Do not list “Medium” first, “Low” second, and “High” third. |
4.3 Organizational Best Practices to Support Data Collection
| Num | Best Practice Recommendation | Rationale |
|---|---|---|
1 | Collect necessary data only. CRFs should focus on collecting only the data that support protocol objectives and endpoints. The protocol should clearly state which data will be collected in the study | Usually, only data that will be used for efficacy analysis and to assess safety of the investigational product should be collected on the CRF, due to the cost and time associated with collecting and fully processing the data. However, some fields on a CRF may be present to support EDC functionality and/or review and cleaning of data through automated edit checks. The protocol (and SAP, when it is prepared in conjunction with the protocol) should be reviewed to ensure that the parameters needed for analysis are collected and can be easily analyzed. The statistician is responsible for confirming that the CRF collects all of the data necessary to support the analysis. |
2 | CRF development should be a controlled, documented process that incorporates (as applicable):
CRF development should be controlled by SOPs covering these topics, as well as site training. | A controlled process for developing CRFs will help ensure that CRFs comply with company standards and processes. |
3 | The CRF design process should include adequate review and approval steps, and each reviewer should be informed on the scope of the review they are expected to provide. The team that designs the data collection instruments for a study should be involved in the development of the protocol and should have appropriate expertise represented on the CRF design team, including the following:
Ideally, the CRF should be developed in conjunction with the protocol (and the SAP if it is available). All research-related data on the CRF should be addressed in the protocol to specify how and when it will be collected. |
Reviewers from different functions increase the probability that the CRF will be easier to complete and support the assessment of safety and efficacy as defined in the protocol and SAP. The CRF design team should ensure that the data can be collected in a manner that is consistent with the implementer’s processes and easy for the site to complete. |
4 | Translations of CRFs into other languages should be done under a controlled process by experts who understand both the study questions and the language and culture for which the CRF is being translated. The translation should be a parallel process following the same set of steps with separate reviews and approvals by the appropriate experts. Translations may require author approval and a separate validation of the translated instrument. CRFs that are translated into other languages should follow the same development process as the original CRF to ensure the integrity of the data collected. Consideration of translation should be part of the CRF development process. Avoid the use of slang or other wording that would complicate or compromise translation into other languages. | Cultural and language issues should be addressed appropriately during the process of translating CRFs to ensure the CRF questions have consistent meaning across languages. |
5 | Data that are collected on CRFs should be databased. For some fields, such as “Were there any Adverse Events", the response—in this case "Yes/No”—may need to be databased, but will not be included in the submission data. Some fields, such as Investigator’s Signature, can be verified by the data entry staff, but an actual signature may not be databased unless there is an e-signature. | If certain data are not required in the CRF, but are needed to aid the investigator or monitor, those data should be recorded on a site worksheet (e.g., entry criteria worksheet, dose titration worksheet). All such site worksheets should be considered source documents or monitoring tools, and should be maintained at the site with the study files. |
6 | Establish and use standardized case report forms. | Using data collection standards across compounds and TAs saves time and money at every step of drug development. Using standards: |
4.4 General Recommendations on Screen Failures
Should the sponsor choose, screen failure data are collected for those who fail screening and who are not subsequently enrolled in the study. Section 10.1 of ICH E3 (Structure and Content of Clinical Study Reports, available at https://www.fda.gov/regulatory-information/) describes the reporting of subject disposition in the clinical study report. This section states that it may be “relevant to provide the number of patients screened for inclusion and a breakdown of the reasons for excluding patients during screening, if this could help clarify the appropriate patient population for eventual drug use.” Although screen failure data may not be relevant for all studies, it is recommended that screen failure data be collected based on the needs of the protocol and drug development programs. Timely collection of screen failure data may also be used to identify eligibility criteria that contribute to enrollment challenges.
Using CDASH, the minimum data to be collected should include a subject identifier and reason(s) for screen failure. Typically, there is a reason on the End of Study form indicating “Screen Failure”. This information allows overall summarization of all subjects screened/enrolled and, when captured, provides easy subject accountability for the clinical study report. Other data may be considered for collection, such as date of informed consent, sex, race, date of birth or age, or other data to further describe the reason for ineligibility (e.g., the lab value that was out of range).
The SDTMIG does not provide a separate domain specifically for screen failure data and does not require that the screen failure data be included in the SDTM. Data for screen failure subjects, if submitted, should be included in the appropriate SDTMIG domains. Refer to the SDTMIG for further guidance on submitting screen failure data.
5 Conformance to the CDASH Standard
5.1 Conformance Rules
Conformance means that:
1. Core designations must be followed. All Highly Recommended and applicable Recommended/Conditional Fields must be present in the case report form (CRF) or available from the operational database.
2. CDISC Controlled Terminology must be used. The CDISC Controlled Terminology that is included in the CDASHIG Metadata Table must be used to collect the data in the CRF. All codelists displayed in the CRF must use or directly map to the current published CDISC Controlled Terminology submission values, when it is available. Subsets of published Controlled Terminology, such as those provided in CDASH terminology, can be used.
a. In Findings domains, values from the relevant CDISC Controlled Terminology lists must also be used to create appropriate Question Text, Prompts, and/or variable names. For example, if the question is about the subject's height, incorporate the value of "Height" from the VSTEST codelist as the Prompt on the CRF, and incorporate "HEIGHT" from VSTESTCD in the variable name.
3. Best practices must be followed. The design of the CRF must follow guidance in Section 4.1, Best Practices for Creating Data Collection Instruments and Section 4.2, CRF Design Best Practices.
4. The wording of CRF questions should be standardized; CDASH Question Text or Prompt must be used to ask the question.
a. In cases where the data collection is done in a denormalized presentation on the CRF, the relevant CDISC Controlled Terminology should be used in the Question Text or Prompt as much as possible. It is acceptable to use synonym text that will directly map to a CDISC Submission Value (including an NCI Preferred Term), if the CDISC Submission Value is not appropriate for data collection. For example, "ALT" may be better than "Alanine Aminotransferase" as the prompt for this lab test. If there is no CDISC Controlled Terminology available, the Question Text or Prompt must be standardized by the implementing organization and used consistently. One of the basic purposes of CDASH is to reduce unnecessary variability between CRFs and to encourage the consistent use of variables to support semantic interoperability; therefore, Question Text and Prompt must be used verbatim.
b. Similarly, where Study Data Tabulation Model Implementation Guide (SDTMIG) variables may exist in the operational database and the value conforms to controlled terminology, it is permissible to use a familiar synonym on the CRF without affecting conformance. For example, on the Demographics page, SEX may be displayed as "Male" or "Female", whereas in the operational database the controlled terminology values of "M" and "F" would be used.
c. In some cases, CDASH Questions Text and Prompt allow for flexibility while still being considered conformant. See Section 2.3, CRF Development Overview, for further details on the usage of Question Text and Prompt.
d. CDASH Model Question Text may contain options for the tense, but if the option is not provided, the tense of the Question Text may be modified to reflect the needs of the study.
e. For cases where the Question Text or Prompt cannot be used due to culture or language, or a CRF must be translated for language or cultural reasons, the implementer must ensure the translation is semantically consistent with the CDASH Question Text and Prompt in the CDASHIG Metadata Table.
f. In cases where a more specific question needs to be asked than what is provided by Question Text or Prompt, CDASH recommends the use of a brief CRF Completion Instruction, as long as the instruction clarifies the data required by the study without altering the meaning of variable as defined by the standard. For example, "Sex at birth" is not the same question as "Sex" (which is loosely defined as "reported sex").
5. CDASHIG variable naming conventions should be used in the operational database. Use a consistent syntax that includes the root variable name and/or controlled terminology, and any other standardized concepts that are needed to support efficient mapping of the collected value to SDTM datasets. The goals are to have beginning-to-end traceability of the variable name from the data capture system to the SDTM datasets, and to support automating electronic data capture (EDC) set-up and downstream processes.
a. It is recognized that (particularly in an EDC system) the variable name of a data collection field, as well as the name in the underlying database, may have various “system” components that become part of the item’s identifier. EDC systems, prior to exporting data in a defined format, may require the variable name to include such database “references” as the EDC page name, the item “group” name, or perhaps a combination.
b. In cases where the data collection is done in a denormalized way, appropriate CDISC Controlled Terminology must be used when it is available. For example, when collecting Vital Signs results in a denormalized eCRF, the variable names can be created by using terms from the Vital Signs Test Code codelist. For example, Temperature result can be collected in a variable called TEMP or TEMP_VSORRES; Systolic Blood Pressure result can be collected in a variable called SYSBP or SYSBP_VSORRES. When a particular system’s constraints limit the variable name to 8 characters, a similar, consistent implementation that preserves either the normalized root variable (e.g., ORRES) or the controlled terminology (e.g., --TESTCD value) should be implemented.
c. Whereas all CDASHIG defined variable names are 8 characters or fewer to accommodate SDTM limits on variable names, QNAMs, and --TESTCDs, the maximum length of a variable name that may be implemented is determined by the data management system used, not by CDASH.
d. When collecting data in a horizontal manner, to facilitate transformation to SDTM datasets, when possible it is recommended to create denormalized CDASH variables in the data collection system by incorporating the SDTMIG variable name target and/or the controlled terminology (e.g., --TESTCD) as part of the CDASH variable names. The domain level metadata labeled as "Horizontal-Generic" in the Implementation Options column of the CDASHIG metadata tables are examples of how to implement this. There is no conformance requirement implied by these examples.
6. Data output by the operational database into an SDTMIG variable ideally should require no additional processing if the CDASHIG and SDTMIG variable names are the same. An SDTM data programmer should be able to assume that data in an SDTMIG variable is SDTMIG-compliant. Minimal processing (e.g., changing case) does not effect conformance. This helps to ensure a quality deliverable, even if the programmer is unfamiliar with data capture practices.
7. Validated questionnaires, ratings, or scales must present the questions and reply choices in the manner in which these were validated. This must be followed to maintain the validity of a validated instrument. (See Section 8.3.12, QRS - Questionnaires, Ratings, and Scales).
a. In some cases, this may result in CRFs that do not conform to CDASH best practices; however, restructuring these questionnaires should not be done because it could invalidate them.
b. The use of such questionnaires in their native format should not be considered to affect conformance to CDASH.
Implementers must determine what additional data fields to add to address study-specific and therapeutic area requirements, and applicable regulatory and business practices. See Section 3.4, How to Create New Data Collection Fields When No CDASHIG Field Has Been Defined, for more information on how to create data collection fields that have not already been described in this implementation guide.
6 Other Recommendations
6.1 Standardized Coding of Collected Data
6.1.1 Data Collection to Facilitate Coding
Adverse Events, Medical History, and Prior and Concomitant Medications are often coded to standard dictionaries (thesauri). There are many coding dictionaries; this section will focus on the Medical Dictionary for Regulatory Activities (MedDRA) and the World Health Organization Drug Dictionary (WHO-DD) as examples, because these are common coding dictionaries.
The Study Data Tabulation Model Implementation Guide (SDTMIG) variable AEDECOD is the dictionary-derived text description of AETERM (the reported term for the adverse event) or AEMODIFY (the modified reported term). When coding with MedDRA, the AEDECOD is the Preferred Term and is a required variable. Corresponding SDTMIG variables CMDECOD (for medications) and MHDECOD (for medical history items) are permissible SDTMIG variables. These are the equivalent of the preferred term in the dictionary used for coding, and when data are coded these SDTMIG variables should be provided.
These --DECOD variables are not necessarily collected on case report forms (CRFs). They are often determined from other collected variables (e.g., AETERM, CMTRT, and MHTERM). Conventions adopted in the collection of these reported terms can have an effect on the resulting --DECOD variables. If collected on a CRF, --DECOD values would be selected from sponsor-defined or CDISC Controlled Terminology.
CRF designers should consult with medical coders, review relevant documentation, and ensure that all elements needed to facilitate the coding process are collected.
6.1.2 Coding Adverse Events and Medical History Items
Data managers are encouraged to collaborate with coding specialists and medical staff to develop guidance for sites in accordance with applicable coding conventions and other company/project agreements and requirements.
Reported terms are often coded without other information for the subject. Therefore, sites should be advised that nothing can be assumed, and that the reported term should include all information relevant to the event being reported. For example, if “Congestion” is reported as an adverse event for a particular subject, together with several other pulmonary events, the coder cannot assume that the congestion is “Lung congestion”, rather than congestion of some other organ (e.g., nose, ear). The reported term “Congestion” will need to be queried before it can be coded.
6.1.3 Medications
With regard to medications, the CDASH standard offers some guidance on the recording of medication names and on the use of additional Recommended/Conditional data collection fields (e.g., CMROUTE, CMINDC) to facilitate coding. See Section 8.1.1, General CDASH Assumptions for Interventions Domains.
The purpose of coding medications is usually to provide a Standardized Medication Name (CMDECOD) and a Medication Class (CMCLAS). Most dictionaries facilitate the derivation of the Standardized Medication Name on identification of the medication that was taken and the reason taken.
Although it would be preferable to collect all active ingredients of a particular medication, in a clinical trial this is impractical. There are numerous CRF design possibilities. When designing a collection tool, it is critical to ensure that details appropriate to the trial and the sponsor’s coding requirements are included. For example, betamethasone dipropionate is used topically; however, if the site records only betamethasone (which can be administered orally, as drops, or inhaled), the topical route of the drug will be lost. In this case, collecting route of administration (CMROUTE) or the indication (CMINDC) would provide the additional information needed to code this medication.
In summary, when medications are to be coded, the indication (CMINDC) and route (CMROUTE) or anatomical location (CMLOC) should be collected along with the medication name.
7 Special-purpose Domains
The Study Data Tabulation Model Implementation Guide (SDTMIG) includes 3 types of special-purpose datasets:
- Domain datasets—Demographics (DM), Comments (CO), Subject Elements (SE), and Subject Visits (SV)—which contain subject-level data
- Trial Design Model (TDM) datasets, which contain trial-level data
- Relationship datasets
These datasets are described in SDTMIG Section 2.3. CDASH does not currently contain information on Trial Design Model or Relationship datasets. Because CDASH standards are for collection of subject-level data, the collection of Trial Design domains is out of the scope for CDASH. CDASHIG contains information on these Special-Purpose Domains: Comments (CO) and Demographics (DM).
7.1 General CDASH Assumptions for Special-purpose Domains
1. Each study must include the DM domain.
2. CDASH does not currently contain metadata information on SDTM Special-Purpose Domains Subject Elements (SE) and Subjects Visits (SV). The SDTM SE and SV domains are commonly derived/created during the SDTM dataset creation process. Each implementer has to determine the best practice within their organization for creating visits and collecting the information on any unplanned visits. See SDTMIG v3.2 Section 5, Subject Elements and Subject Visits, for more information.
7.2 CO - Comments
Description/Overview for the CDASHIG CO - Comments Domain
The CDASHIG Comments (CO) domain describes free text collected alongside other data on typical case report form (CRF) pages (e.g., Adverse Events) when there is not a specified variable for the free text. The CDASHIG CO domain has no mandatory data elements for inclusion in a separate Comments CRF, and the recommendation is to avoid creating a General Comments CRF.
Specification for the CDASHIG CO - Comments Domain
Comments Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Special- Purpose |
CO |
N/A |
N/A |
1 |
STUDYID |
Study Identifier |
A unique identifier for a study. |
What is the study identifier? |
[Protocol/Study] |
Char |
HR |
N/A |
STUDYID |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Special- Purpose |
CO |
N/A |
N/A |
2 |
SITEID |
Study Site Identifier |
A unique identifier for a site within a study. |
What is the site identifier? |
Site (Identifier) |
Char |
HR |
N/A |
DM.SITEID |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted for the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Special- Purpose |
CO |
N/A |
N/A |
3 |
SUBJID |
Subject Identifier for the Study |
A unique subject identifier within a site and a study. |
What [is/was] the (study) [subject/participant] identifier? |
[Subject/Participant] (Identifier) |
Char |
HR |
Record the identifier for the subject. |
DM.SUBJID |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. | Special- Purpose |
CO |
N/A |
N/A |
4 |
COVAL |
Comment |
A free text comment. |
[protocol-specified targeted question] |
[abbreviated version of the protocol- specified targeted question] |
Char |
O |
[protocol specific] |
CO.COVAL |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. RDOMAIN contains the domain to which is related to the comment. |
N/A |
N/A |
If an additional free-text field is needed to provide more information about a particular record, use the COVAL variable to collect the free text, and associate the free text (comment) with the original record using RDOMAIN, IDVAR, IDVARVAL. |
Assumptions for the CDASHIG CO - Comments Domain
Solicited Comments vs. Unsolicited Comments
Solicited comments are defined as those comments entered in free-text data collection fields (e.g., “Please comment”) that are intentionally included on the CRF. These data collection fields provide the site with a predefined space to further explain or clarify an associated record within the CRF. For example, the Vital Signs CRF may include a solicited comment data collection field that enables recording of free text, such as “Reason for performing assessment out of window”.
Unsolicited comments are those comments entered outside of predefined data collection fields (also referred to as marginal comments, as they are sometimes written in the margins of paper CRFs). These may include marginal CRF comments written by site staff, notes written by the subject on paper diaries, or additional information collected through an electronic data capture (EDC) system function which collects comments that are not included as data collection fields on the eCRF. Although such comments may be intended to reduce queries, in practice they often lead to clinical data not being entered into the correct data collection field and may cause additional work in the data management process. The collection of unsolicited comments should be discouraged. If allowed, unsolicited comments should be reviewed and resolved during the conduct of the study.
Some unsolicited comments may be intended to avoid queries, for example “Subject visit was delayed due to his holidays”, and may not be regarded as clinical data. When these comments are permitted during data collection, the sponsor should have a process by which the comments are reviewed and processed. This should include a method to query and move relevant data to the appropriate form.
The Investigative site should be trained to enter clinical data in the appropriate data collection fields rather than making marginal notes on the CRF, and to use appropriate methods and tools to communicate to the monitor information that should not be included in the clinical data.
Free Text vs. Value List Fields
Clinical data must be entered in appropriate data collection fields; otherwise, there is a potential for data that should be entered on other CRFs to be hidden within the comment. For example, if a general comment of “Subject visit was delayed as he had the flu” was captured, this would necessitate that someone review the data and query the site to enter “flu” in an Adverse Event CRF and not leave it as a comment. An additional concern with free-text comments is the potential for inappropriate or sensitive information to be included within general comments data collection fields. For example, a comment could contain a subject’s name or may have unblinding information. Free-text comments are also inefficient for processing due to their variable and unstructured nature; they offer limited or no value for statistical analysis, as they cannot be tabulated. CRF development teams are encouraged to strive for data collection methods that maximize the use of predefined lists of responses rather than relying solely on free-text comment fields. The recommendation is that CRF development teams consider what additional questions may be needed within a specific CRF, and what the typical responses would be. They can then create a standardized list of responses for those questions, and make the data collected more useful for analysis.
Considerations Regarding General Comments CRFs
Solicited comments often used to be collected on a General Comments CRF. In recent years, though, most organizations have discontinued this practice.
The CDASHIG CO domain has no mandatory data elements and is not intended to encourage the creation of a General Comments CRF. CDASHIG recommends against the use of a General Comments CRF. This recommendation is not meant to discourage investigators from providing unsolicited comments where they are appropriate, nor to discourage solicited free-text comment data collection fields that may appear within any CRF. Free-text comment fields should be used to solicit comments where they are needed. When comments are collected, they should use a variable naming convention that conforms to CDASH (e.g., COVAL may be used in any CRF because it is part of the SDTMIG specification).
Example CRF for the CDASHIG CO - Comments Domain
Example 1
This CRF shows the use of a targeted comment to collect the reason a pharmacokinetics (PK) sample was drawn more than 5 minutes late.
Title: Pharmacokinetic Sample Collection with Comments
|
Example CRF Completion Instructions
|
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
PCPERF |
1 |
Were blood samples drawn? |
Collected |
Indicate that all of the blood samples in this group were collected. |
Text |
PCSTAT |
If PCPERF = N, then set PCSTAT = "NOT DONE" and PCTESTCD = "QSALL". If PCPERF = "Y", then NOT SUBMITTED. |
(NY) |
No; Yes |
Qtext |
|||
PCDAT |
2 |
What was the date of the blood sample collection? |
Collection Date |
Record the date when the blood sample collection occurred using this format (DD-MON- YYYY). |
Date |
PCDTC |
|||||||
PCTIM |
3 |
What was the (start) time of the Pre-Dose blood sample collection? |
Collection Time |
Record the [start] time of the Pre-Dose blood sample collection (as complete as possible). |
Time |
PCDTC |
PCDTC where PCTPT = "Pre-Dose" |
Qtext |
|||||
COVAL |
4 |
If late, comment |
Pre-Dose Collection Comment |
If the collection did not occur at the planned time point, please comment. |
Text |
CO.COVAL |
Qtext |
||||||
PCTIM |
5 |
What was the (start) time of the 30 Minutes Post Dose blood sample collection? |
Collection Time |
Record the [start] time of the 30 Minutes Post Dose blood sample collection (as complete as possible). |
Time |
PCDTC |
PCDTC where PCTPT = "30 Minutes Post Dose" |
Qtext |
|||||
COVAL |
6 |
If late, comment |
30 Minute Post Dose Collection Comment |
If the collection did not occur at the planned time point, please comment. |
Text |
CO.COVAL |
Qtext |
||||||
PCTIM |
7 |
What was the (start) time of the 90 Minutes Post Dose blood sample collection? |
Collection Time |
Record the [start] time of the 90 Minutes Post Dose blood sample collection (as complete as possible). |
Time |
PCDTC |
PCDTC where PCTPT = "90 Minutes Post Dose" |
Qtext |
|||||
COVAL |
8 |
If late, comment |
90 Minute Post Dose Collection Comment |
If the collection did not occur at the planned time point, please comment. |
Text |
CO.COVAL |
7.3 DM - Demographics
Description/Overview for the CDASHIG DM - Demographics Domain
The Demographics (DM) CDASHIG domain includes essential data collection fields that describe each subject in a clinical study. The collection of some demographics data is useful to perform simple analyses based upon population stratification.
Privacy concerns surrounding the DM and Subject Characteristics (SC) data were taken into account when these domains were created. For example, there are optional CDASHIG variables to collect the components of birthdate (e.g., BRTHDD, BRTHMO, BRTHYY); therefore, limited elements of birthday may be collected and later mapped to the SDTMIG variable BRTHDTC. This approach provides flexibility in categorizing some variables to facilitate compliance with local privacy issues.
Collection of Age vs. Date of Birth
It is recognized that sponsors may collect the Age or Date of Birth of the subject. In multiregional studies, sponsors may need to enable the collection of either in order to comply with local regulations. But only one or the other should be collected for any given subject. When only AGE is collected, the sponsor is left with a window of uncertainty of, at most, 365 days. Although knowing the precise date of birth provides the ability to calculate accurately an age for any date, a precise (and complete) date of birth may be considered “personally identifying information” for some privacy oversight boards or government regulators.
Collect the date of birth to the extent that the local regulatory authorities will allow.
- The best method is to collect a complete date of birth, and derive age.
- When there are privacy concerns with collecting the complete date of birth, the recommendation is to collect year of birth at a minimum.
- In cases when neither of the above can be implemented (e.g., cultural or regional considerations) then Age and Age Unit should be collected, and Date of Collection should be collected or derived from the Visit Date.
- Use the AGETXT variable only in very rare cases when only an age range or age description can be determined.
Date of Birth should be implemented such that incomplete dates may be entered, as allowed by the data capture system.
See Section 3.6, Collection, Conversion, and Imputation of Dates, for more information.
Collection of Sex
The collection of some demographics data is useful to perform simple analyses based upon population stratification.
Collection of Ethnicity and Race
The US Food and Drug Administration (FDA) requirement is to collect Ethnicity before Race. A secondary analysis is often made using the phenotypic race of the subject. Collect Race if required for the protocol and not prohibited by local laws and regulations.
In 2016, the US Office of Management and Budget (OMB) revised its recommendations for the collection and use of race and ethnicity data by Federal agencies. The FDA follows this directive and recommends “the use of the standardized OMB race and ethnicity categories for data collection in clinical trials for two reasons. The use of the recommended OMB categories will help ensure consistency in demographic subset analyses across studies used to support certain marketing applications to FDA and across data collected by other government agencies”.
The Race values listed in the FDA Guidance (available at https://www.fda.gov/) are:
- American Indian or Alaska Native
- Asian
- Black or African American
- Native Hawaiian or Other Pacific Islander
- White
Note: For studies where data are collected outside the US, the recommended categories are the same, except for “Black” instead of “Black or African American”.
CDASH provides only 1 variable for Race. Sponsors wishing to capture more than 1 race will need to create non- standard variables to store the collection of the multiple races and map appropriately to the SDTMIG DM domain.
Race Other has been included as a free-text field to capture responses. The use of this variable is optional and should be used with caution. When submitting data to the FDA, all races must be mapped to the 5 values recognized by the FDA; providing an Other, Specify field might lead to mapping errors or difficulties. RACE is Recommended/Conditional (R/C) because some sponsors prefer to derive values that are compliant with the codelist RACE (e.g., as derived from values collected in CRACE).
The category of ethnicity is similar to race but, as defined by the US Centers for Disease Control and Prevention (CDC), is an arbitrary classification based on cultural, religious, or linguistic traditions; ethnic traits; background; or allegiance or association. In a fairly heterogeneous country such as the United States, ethnicity data (e.g., "Hispanic or Latino" and "Not Hispanic or Latino") might be useful to confirm that ethnic groups are not being discriminated against by being unfairly excluded from clinical research. In other countries, regulatory bodies (e.g., Japanese Ministry of Health, Labour and Welfare) may expect the reporting of ethnicity values which appropriately reflect the population of their areas. This information may be collected using the CETHNIC variable with its corresponding codelist, ETHNICC.
If more detailed information on race or ethnicity is required to further characterize study subjects, it is recommended that the presented choices be “collapsible” up to one of the 5 FDA designations for race, as well as the 2 categories for representing ethnicity, as needed for reporting to FDA. If more detailed categorizations are desired, the recommendation is to use the HL7 Reference Information Model's race and ethnicity vocabulary tables (available at https://www.hl7.org/), which are designed to collapse up in this manner. For the collection of such added detail or granularity, as the sponsor may require, CDASH provides the variables CRACE and CETHNIC, respectively.
Collection of Special Optional Fields in Demographics
CDASHIG allows for collection of the Date of Informed Consent using the variable RFICDAT. (If a sponsor chooses to collect Informed Consent using this variable, the data should not also be collected using DSSTDAT from the Disposition (DS) Domain.) The data from RFICDAT would then be mapped to the SDTMIG variable DSSTDTC and the companion variables (e.g., DSTERM, DSDECOD) must be populated accordingly.
The CDASH Model also defines a field for Death Date (DTHDAT) as a timing variable. It may be collected on any CRF deemed appropriate by the sponsor. The SDTMIG variables DTHDTC and DTHFL are mapped to the DM domain during the SDTM submission dataset creation process. The CDASH field Death Date may be mapped to other SDTMIG domains (e.g., DS), as deemed appropriate by the sponsor.
See Section 4.1, Best Practices for Creating Data Collection Instruments, Num 4 for additional guidance recommending that the same data not be collected more than 1 time per subject.
Specification for the CDASHIG DM - Demographics Domain
Demographics Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Special- Purpose |
DM |
N/A |
N/A |
1 |
STUDYID |
Study Identifier |
A unique identifier for a study. |
What is the study identifier? |
[Protocol/Study] |
Char |
HR |
N/A |
STUDYID |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Special- Purpose |
DM |
N/A |
N/A |
2 |
SITEID |
Study Site Identifier |
A unique identifier for a site within a study. |
What is the site identifier? |
Site (Identifier) |
Char |
HR |
N/A |
DM.SITEID |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted for the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Special- Purpose |
DM |
N/A |
N/A |
3 |
SUBJID |
Subject Identifier for the Study |
A unique subject identifier within a site and a study. |
What [is/was] the (study) [subject/participant] identifier? |
[Subject/Participant] (Identifier) |
Char |
HR |
Record the identifier for the subject. |
DM.SUBJID |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Special- Purpose |
DM |
N/A |
N/A |
4 |
BRTHDAT |
Birth Date |
A subject's date of birth (with or without the time of birth). The complete Date of Birth is made from the temporal components of Birth Year, Birth Month, Birth Day and Birth Time. | What is the subject's date of birth? |
Birth Date |
Char |
R/C |
Record the date of birth to the level of precision known (e.g., day/month/year, year, month/year, etc.) in this format (DD-MON- YYYY). |
BRTHDTC |
This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable BRTHDTC in ISO 8601 format. |
N/A |
N/A |
BRTHDAT is the collected field used for recording the full birth date. The sponsor may choose to database the date of birth as a single variable (BRTHDAT), or as separate variables for each component of the date/time (BRTHYY, BRTHMO, BRTHDD, BRTHTIM). The sponsor may choose a method based on database considerations, or for regulatory reasons. It is expected that what is collected for BRTHDAT (complete date or whichever components are collected) is reported in the SDTMIG variable BRTHDTC in the ISO 8601 format. If data are collected in a manner resulting in a reduced precision level, then the AGE (SDTM expected variable), if not collected on the CRF, should be derived using a documented algorithm that describes how the age was determined and/or imputed for those birth dates collected with reduced precision. |
Special- Purpose |
DM |
N/A |
N/A |
5 |
BRTHDD |
Birth Day |
Day of birth of the subject in an unambiguous date format (e.g., DD). |
What is the subject's day of birth? |
Birth Day |
Char |
R/C |
Record the subject's day of birth (e.g., 01 or 31). |
BRTHDTC |
This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable BRTHDTC in ISO 8601 format. | N/A |
N/A |
Day of Birth is the collected variable used for recording the day component of the Date of Birth. The sponsor may choose to database the date of birth as a single variable (BRTHDAT), or as separate variables for each component of the date/time (BRTHYY, BRTHMO, BRTHDD, BRTHTIM). The sponsor may choose a method based on database considerations, or for regulatory reasons. It is expected that what is collected for BRTHDAT (complete date or whichever components are collected) is reported in the SDTMIG variable BRTHDTC in the ISO 8601 format. If data are collected in a manner resulting in a reduced precision level, then the AGE (SDTM expected variable), if not collected on the CRF, should be derived using a documented algorithm that describes how the age was derived and/or imputed for those birth dates collected with reduced precision. |
Special- Purpose |
DM |
N/A |
N/A |
6 |
BRTHMO |
Birth Month |
Month of birth of the subject in an unambiguous date format (e.g., MMM). |
What is the subject's month of birth? |
Birth Month |
Char |
R/C |
Record the subject's month of birth [e.g., (in local language short month format) (JAN- DEC) or (ENE- DIE) or (JAN- DEZ), etc.]. |
BRTHDTC |
This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable BRTHDTC in ISO 8601 format. | N/A |
N/A |
Month of Birth is the collected variable used for recording the month component of the Date of Birth. The sponsor may choose to database the date of birth as a single variable (BRTHDAT), or as separate variables for each component of the date/time (BRTHYY, BRTHMO, BRTHDD, BRTHTIM). The sponsor may choose a method based on database considerations, or for regulatory reasons. It is expected that what is collected for BRTHDAT (complete date or whichever components are collected) is reported in the SDTMIG variable BRTHDTC in the ISO 8601 format. If data are collected in a manner resulting in a reduced precision level, then the AGE (SDTM expected variable), if not collected on the CRF, should be derived using a documented algorithm that describes how the age was derived and/or imputed for those birth dates collected with reduced precision. |
Special- Purpose |
DM |
N/A |
N/A |
7 |
BRTHYY |
Birth Year |
The year of birth of the subject in an unambiguous date format (e.g., YYYY). |
What is the subject's year of birth? |
Birth Year |
Char |
R/C |
Record the subject's year of birth (e.g., YYYY, a four-digit year). |
BRTHDTC |
This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable BRTHDTC in ISO 8601 format. | N/A |
N/A |
Year of Birth is the collected variable used for recording the year component of the Date of Birth. The sponsor may choose to database the date of birth as a single variable (BRTHDAT), or as separate variables for each component of the date/time (BRTHYY, BRTHMO, BRTHDD, BRTHTIM). The sponsor may choose a method based on database considerations, or for regulatory reasons. It is expected that what is collected for BRTHDAT (complete date or whichever components are collected) is reported in the SDTMIG variable BRTHDTC in the ISO 8601 format. If data are collected in a manner resulting in a reduced precision level, then the AGE (SDTM expected variable), if not collected on the CRF, should be derived using a documented algorithm that describes how the age was derived and/or imputed for those birth dates collected with reduced precision. |
Special- Purpose |
DM |
N/A |
N/A |
8 |
BRTHTIM |
Birth Time |
The time of birth of the subject in an unambiguous time format (e.g., hh:mm) |
What is the subject's time of birth? |
Birth Time |
Char |
O |
Record the time of birth (as completely as possible). |
BRTHDTC |
This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable BRTHDTC in ISO 8601 format. | N/A |
N/A |
The level of detail collected by Time of Birth may be necessary for analysis for some pediatric, natal or neonatal studies. The sponsor may choose to database the date of birth as a single variable (BRTHDAT), or as separate variables for each component of the date/time (BRTHYY, BRTHMO, BRTHDD, BRTHTIM). The sponsor may choose a method based on database considerations, or for regulatory reasons. It is expected that what is collected for BRTHDAT (complete date or whichever components are collected) is reported in the SDTMIG variable BRTHDTC in the ISO 8601 format. If data are collected in a manner resulting in a reduced precision level, then the AGE (SDTMIG expected variable), if not collected on the CRF, should be derived using a documented algorithm that describes how the age was derived and/or imputed for those birth dates collected with reduced precision. |
Special- Purpose |
DM |
N/A |
N/A |
9 |
AGE |
Age |
The age of the subject expressed in AGEU. |
What is the subject's age? |
Age |
Num |
O |
Record age of the subject. |
AGE |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
If Age is collected, it should be collected as a number and, to be correctly interpreted, the age value should be associated to a variable for the Age Unit. It may be necessary to know when the age was collected as an age may need to be recalculated for analysis, such as deriving age at a reference start time (RFSTDTC for SDTM). BRTHDTC may not be available in all cases (due to subject privacy concerns).If AGE is collected, then it is recommended that the date of collection also be recorded, either separately or by association to the date of the visit. |
Special- Purpose |
DM |
N/A |
N/A |
10 |
AGEU |
Age Units |
Those units of time that are routinely used to express the age of a person. |
What is the age unit used? |
Age Unit |
Char |
O |
Record the appropriate age unit (e.g., YEARS, MONTHS, WEEKS, etc.). |
AGEU |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
(AGEU) |
N/A |
If Age is captured on the CRF, the age unit must be known to make the age value meaningful. The age unit might be collected on the CRF, in those cases where the protocol allows for any age group, or it may be preprinted on the CRF (typically with the unit of "years"). |
Special- Purpose |
DM |
N/A |
N/A |
11 |
DMDAT |
Demographics Collection Date |
The date of collection represented in an unambiguous date format (e.g., DD-MON-YYYY) |
What is the date of collection? |
Collection Date |
Char |
R/C |
Record the date of collection using this format (DD- MON-YYYY). |
DMDTC |
This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable DMDTC in ISO 8601 format. | N/A |
N/A |
The date of collection may be determined from the date of visit; if so, a separate date field is not needed. |
Special- Purpose |
DM |
N/A |
N/A |
12 |
SEX |
Sex |
Sex of the subject as determined by the investigator. |
What is the sex of the subject? |
Sex |
Char |
R/C |
Record the appropriate sex (e.g., F (female), M (male). |
SEX |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
(SEX) |
N/A |
Collect the subject's sex or gender, as reported by the investigator. This is a phenotypic assessment and not a genotypic assessment. |
Special- Purpose |
DM |
N/A |
N/A |
13 |
ETHNIC |
Ethnicity |
A social group characterized by a distinctive social and cultural tradition maintained from generation to generation, a common history and origin and a sense of identification with the group; members of the group have distinctive features in their way of life, shared experiences and often a common genetic heritage; these features may be reflected in their experience of health and disease. | Do you consider yourself Hispanic/Latino or not Hispanic/Latino? |
Ethnicity |
Char |
O |
Study participants should self-report ethnicity, with ethnicity being asked about before race. |
ETHNIC |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
(ETHNIC) |
N/A |
For use when values are being collected using the exact non-extensible ETHNIC codelist (C66790) values. Sponsors should refer to the FDA's Collection of Race and Ethnicity Data in Clinical Trials guidance regarding the collection of ethnicity http://www.fda.gov/). |
Special- Purpose |
DM |
N/A |
N/A |
14 |
CETHNIC |
Collected Ethnicity |
A social group characterized by a distinctive social and cultural tradition maintained from generation to generation, a common history and origin and a sense of identification with the group; members of the group have distinctive features in their way of life, shared experiences and often a common genetic heritage; these features may be reflected in their experience of health and disease. | What is the ethnicity of the subject? |
Ethnicity |
Char |
O |
Study participants should self-report ethnicity, with ethnicity being asked about before race. |
SUPPDM.QVAL |
This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPDM dataset as the value of SUPPDM.QVAL where SUPPDM.QNAM = "CETHNIC" and SUPPDM.QLABEL= "Collected Ethnicity". See SDTMIG v3.2 Section 5, DM Domain. Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (ETHNICC) |
N/A |
Use when values are collected using the NCI Thesaurus codelist for Ethnicity As Collected (C128690), the extended HL7 hierarchy of codelist values, or other regulatory agency- specific controlled terminology for Ethnic Group. Sponsors may append a suffix to denote multiple collected ethnicities (e.g. CETHNIC1, CETHNIC2). |
Special- Purpose |
DM |
N/A |
N/A |
15 |
RACE |
Race |
An arbitrary classification based on physical characteristics; a group of persons related by common descent or heredity (U.S. Center for Disease Control). | Which of the following five racial designations best describes you? (More than one choice is acceptable.) | Race |
Char |
R/C |
Study participants should self-report race, with race being asked about after ethnicity. |
RACE |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
(RACE) |
N/A |
Use RACE when the 5 designations for race used by the FDA are collected (American Indian or Alaska Native; Asian; Black or African American; Native Hawaiian or Other Pacific Islander ; White).* Sponsors should refer to the FDA Guidance. See SDTMIG v3.2 Section 5, DM Domain. *For studies where data are collected outside the US, the recommended categories are the same except for Black instead of Black or African American. If multiple races are collected, an alternate sponsor-defined variable structure would be required. Sponsors may record multiple self-reported races for a subject by appending a suffix to denote multiple collected races (e.g. RACE1, RACE2) and populate RACE with the value MULTIPLE. |
Special- Purpose |
DM |
N/A |
N/A |
16 |
CRACE |
Collected Race |
An arbitrary classification based on physical characteristics; a group of persons related by common descent or heredity (US Centers for Disease Control). | Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Char |
R/C |
Study participants should self-report race, with race being asked about after ethnicity. (The FDA guidance suggests that individuals be permitted to designate a multiracial identity. Check all that apply at the time of collection). | SUPPDM.QVAL |
This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPDM dataset as the value of SUPPDM.QVAL when SUPPDM.QNAM = "CRACE" and SUPPDM.QLABEL="Collected Race". See SDTMIG v3.2 Section 5 for the DM Domain. | (RACEC) |
N/A |
Use CRACE when more detailed race categorizations are desired (e.g., more than the 5 minimum designations for race used by the FDA). The use of race and vocabulary tables located within the HL7 Reference Information Model Structural Vocabulary Tables is recommended, as they are designed to collapse up to the SDTM variable RACE with CT (e.g., American Indian or Alaska Native Asian; Black or African American; Native Hawaiian or Other Pacific Islander; White ).* If sponsors choose to map the extended Race codelist values to RACE CT (e.g., Japanese to Asian), then this mapped variable would be reported using the SDTMIG variable RACE. Sponsors should refer to the FDA Guidance. *For studies where data are collected outside the US, the recommended categories are the same except for Black instead of Black or African American. If multiple races are collected, an alternate sponsor-defined variable structure would be required. Sponsors may record multiple self- reported races for a subject by appending a suffix to denote multiple collected races (e.g. CRACE1, CRACE2) and populate CRACE with the value MULTIPLE. |
Special- Purpose |
DM |
N/A |
N/A |
17 |
RACEOTH |
Race Other |
A free-text field to be used when none of the preprinted values for RACE are applicable or if another, unprinted selection should be added to those preprinted values. | What was the other race? |
Specify Other Race |
Char |
O |
If none of the pre- printed values for RACE are applicable or if another, unprinted selection should be added to those pre-printed values, record the value in this free text field. | SUPPDM.QVAL |
This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPDM dataset as the value of SUPPDM.QVAL where SUPPDM.QNAM = "RACEOTH" and SUPP.QLABEL="RACE OTHER". See SDTMIG v3.2 Section 5, DM Domain. Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | N/A |
N/A |
When creating the DM form, it is suggested that the 5 standard race categories be included. Sponsors may choose to include another value of Specify, Other with a free- text field for extending the list of values. The RACEOTH variable contains the free text added by the site. The value(s) added in the optional variable might or might not "collapse up" into one of the 5 categories specified by the FDA Guidance. See SDTMIG v3.2, Section 5 - DM Domain for examples of reporting this implementation. |
Assumptions for the CDASHIG DM - Demographics Domain
The CDASHIG DM domain is a special-purpose domain that collects specific data elements that are mapped to the SDTMIG DM domain. Additional data elements that are not within the scope of the demographics must be mapped to other domains.
Example CRFs for the CDASHIG DM - Demographics Domain
Example 1Title: Demographics
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTM Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
BRTHDAT |
1 |
What is the subject's date of birth? |
Birth Date |
Record the date of birth to the level of precision known (e.g., day/month/year, year, month/year) in this format (DD-MON- YYYY). |
Date |
BRTHDTC |
|||||||
SEX |
2 |
What is the sex of the subject? |
Sex |
Record the appropriate sex (e.g., Female, Male). |
Text |
SEX |
(SEX) |
Male; Female; Unknown; Undifferentiated |
radio |
||||
ETHNIC |
3 |
Do you consider yourself Hispanic/Latino or not Hispanic/Latino? |
Ethnicity |
Study participants should self-report ethnicity, with ethnicity being asked about before race. |
Text |
ETHNIC |
(ETHNIC) |
Hispanic or Latino; Not Hispanic or Latino; Not Reported |
radio |
||||
RACE |
4 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Study participants should self-report race, with race being asked about after ethnicity. |
Text |
RACE |
(RACE) |
American Indian or Alaska Native; Asian; Black or African American; Native Hawaiian or Other Pacific Islander; White |
check |
Example 2
|
Sponsors may append a suffix to denote multiple collected races and ethnicities (e.g. RACE1, RACE2, CRACE1, CRACE2, CETHNIC1, and CETHNIC2). The appended suffixes shown for the CDASH Variable Name, QNAM and QLABEL in this aCRF are just examples and are not indicative of any proscribed values that must be followed. |
Title: Demographics with Additional Granularity for Ethnicity and Race
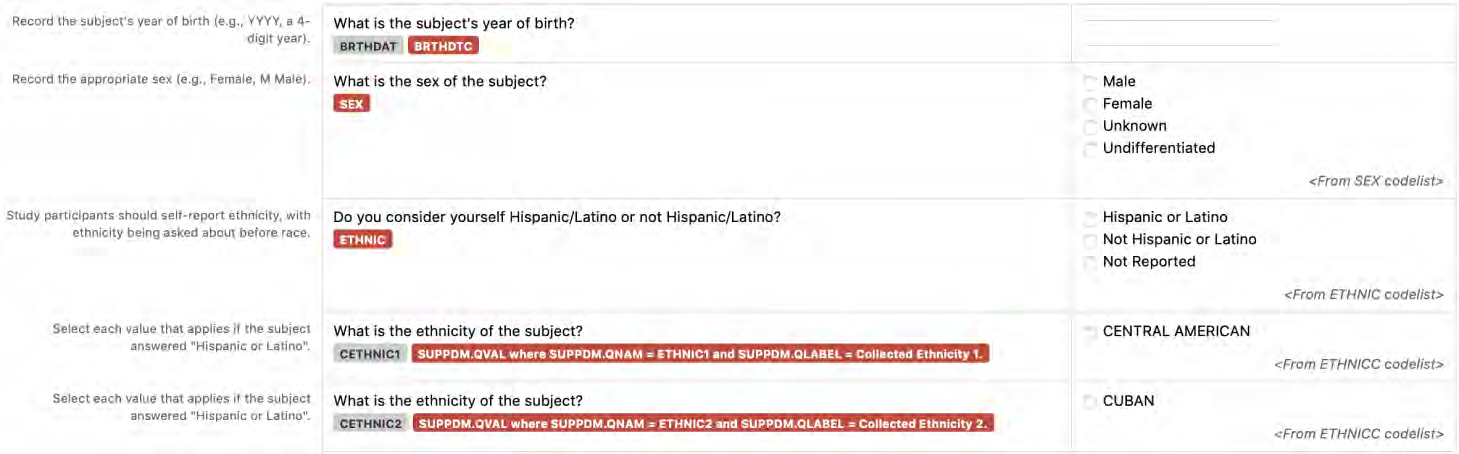
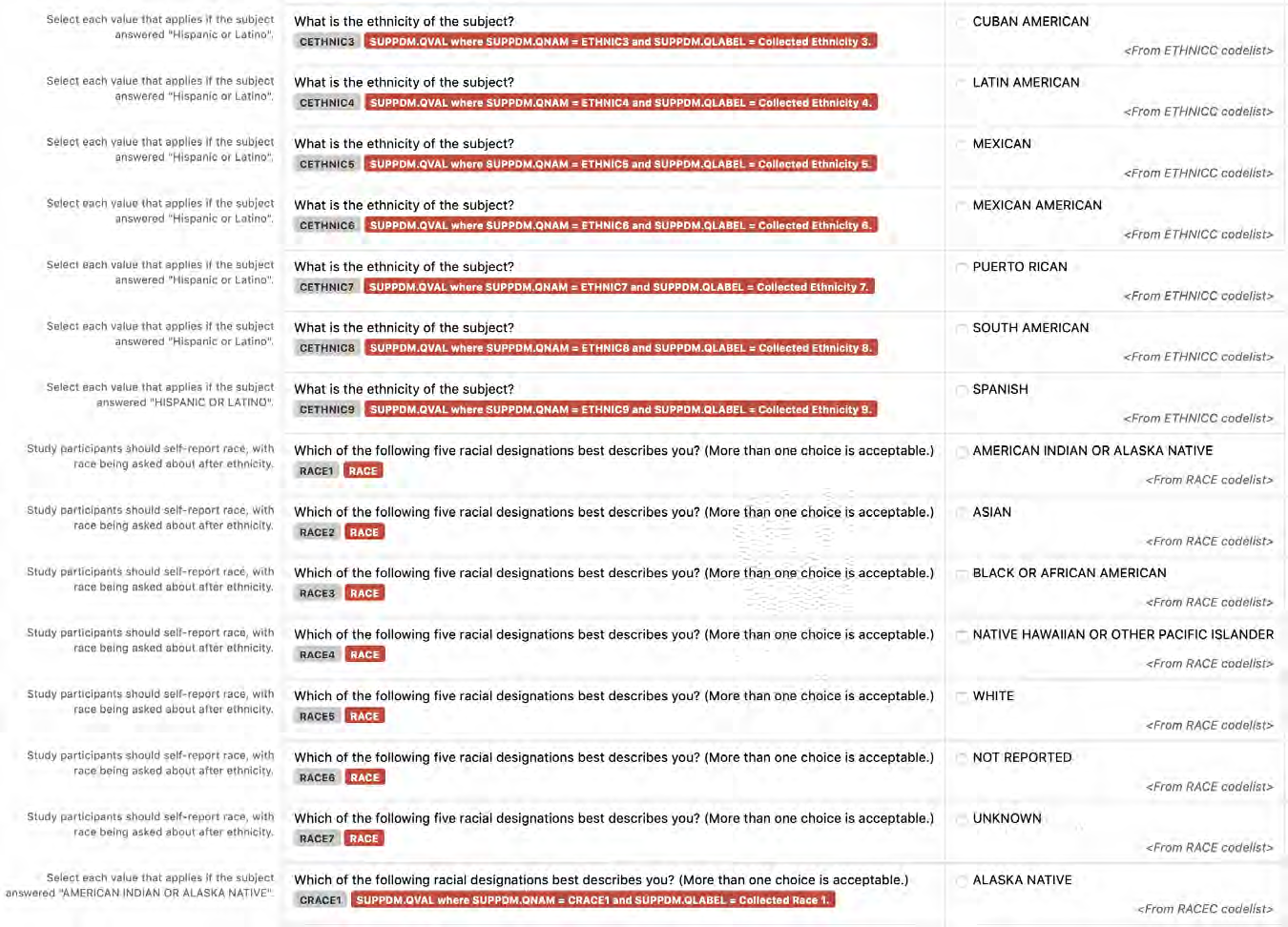
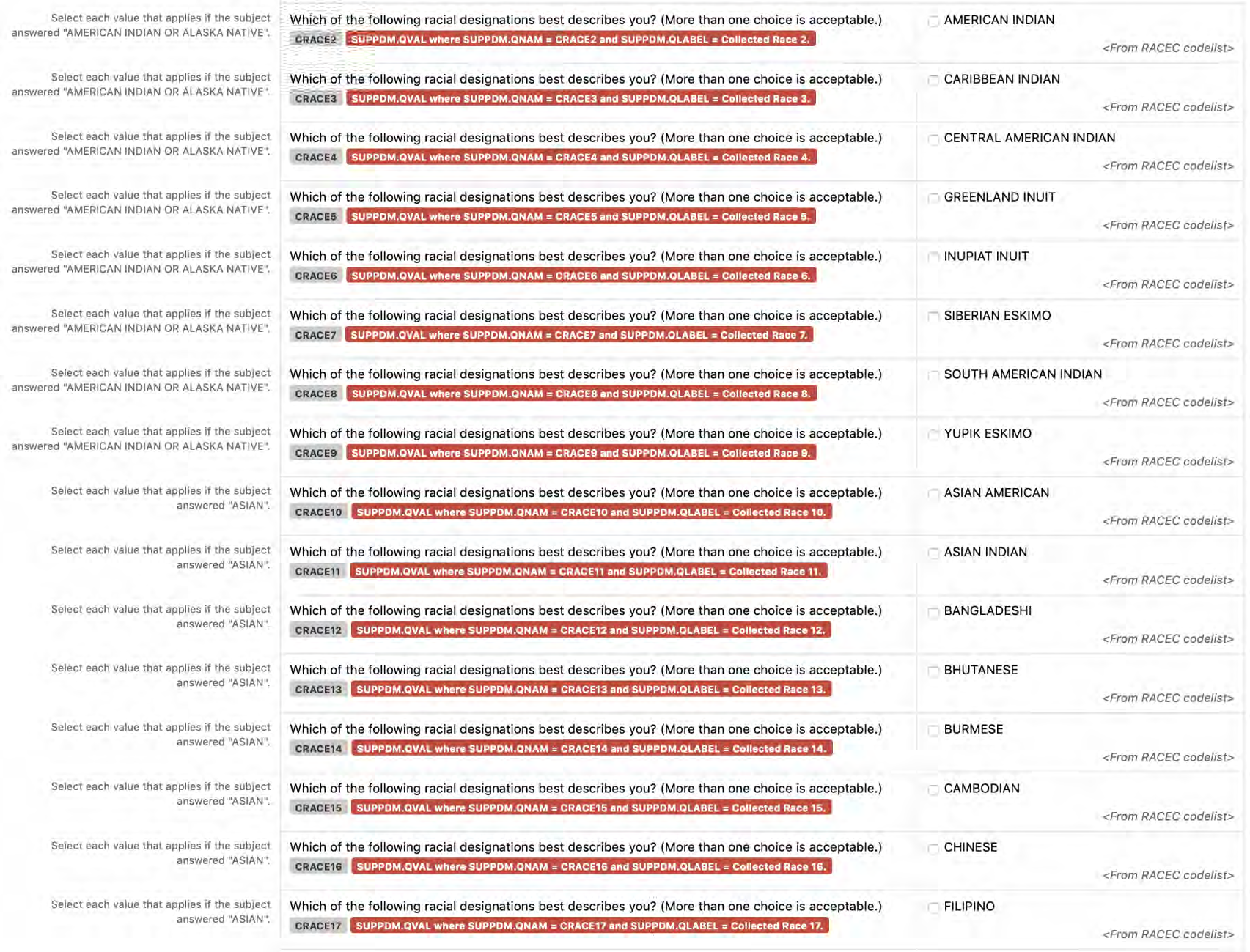
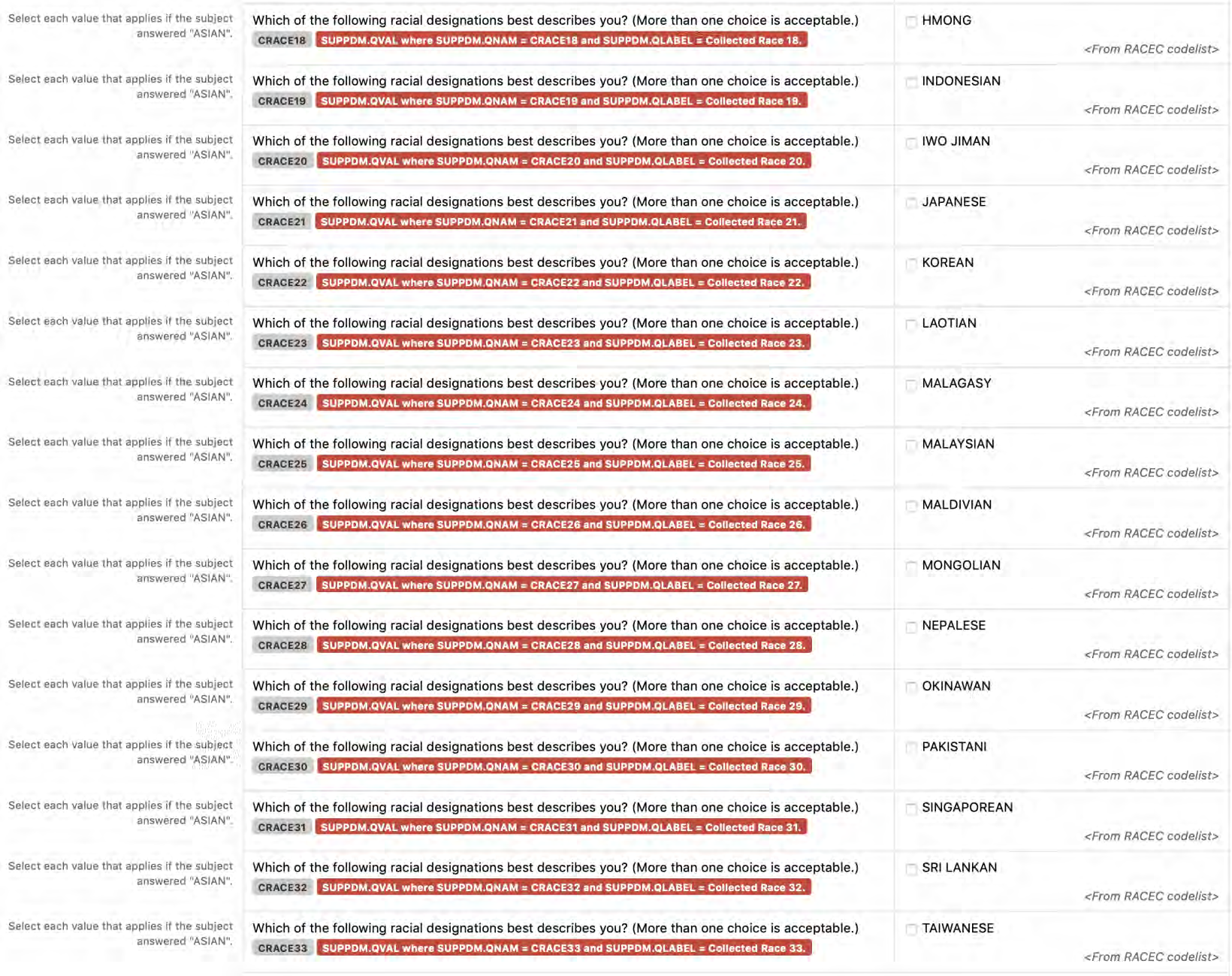

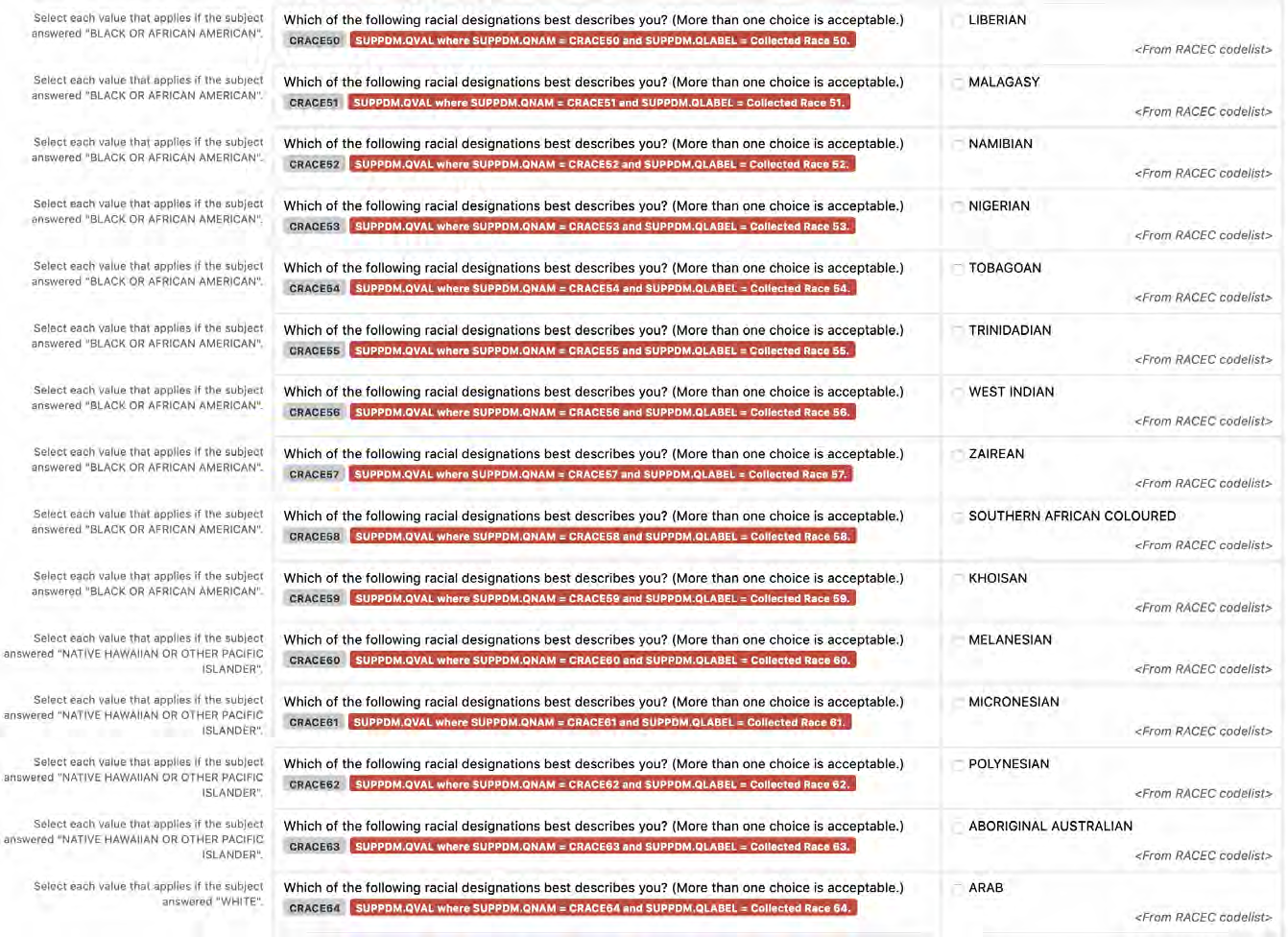
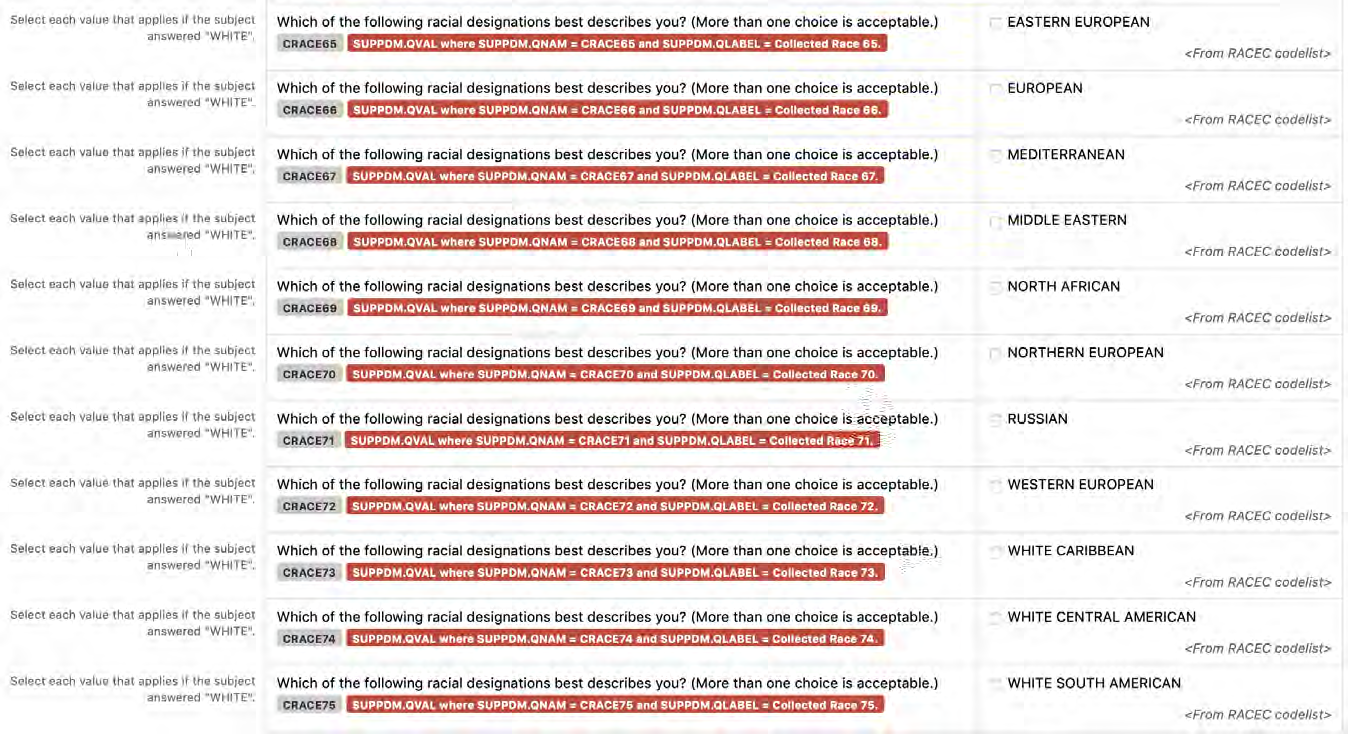
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTM Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
BRTHDAT |
1 |
What is the subject's year of birth? |
Birth Date |
Record the subject's year of birth (e.g., YYYY, a 4-digit year). |
Text |
BRTHDTC |
|||||||
SEX |
2 |
What is the sex of the subject? |
Sex |
Record the appropriate sex (e.g., Female, M Male). |
Text |
SEX |
(SEX) |
Male; Female; Unknown; Undifferentiated |
radio |
||||
ETHNIC |
3 |
Do you consider yourself Hispanic/Latino or not Hispanic/Latino? |
Ethnicity |
Study participants should self-report ethnicity, with ethnicity being asked about before race. |
Text |
ETHNIC |
(ETHNIC) |
Hispanic or Latino; Not Hispanic or Latino; Not Reported |
radio |
||||
CETHNIC1 |
4 |
What is the ethnicity of the subject? |
Ethnicity |
Select each value that applies if the subject answered "Hispanic or Latino". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = ETHNIC1 and SUPPDM.QLABEL = Collected Ethnicity 1. |
(ETHNICC) |
CENTRAL AMERICAN |
checkbox | |||
CETHNIC2 |
5 |
What is the ethnicity of the subject? |
Ethnicity |
Select each value that applies if the subject answered "Hispanic or Latino". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = ETHNIC2 and SUPPDM.QLABEL = Collected Ethnicity 2. |
(ETHNICC) |
CUBAN | checkbox | |||
CETHNIC3 |
6 |
What is the ethnicity of the subject? |
Ethnicity |
Select each value that applies if the subject answered "Hispanic or Latino". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = ETHNIC3 and SUPPDM.QLABEL = Collected Ethnicity 3. |
(ETHNICC) |
CUBAN AMERICAN |
checkbox |
|||
CETHNIC4 |
8 |
What is the ethnicity of the subject? |
Ethnicity |
Select each value that applies if the subject answered "Hispanic or Latino". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = ETHNIC4 and SUPPDM.QLABEL = Collected Ethnicity 4. |
(ETHNICC) |
LATIN AMERICAN |
checkbox |
|||
CETHNIC5 |
9 |
What is the ethnicity of the subject? |
Ethnicity |
Select each value that applies if the subject answered "Hispanic or Latino". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = ETHNIC5 and SUPPDM.QLABEL = Collected Ethnicity 5. |
(ETHNICC) |
MEXICAN |
checkbox |
|||
CETHNIC6 |
10 |
What is the ethnicity of the subject? |
Ethnicity |
Select each value that applies if the subject answered "Hispanic or Latino". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = ETHNIC6 and SUPPDM.QLABEL = Collected Ethnicity 6. |
(ETHNICC) |
MEXICAN AMERICAN |
checkbox | |||
CETHNIC7 |
11 |
What is the ethnicity of the subject? |
Ethnicity |
Select each value that applies if the subject answered "Hispanic or Latino". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = ETHNIC7 and SUPPDM.QLABEL = Collected Ethnicity 7. |
(ETHNICC) |
PUERTO RICAN |
checkbox |
|||
CETHNIC8 |
12 |
What is the ethnicity of the subject? |
Ethnicity |
Select each value that applies if the subject answered "Hispanic or Latino". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = ETHNIC8 and SUPPDM.QLABEL = Collected Ethnicity 8. |
(ETHNICC) |
SOUTH AMERICAN |
checkbox |
|||
CETHNIC9 |
13 |
What is the ethnicity of the subject? |
Ethnicity |
Select each value that applies if the subject answered "HISPANIC OR LATINO". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = ETHNIC9 and SUPPDM.QLABEL = Collected Ethnicity 9. |
(ETHNICC) |
SPANISH |
checkbox |
|||
RACE1 |
14 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Study participants should self-report race, with race being asked about after ethnicity. |
Text |
RACE |
(RACE) |
AMERICAN INDIAN OR ALASKA NATIVE |
checkbox | ||||
RACE2 |
15 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Study participants should self-report race, with race being asked about after ethnicity. |
Text |
RACE |
(RACE) |
ASIAN | checkbox | ||||
RACE3 |
16 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Study participants should self-report race, with race being asked about after ethnicity. |
Text |
RACE | (RACE) |
BLACK OR AFRICAN AMERICAN | checkbox | ||||
RACE4 |
17 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Study participants should self-report race, with race being asked about after ethnicity. |
Text |
RACE | (RACE) |
NATIVE HAWAIIAN OR OTHER PACIFIC ISLANDER | checkbox | ||||
RACE5 |
18 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Study participants should self-report race, with race being asked about after ethnicity. |
Text |
RACE | (RACE) |
WHITE | checkbox | ||||
RACE6 |
19 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Study participants should self-report race, with race being asked about after ethnicity. |
Text |
RACE | (RACE) |
NOT REPORTED | checkbox |
||||
RACE7 |
20 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Study participants should self-report race, with race being asked about after ethnicity. |
Text |
RACE | (RACE) |
UNKNOWN | checkbox | ||||
CRACE1 |
21 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "AMERICAN INDIAN OR ALASKA NATIVE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE1 and SUPPDM.QLABEL = Collected Race 1. |
(RACEC) |
ALASKA NATIVE |
checkbox |
|||
CRACE2 |
22 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "AMERICAN INDIAN OR ALASKA NATIVE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE2 and SUPPDM.QLABEL = Collected Race 2. |
(RACEC) |
AMERICAN INDIAN |
checkbox |
|||
CRACE3 |
23 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "AMERICAN INDIAN OR ALASKA NATIVE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE3 and SUPPDM.QLABEL = Collected Race 3. |
(RACEC) |
CARIBBEAN INDIAN |
checkbox |
|||
CRACE4 |
24 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "AMERICAN INDIAN OR ALASKA NATIVE". | Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE4 and SUPPDM.QLABEL = Collected Race 4. |
(RACEC) |
CENTRAL AMERICAN INDIAN |
checkbox |
|||
CRACE5 |
25 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "AMERICAN INDIAN OR ALASKA NATIVE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE5 and SUPPDM.QLABEL = Collected Race 5. |
(RACEC) |
GREENLAND INUIT |
checkbox |
|||
CRACE6 | 26 | Which of the following racial designations best describes you? (More than one choice is acceptable.) | Race | Select each value that applies if the subject answered "AMERICAN INDIAN OR ALASKA NATIVE". | Text | SUPPDM.QVAL | SUPPDM.QVAL where SUPPDM.QNAM = CRACE6 and SUPPDM.QLABEL = Collected Race 6. | (RACEC) | INUPIAT INUIT |
checkbox |
|||
CRACE7 | 27 | Which of the following racial designations best describes you? (More than one choice is acceptable.) | Race | Select each value that applies if the subject answered "AMERICAN INDIAN OR ALASKA NATIVE". | Text | SUPPDM.QVAL | SUPPDM.QVAL where SUPPDM.QNAM = CRACE7 and SUPPDM.QLABEL = Collected Race 7. | (RACEC) | SIBERIAN ESKIMO |
checkbox |
|||
CRACE8 | 28 | Which of the following racial designations best describes you? (More than one choice is acceptable.) | Race | Select each value that applies if the subject answered "AMERICAN INDIAN OR ALASKA NATIVE". | Text | SUPPDM.QVAL | SUPPDM.QVAL where SUPPDM.QNAM = CRACE8 and SUPPDM.QLABEL = Collected Race 8. | (RACEC) | SOUTH AMERICAN INDIAN |
checkbox |
|||
CRACE9 | 29 | Which of the following racial designations best describes you? (More than one choice is acceptable.) | Race | Select each value that applies if the subject answered "AMERICAN INDIAN OR ALASKA NATIVE". | Text | SUPPDM.QVAL | SUPPDM.QVAL where SUPPDM.QNAM = CRACE9 and SUPPDM.QLABEL = Collected Race 9. | (RACEC) | YUPIK ESKIMO |
checkbox |
|||
CRACE10 | 30 | Which of the following racial designations best describes you? (More than one choice is acceptable.) | Race | Select each value that applies if the subject answered "ASIAN". | Text | SUPPDM.QVAL | SUPPDM.QVAL where SUPPDM.QNAM = CRACE10 and SUPPDM.QLABEL = Collected Race 10. | (RACEC) | ASIAN AMERICAN |
checkbox |
|||
CRACE11 |
31 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE11 and SUPPDM.QLABEL = Collected Race 11. |
(RACEC) |
ASIAN INDIAN |
checkbox |
|||
CRACE12 |
32 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE12 and SUPPDM.QLABEL = Collected Race 12. |
(RACEC) |
BANGLADESHI |
checkbox |
|||
CRACE13 |
33 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE13 and SUPPDM.QLABEL = Collected Race 13. |
(RACEC) |
BHUTANESE |
checkbox |
|||
CRACE14 |
34 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE14 and SUPPDM.QLABEL = Collected Race 14. |
(RACEC) |
BURMESE |
checkbox |
|||
CRACE15 |
35 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE15 and SUPPDM.QLABEL = Collected Race 15. |
(RACEC) |
CAMBODIAN |
checkbox |
|||
CRACE16 |
36 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE16 and SUPPDM.QLABEL = Collected Race 16. |
(RACEC) |
CHINESE |
checkbox |
|||
CRACE17 |
37 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE17 and SUPPDM.QLABEL = Collected Race 17. |
(RACEC) |
FILIPINO |
checkbox |
|||
CRACE18 |
38 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE18 and SUPPDM.QLABEL = Collected Race 18. |
(RACEC) |
HMONG |
checkbox |
|||
CRACE19 |
39 |
Which of the following five racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE19 and SUPPDM.QLABEL = Collected Race 19. |
(RACEC) |
INDONESIAN |
checkbox |
|||
CRACE20 |
40 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE20 and SUPPDM.QLABEL = Collected Race 20. |
(RACEC) |
IWO JIMAN |
checkbox |
|||
CRACE21 |
41 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE21 and SUPPDM.QLABEL = Collected Race 21. |
(RACEC) |
JAPANESE |
checkbox |
|||
CRACE22 |
42 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE22 and SUPPDM.QLABEL = Collected Race 22. |
(RACEC) |
KOREAN |
checkbox |
|||
CRACE23 |
43 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE23 and SUPPDM.QLABEL = Collected Race 23. |
(RACEC) |
LAOTIAN |
checkbox | |||
CRACE24 |
44 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE24 and SUPPDM.QLABEL = Collected Race 24. |
(RACEC) |
MALAGASY |
checkbox |
|||
CRACE25 |
45 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE25 and SUPPDM.QLABEL = Collected Race 25. |
(RACEC) |
MALAYSIAN |
checkbox |
|||
CRACE26 |
46 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE26 and SUPPDM.QLABEL = Collected Race 26. |
(RACEC) |
MALDIVIAN |
checkbox |
|||
CRACE27 |
47 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE27 and SUPPDM.QLABEL = Collected Race 27. |
(RACEC) |
MONGOLIAN |
checkbox |
|||
CRACE28 |
48 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE28 and SUPPDM.QLABEL = Collected Race 28. |
(RACEC) |
NEPALESE | checkbox | |||
CRACE29 |
49 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE29 and SUPPDM.QLABEL = Collected Race 29. |
(RACEC) |
OKINAWAN | checkbox | |||
CRACE30 |
50 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE30 and SUPPDM.QLABEL = Collected Race 30. |
(RACEC) |
PAKISTANI | checkbox | |||
CRACE31 |
51 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE31 and SUPPDM.QLABEL = Collected Race 31. |
(RACEC) |
SINGAPOREAN |
checkbox | |||
CRACE32 |
52 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE32 and SUPPDM.QLABEL = Collected Race 32. |
(RACEC) |
SRI LANKAN | checkbox | |||
CRACE33 |
53 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE33 and SUPPDM.QLABEL = Collected Race 33. |
(RACEC) |
TAIWANESE | checkbox | |||
CRACE34 |
54 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE34 and SUPPDM.QLABEL = Collected Race 34. |
(RACEC) |
THAI | checkbox | |||
CRACE35 |
55 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "ASIAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE35 and SUPPDM.QLABEL = Collected Race 35. |
(RACEC) |
VIETNAMESE | checkbox | |||
CRACE36 |
56 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE36 and SUPPDM.QLABEL = Collected Race 36. |
(RACEC) |
AFRICAN | checkbox | |||
CRACE37 |
57 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE37 and SUPPDM.QLABEL = Collected Race 37. |
(RACEC) |
AFRICAN AMERICAN | checkbox | |||
CRACE38 |
58 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE38 and SUPPDM.QLABEL = Collected Race 38. |
(RACEC) |
AFRICAN CARIBBEAN | checkbox | |||
CRACE39 |
59 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE39 and SUPPDM.QLABEL = Collected Race 39. |
(RACEC) |
BAHAMIAN |
checkbox |
|||
CRACE40 |
60 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE40 and SUPPDM.QLABEL = Collected Race 40. |
(RACEC) |
BARBADIAN | checkbox |
|||
CRACE41 |
61 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE41 and SUPPDM.QLABEL = Collected Race 41. |
(RACEC) |
BLACK | checkbox |
|||
CRACE42 |
62 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE42 and SUPPDM.QLABEL = Collected Race 42. |
(RACEC) |
BLACK CENTRAL AMERICAN | checkbox |
|||
CRACE43 |
63 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE43 and SUPPDM.QLABEL = Collected Race 43. |
(RACEC) |
BLACK SOUTH AMERICAN | checkbox |
|||
CRACE44 |
64 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE44 and SUPPDM.QLABEL = Collected Race 44. |
(RACEC) |
BOTSWANAN | checkbox |
|||
CRACE45 |
65 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE45 and SUPPDM.QLABEL = Collected Race 45. |
(RACEC) |
DOMINICA ISLANDER | checkbox |
|||
CRACE46 |
66 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE46 and SUPPDM.QLABEL = Collected Race 46. |
(RACEC) |
DOMINICAN | checkbox |
|||
CRACE47 |
67 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE47 and SUPPDM.QLABEL = Collected Race 47. |
(RACEC) |
ETHIOPIAN | checkbox |
|||
CRACE48 |
68 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE48 and SUPPDM.QLABEL = Collected Race 48. |
(RACEC) |
HAITIAN | checkbox |
|||
CRACE49 |
69 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN".v |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE49 and SUPPDM.QLABEL = Collected Race 49. |
(RACEC) |
JAMAICAN | checkbox |
|||
CRACE50 |
70 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE50 and SUPPDM.QLABEL = Collected Race 50. |
(RACEC) |
LIBERIAN | checkbox |
|||
CRACE51 |
71 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE51 and SUPPDM.QLABEL = Collected Race 51. |
(RACEC) |
MALAGASY | checkbox |
|||
CRACE52 |
72 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE52 and SUPPDM.QLABEL = Collected Race 52. |
(RACEC) |
NAMIBIAN | checkbox |
|||
CRACE53 |
73 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE53 and SUPPDM.QLABEL = Collected Race 53. |
(RACEC) |
NIGERIAN | checkbox |
|||
CRACE54 |
74 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE54 and SUPPDM.QLABEL = Collected Race 54. |
(RACEC) |
TOBAGOAN | checkbox |
|||
CRACE55 |
75 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE55 and SUPPDM.QLABEL = Collected Race 55. |
(RACEC) |
TRINIDADIAN | checkbox |
|||
CRACE56 |
76 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE56 and SUPPDM.QLABEL = Collected Race 56. |
(RACEC) |
WEST INDIAN | checkbox |
|||
CRACE57 |
77 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE57 and SUPPDM.QLABEL = Collected Race 57. |
(RACEC) |
ZAIREAN | checkbox |
|||
CRACE58 |
78 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE58 and SUPPDM.QLABEL = Collected Race 58. |
(RACEC) |
SOUTHERN AFRICAN COLOURED | checkbox |
|||
CRACE59 |
79 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "BLACK OR AFRICAN AMERICAN". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE59 and SUPPDM.QLABEL = Collected Race 59. |
(RACEC) |
KHOISAN | checkbox |
|||
CRACE60 |
80 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "NATIVE HAWAIIAN OR OTHER PACIFIC ISLANDER". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE60 and SUPPDM.QLABEL = Collected Race 60. |
(RACEC) |
MELANESIAN | checkbox |
|||
CRACE61 |
81 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "NATIVE HAWAIIAN OR OTHER PACIFIC ISLANDER". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE61 and SUPPDM.QLABEL = Collected Race 61. |
(RACEC) |
MICRONESIAN | checkbox |
|||
CRACE62 |
82 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "NATIVE HAWAIIAN OR OTHER PACIFIC ISLANDER". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE62 and SUPPDM.QLABEL = Collected Race 62. |
(RACEC) |
POLYNESIAN | checkbox |
|||
CRACE63 |
83 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "NATIVE HAWAIIAN OR OTHER PACIFIC ISLANDER". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE63 and SUPPDM.QLABEL = Collected Race 63. |
(RACEC) |
ABORIGINAL AUSTRALIAN | checkbox |
|||
CRACE64 |
84 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "WHITE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE64 and SUPPDM.QLABEL = Collected Race 64. |
(RACEC) |
ARAB | checkbox |
|||
CRACE65 |
85 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "WHITE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE65 and SUPPDM.QLABEL = Collected Race 65. |
(RACEC) |
EASTERN EUROPEAN | checkbox |
|||
CRACE66 |
86 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "WHITE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE66 and SUPPDM.QLABEL = Collected Race 66. |
(RACEC) |
EUROPEAN | checkbox |
|||
CRACE67 |
87 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "WHITE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE67 and SUPPDM.QLABEL = Collected Race 67. |
(RACEC) |
MEDITERRANEAN | checkbox |
|||
CRACE68 |
88 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "WHITE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE68 and SUPPDM.QLABEL = Collected Race 68. |
(RACEC) |
MIDDLE EASTERN | checkbox |
|||
CRACE69 |
89 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "WHITE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE69 and SUPPDM.QLABEL = Collected Race 69. |
(RACEC) |
NORTH AFRICAN | checkbox |
|||
CRACE70 |
90 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "WHITE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE70 and SUPPDM.QLABEL = Collected Race 70. |
(RACEC) |
NORTHERN EUROPEAN | checkbox |
|||
CRACE71 |
91 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "WHITE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE71 and SUPPDM.QLABEL = Collected Race 71. |
(RACEC) |
RUSSIAN | checkbox |
|||
CRACE72 |
92 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "WHITE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE72 and SUPPDM.QLABEL = Collected Race 72. |
(RACEC) |
WESTERN EUROPEAN | checkbox |
|||
CRACE73 |
93 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "WHITE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE73 and SUPPDM.QLABEL = Collected Race 73. |
(RACEC) |
WHITE CARIBBEAN | checkbox |
|||
CRACE74 |
94 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "WHITE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE74 and SUPPDM.QLABEL = Collected Race 74. |
(RACEC) |
WHITE CENTRAL AMERICAN | checkbox |
|||
CRACE75 |
95 |
Which of the following racial designations best describes you? (More than one choice is acceptable.) |
Race |
Select each value that applies if the subject answered "WHITE". |
Text |
SUPPDM.QVAL |
SUPPDM.QVAL where SUPPDM.QNAM = CRACE75 and SUPPDM.QLABEL = Collected Race 75. |
(RACEC) |
WHITE SOUTH AMERICAN | checkbox |
8 General Observation Classes
This section reflects common practices implemented by a significant number of the organizations/companies that provided information and examples. Most subject-level data collected during a study are represented using 1 of the 3 Study Data Tabulation Model (SDTM) general observation classes: Interventions, Events, and Findings. Section 8.1 describes the CDASH Intervention domains, Section 8.2 the CDASH Events domains, and Section 8.3 the CDASH Findings Domains.
Within each domain class, the domains are presented in alphabetical order. Readers should refer to Section 6 of the SDTM Implementation Guide (SDTMIG) for additional information on these classes. Within each domain, a link is provided to the CDASHIG Domain Metadata table. The CDASHIG Domain Metadata tables include the variables that are commonly used by a significant number of the organizations/companies that provided information and examples.
Implementers may add other variables from the CDASH Model as needed, following the instructions in Section 3.4, How to Create New Data Collection Fields When No CDASHIG Field Has Been Defined.
8.1 Interventions Class Domains
The Interventions class includes CDASH domains that define collection standards for investigational treatments, therapeutic treatments, and procedures that are intentionally administered to the subject either as specified by the study protocol (e.g., exposure), coincident with the study assessment period (e.g., concomitant medications), or self- administered by the subject (e.g., alcohol, tobacco, caffeine).
8.1.1 General CDASH Assumptions for Interventions Domains
- Sponsors may use terms like concomitant medications, treatments, or therapies, as appropriate for the study. The following text may use one of these terms, but sponsors can always use the term most appropriate for their study.
- The term prior refers to medications/treatments that were started before study participation, because limited information may be available on prior medications taken by a subject; the core requirements were constrained to reflect this limitation.
- Sponsors should define the appropriate collection period for prior and concomitant medications/treatments in the study protocol.
1. CDASH --YN variables with the question text "Were there any interventions?" (e.g., “Were there any procedures?”, “Were there any concomitant medications?") are intended to assist in the cleaning of data and in confirming that there are no missing values. These variables are not included as part of the SDTM Intervention domains for submission and are annotated as NOT SUBMITTED on the case report form (CRF).
2. CDASH --CAT and/or -SCAT are generally not entered on the CRF by the sites. Implementers may prepopulate and display these category values to help the site understand what data should be recorded on the CRF. Implementers may also prepopulate hidden variables with the values assigned within their operational database. Categories and subcategories are determined per protocol design, and could be populated during SDTM submission dataset creation.
3. Date and Time Variables
a. CDASH date variables (e.g., --DAT, --STDAT, --ENDAT) are concatenated with CDASH Time variables (e.g., --TIM, --STTIM, --ENTIM, if time is applicable) into the appropriate SDTM --DTC variables (e.g., --DTC, --STDTC, --ENDTC) using the ISO 8601 format.
b. Collecting the time an intervention was started is only appropriate if it can be realistically determined and if there is a scientific reason for needing to know this level of detail. An example is where the subject is under the direct care of the site at the time the intervention was started, and the study design is such that it is important to know the intervention start time with respect to dosing.
4. The CDASH variable -- REASND is used with SDTM variable --STAT only. The value "NOT DONE" in - -STAT indicates that the subject was not questioned about the intervention or that data were not collected; it does not mean that the subject had no interventions.
5. The CDASH --SPID variable may be populated by the sponsor's data collection system. If collected, it can be beneficial to use an identifier in a data query to communicate clearly to the site the specific record in question. This field may be populated by the sponsor's data collection system.
6. Coding
a. When free-text intervention treatments are recorded, the location may be included in the --TRT variable to facilitate coding (e.g., Liver Biopsy). Location may be collected when the sponsor needs to identify the specific anatomical location of the intervention. This location information does not need to be removed from the verbatim --TRT when creating SDTMIG submission datasets.
b. The Non-Standard (or SUPPQUAL) Variables --ATC1 through --ATC5 and --ATC1CD through -- ATC5CD are used only when the intervention is coded using the World Health Organization's Anatomical Therapeutic Chemical (ATC) classification system (https://www.who.int/medicines/regulation/). The numbers indicate the anatomical main group: "1" = the anatomical main group, "2" = the therapeutic main group, "3" = the therapeutic/pharmacological subgroup, "4" = chemical/therapeutic/pharmacological subgroup, "5" = chemical substance.
c. The implementer may also add MedDRA coding elements as non-standard variables to the Intervention domain if this dictionary is used for coding.
7. Location (--LOC) and related variables (--LAT, --DIR, -- PORTOT)
a. Because the complete lists of controlled terminology for these variables may be too extensive to be relevant for a particular study CRF, sponsors may choose to include only subsets of the controlled terminology on the CRF.
b. --LOC could be a defaulted or hidden field on the CRF for prespecified [--TRT]/Intervention Topic].
8. Relative Timing Variables (See SDTMIG v3.2 Section 4.1.4.7 for more information and details)
a. For each study, the sponsor defines the study reference period in the DM domain using SDTMIG variables RFSTDTC and RFENDTC. Other sponsor-specified reference time points can be defined for other data collection situations. The CDASH variables --PRIOR and --ONGO may be collected in lieu of "start date" or "end date".
b. The CDASH variable --PRIOR is used to indicate if the --TRT (the topic item) started prior to either the study reference period or another sponsor-defined reference time point. When the study reference period is used as the anchor, --PRIOR may be used to derive a value (from the Controlled Terminology codelist STENRF) into the SDTM relative timing variable --STRF. When populating --STRF, if the value of --PRIOR is "Y", the value of “BEFORE” may be mapped to --STRF. The value in DM.RFSTDTC serves as the anchor for --STRF.
c. When a reference time point is used instead of the study reference period, --PRIOR may be used to derive a value into the SDTM relative timing variable --STRTPT. If the value of --PRIOR is "Y", the value of "BEFORE" may be derived into --STRTPT. Note: --STRTPT must refer to the “time point anchor” as described in --STTPT. The value in --STTPT can be either text (e.g., "VISIT 1") or a date (in ISO 8601 format).
d. The CDASH variable --ONGO is used to indicate if the value in --TRT is continuing beyond the study reference period or beyond another sponsor-defined reference time point. When the study reference period is used as the anchor, --ONGO may be used to derive a value into the SDTM relative timing variable --ENRF. If the value of --ONGO = "Y", the value of "AFTER" may be mapped to --ENRF.
e. When a reference time point is used instead of the study reference period, --ONGO may be used to derive a value into the SDTM relative timing variable --ENRTPT. If the value of --ONGO is "Y", the value of "ONGOING" may be mapped to --ENRTPT. Note: --ENRTPT must refer to the “time point anchor” as described in --ENTPT. The value in --ENTPT can be either text (e.g., "TRIAL EXIT") or a date (in ISO 8601 format).
8.1.2 CM - Prior and Concomitant Medications
Description/Overview for the CDASHIG CM - Prior and Concomitant Medications Domain
The same basic data collection variables should be collected for all medications/treatments/therapies (Prior, General Concomitant Medications, and Medications of Interest). If additional fields are needed to collect other data about a medication of interest, those should be added as non-standard fields.
Note:
Specification for the CDASHIG CM - Prior and Concomitant Medications Domain
Prior and Concomitant Medications Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Interventions | CM | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Interventions | CM | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on CRFs that are shipped to each site. EDC: This should be prepopulated. |
Interventions | CM | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Interventions | CM | N/A | N/A | 4 | CMCAT | Category for Medication | A grouping of topic-variable values based on user-defined characteristics. | What is the category for the (concomitant) [medication/treatme nt/therapy]? | (Concomitant) [Medication/Treatment/ Therapy Category]; NULL | Char | O | Record the (concomitant) [medication/treat ment/therapy] category, if not pre-printed on the CRF. | CMCAT | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Interventions | CM | N/A | N/A | 5 | CMSCAT | Subcategory for Medication | A sub-division of the CMCAT values based on user- defined characteristics. | What is the subcategory for the (concomitant) [medication/treatme nt/therapy]? | (Concomitant) [Medication/Treatment/ Therapy subcategory]; NULL | Char | O | Record (concomitant) [medication/treat ment/therapy] subcategory, if not pre-printed on the CRF. | CMSCAT | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be pre- printed on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. |
Interventions | CM | N/A | N/A | 6 | CMYN | Any Concomitant Medications Taken | An indication whether or not any (concomitant) medications/treatm ents/therapies were taken/given. | Were/Was any (concomitant) [medication(s)/treat ment(s)/therapy(ies)] taken? | Any (Concomitant) [Medication(s)/ Treatment(s)/ Therapy(ies)] | Char | O | Indicate if the subject took any (concomitant) [medications/treat ments]. If Yes, include the appropriate details where indicated on the CRF. | N/A | Does not map to an SDTMIG variable. The SDTM Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification that all other fields on the CRF were deliberately left blank. |
Interventions | CM | N/A | N/A | 7 | CMSPID | CM Sponsor- Defined Identifier | A sponsor-defined identifier. In CDASH, This is typically used for preprinted or auto- generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor-defined question] | [Sponsor defined] | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | CMSPID | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Because SPID is a sponsor-defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile concomitant medication/treatment records with AEs and/or MH. May be used to record preprinted number (e.g. line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Interventions | CM | N/A | N/A | 8 | CMTRT | Reported Name of Drug, Med, or Therapy | Verbatim medication name or treatments (include only treatments with data collection characteristics similar to medications). | What was the (concomitant) [medication/treatme nt/therapy] (name/term)? | (Concomitant) [Medication/Treatment/ Therapy] | Char | HR | Record only 1 [medication/treat ment/therapy] per line. Provide the full trade or proprietary name of the [medication/treat ment/therapy]; otherwise the generic name may be recorded. | CMTRT | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | N/A | N/A | In most cases, the verbatim drug names or treatment will be coded to a standard dictionary such as WHODrug after the data have been collected on the CRF. For the collection of verbatim drug name or treatments, the recommendation is to ask sites to provide the full trade or proprietary name since it is more exact than the generic. The full trade name provides the base generic and the appropriate salt for that particular drug. In addition, for coding purposes it helps with ATC selection. For example, Tylenol with codeine #1 has a different ATC code than Tylenol with codeine #3. This field can be used for either prior or concomitant medication/treatments. |
Interventions | CM | N/A | N/A | 9 | CMPRESP | CM Pre- Specified | An indication that a specific intervention or a group of Interventions is prespecified on a CRF. | N/A | N/A | Char | O | N/A | CMPRESP | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | (NY) | N/A | For prespecified interventions, a hidden field on a CRF defaulted to "Y", or added during the SDTM dataset creation. If a study collects both prespecified interventions as well as free-text interventions, the value of CMPRESP should be "Y" for all prespecified interventions and null for interventions reported as free text. |
Interventions | CM | N/A | N/A | 10 | CMOCCUR | CM Occurrence | An indication whether the prespecified medication/treatme nt/therapy (CMTRT) or the group of medications/treatm ents/therapies was administered when information about the occurrence of a specific intervention is solicited. | Did the subject take [prespecified (concomitant) medication/treatmen t/therapy/dose]?; Has the subject taken [prespecified (concomitant) medication/treatmen t/therapy/dose]? | [Specific (Concomitant) [Medication/ Treatment/Therapy] | Char | O | Indicate if [specific medication/treatm ent] was taken by checking Yes or No. | CMOCCUR | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". If the response was not asked or answered, populate the SDTMIG variable CMSTAT with "NOT DONE". | (NY) | N/A | CMOCCUR is used to report the occurrence of a prespecific medication/treatment. CMOCCUR is not used for spontaneously free text reported concomitant medication/treatments. The site should be able to indicate that the response was not asked or answered. |
Interventions | CM | N/A | N/A | 11 | CMINGRD | Concomitant Meds Active Ingredients | Medication Ingredients. | What were the active ingredients? | Active Ingredients | Char | O | Prior to a subject's clinical visit, remind all subjects to bring all medications bottles, packs etc. they are taking with them to their clinical visit. Record all active ingredient(s) off the medication label and separate each ingredient with a comma for the name of drug medication or treatment taken. For example, the medication Dolmen, if manufactured in Spain, the active ingredients should be collected as noted below: Active Ingredient: Acetylsalicylic Acid, Ascorbic acid, codeine phosphate. | N/A | Does not map to an SDTMIG variable. The SDTM Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. | N/A | N/A | This may be collected in addition to the Medication/Treatment Name. Collecting this provides more detailed information when coding to a medication dictionary like WHODrug Dictionary Enhanced Format C, which now codes to the ingredient level for many trade-named medications. For example, depending on the country where it is manufactured, the active ingredients in the medication Dolmen may be different: In Spain, Acetylsalicylic Acid, Ascorbic acid, codeine phosphate; in Italy and Czech Republic, contains Tenoxicam; in Estonia and Latvia, contains Dexketoprofen trometamol. |
Interventions | CM | N/A | N/A | 12 | CMINDC | CM Indication | The condition, disease, symptom, or disorder that the concomitant (non- study) medication/treatme nt/therapy was used to address or investigate (e.g., why the medication/treatme nt/therapy was taken or administered). | For what indication was the (concomitant) [medication/treatme nt/therapy] taken? | Indication | Char | R/C | Record the reason the medication was taken based on clinical investigator's evaluation. If taken to treat a condition, and a diagnosis was made, the indication should be the diagnosis. If taken to treat a condition, and no diagnosis was made, the indication should be the signs and symptoms. If taken as prophylaxis, report as "Prophylaxis for " and include a description of the condition(s). | CMINDC | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | N/A | N/A | This is not the pharmacological/therap eutic classification of an agent (e.g., antibiotic, analgesic), but the reason for its administration to the subject. This additional information is collected on the CRF when sponsors want to capture the reason(s) why a subject took a medication/treatment. This information could be used as deemed appropriate for coding, analysis (e.g., in the classification of medications), for reconciling the medications/treatments taken by a subject with provided medical history, and/or AEs/SAEs as part of the data clean-up and monitoring process. |
Interventions | CM | N/A | N/A | 13 | CMAENO | Related Adverse Event ID | Identifier for the adverse event that is the indication for this medication/treatme nt/therapy. | What was the identifier for the adverse event(s) for which the (concomitant) [medication/treatme nt/therapy] was taken? | Adverse Event Identifier | Char | O | Record the identifier of the Adverse Event for which this (concomitant) [medication/treat ment/therapy] was taken. | N/A | This does not map directly to an SDTMIG variable. For the SDTM submission datasets, may be used to create RELREC to link this record with a record in the Adverse Events domain. | N/A | N/A | Intent is to establish a link between the medication/treatment and the AE that was reported. CMAENO can be used to identify a relationship between records in CM dataset and records in the AE dataset. See SDTMIG v3.2 Section 8.2 for information on RELREC. |
Interventions | CM | N/A | N/A | 14 | CMMHNO | Related Medical History Event ID | Identifier for the medical history condition that is the indication for this medication/treatme nt/therapy. | What was the identifier for the medical history event(s) for which the (concomitant) [medication/treatme nt/therapy] was taken? | Medical History Event Identifier | Char | O | Record the identifier of the medical history event for which this (concomitant) [medication/treat ment/therapy] was taken. | N/A | This does not map directly to an SDTMIG variable. For the SDTM submission datasets, may be used to create RELREC to link this record with a record in the Medical History domain. | N/A | N/A | Intent is to establish a link between the medical history condition and the medication/treatment taken for the medical history condition. CMMHNO can be used to identify a relationship between records in the CM dataset and records in the MH dataset. See SDTMIG v3.2 Section 8.2 for information on RELREC. |
Interventions | CM | N/A | N/A | 15 | CMDOSE | CM Dose per Administration | The dose of medication/treatme nt (e.g., --TRT ) given at one time represented as a numeric value. | What was the individual dose (of the concomitant [medication/treatme nt/therapy] per administration)? | [Dose/Amount] (per administration) | Num | O | Record the dose of (concomitant) [medication/treat ment] taken per administration (e.g., 200). | CMDOSE | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | N/A | N/A | Used when the dose/amount taken/administered/con sumed has only numeric entries. If non- numeric entries are possible, use the CDASH field CMDSTXT. |
Interventions | CM | N/A | N/A | 16 | CMDSTXT | Concomitant Meds Dose Description | The dose of medication/treatme nt taken per administration. | What was the individual dose of the (concomitant) [medication/treatme nt/therapy]? | Dose | Char | O | Record the dose of (concomitant) [medication/treat ment] taken per administration (e.g., 200). | CMDOSTXT ; CMDOSE | This does not map directly to an SDTMIG variable. Numeric values map to CMDOSE in SDTM. Non- numeric values (e.g., 200-400) map to CMDOSTXT in SDTM. | N/A | N/A | Defining this data collection field as a dose text field allows for flexibility in capturing dose entries as numbers, text or ranges. The data collected in this dose text-format field should be separated or mapped to either SDTMIG CMDOSE if numeric or CMDOSTXT if text. |
Interventions | CM | N/A | N/A | 17 | CMDOSTOT | CM Total Daily Dose | The total amount of CMTRT taken over a day using the units in CMDOSU. | What was the total daily dose of the (concomitant) [medication/treatme nt/therapy]? | Total Daily Dose | Num | O | Record the total dose of (concomitant) [medication/treat ment/therapy] taken daily. | CMDOSTO T | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | N/A | N/A | For use when only total daily dose is collected in the CRF. For general medications/treatments , it is not recommended to use Total Daily Dose. Instead, this can be calculated from other fields such as Units, Dose, and Frequency. |
Interventions | CM | N/A | N/A | 18 | CMDOSU | CM Dose Units | The unit associated with the concomitant medication/treatme nt/therapy taken (e.g., mg in "2 mg 3 times per day"). | What is the unit (for the dose of concomitant [medication/treatme nt/therapy])? | (Dose) Unit | Char | R/C | Record the dose unit of the dose of concomitant [medication/treat ment/therapy] taken (e.g., mg.). | CMDOSU | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | (UNIT) | (CMDOSU) | When sponsors collect data for amount of dose taken (i.e., Dose, Total Daily Dose), Unit must be collected as well (if applicable). |
Interventions | CM | N/A | N/A | 19 | CMDOSFR M | CM Dose Form | The pharmaceutical dosage form in which the CMTRT is physically presented. | What was the dose form of the (concomitant) [medication/treatme nt/therapy]? | Dose Form | Char | O | Record the pharmaceutical dosage form (e.g., TABLET CAPSULE, SYRUP) of delivery for the concomitant [medication/treat ment/therapy] taken. | CMDOSFR M | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | (FRM) | (CMDOSFRM) | Some drugs have multiple forms and this field may be needed to code the drug to an ATC level. However, in general, this level of detail should not be necessary except for medications/treatments of interest. |
Interventions | CM | N/A | N/A | 20 | CMDOSFR Q | CM Dosing Frequency per Interval | The number of doses given/administered /taken during a specific interval. | What was the frequency of the (concomitant) [medication/treatme nt/therapy]? | Frequency | Char | O | Record how often the (concomitant) [medication/treat ment/therapy] was taken (e.g., BID, PRN). | CMDOSFR Q | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | (FREQ) | (CMDOSFRQ) | The frequency of the concomitant medication/treatment. When collected, the recommendation is to collect dosing information in separate fields (e.g., CMDOSE, CMDOSEU, CMDOSFRQ) for specific and consistent data collection and to enable programmatically utilizing these data. |
Interventions | CM | N/A | N/A | 21 | CMROUTE | CM Route of Administration | The route of administration of the (concomitant) [medication/treatm ent/therapy]. | What was the route of administration of the (concomitant) [medication/treatme nt/therapy]? | Route | Char | R/C | Provide the route of administration for the (concomitant) [medication/treat ment/therapy]. | CMROUTE | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | (ROUTE) | (CMROUTE) | This additional information may be important to collect on the CRF when the sponsor would want to capture a medication's/treatment' s route of administration for purposes such as coding and the medication/treatment may have more than one route. Some companies may use route in coding medications/treatments to be able to choose a precise preferred name and ATC code. |
Interventions | CM | N/A | N/A | 22 | CMSTDAT | Concomitant Meds Start Date | The start date when the concomitant medication/treatme nt/therapy was first taken, represented in an unambiguous date format (e.g., DD-MON-YYYY). | What was the (concomitant) [medication/treatme nt/therapy/dose] start date? | Start Date | Char | R/C | Record the date the concomitant [medication/treat ment] was first taken using this format (DD-MON- YYYY). If the subject has been taking the concomitant [medication/treat ment] for a considerable amount of time prior to the start of the study, it is acceptable to have an incomplete date. Concomitant [medication/treat ment] taken during the study are expected to have a complete start date. Prior concomitant [medication/treat ment] that are exclusionary should have both a start and end date. | CMSTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable CMSTDTC in ISO 8601 format. | N/A | N/A | The assumption is that sponsors should either have a Start Date or will indicate that the medication or therapy was started before, during or after the study period. The preferred method is to collect a complete Start Date. Partial dates (e.g., providing year only) for medications/treatment started a considerable amount of time prior to the start of study are acceptable. |
Interventions | CM | N/A | N/A | 23 | CMSTTIM | Concomitant Meds Start Time | The time the concomitant medication/treatme nt/therapy was started, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the (concomitant) [medication/treatme nt/therapy/dose] start time? | Start Time | Char | R/C | Record the time (as complete as possible) that the concomitant [medication/treat ment] was started. | CMSTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable CMSTDTC in ISO 8601 format. | N/A | N/A | Recommend collecting the time a concomitant medication/treatment was started only when a protocol or data collection scenarios supports it. Typically, a start time is not collected unless the subject is under the direct care of the site at the time a concomitant medication/treatment administered or the subject records the start time in a diary. See Section 3.7, Mapping Relative Times from Collection to Submissions, and SDTMIG v3.2 Section 4.1.4.7 for more information. |
Interventions | CM | N/A | N/A | 24 | CMPRIOR | Prior Concomitant Meds | Indication the concomitant medication/treatme nt/therapy was given or taken prior to [CMSTTPT] or prior to the date in DM.RFSTDTC. | Was the (concomitant) [medication/treatme nt/therapy] given/taken prior to [CMSTTPT]?; Was the (concomitant) [medication/treatme nt/therapy] given/taken prior to study start? | Prior to [CMSTTPT]; Prior to Study | Char | O | Check if the concomitant [medication/treat ment/therapy] was started before the study. | CMSTRF; CMSTRTPT | This does not map directly to an SDTMIG variable. May be used to populate a value into an SDTM relative timing variable such as CMSTRF or CMSTRTPT. When populating CMSTRF, or CMSTRTPT, if the value of the CDASH field CMPRIOR is "Y" a value from the CDISC CT (STENRF) may be used. When CMPRIOR refers to the Study Reference Period (defined in DM.RFSTDTC to DM.RFENDTC) the SDTM variable CMSTRF should be populated. When CMPRIOR is compared to another time point, the SDTM variables CMSTRTPT and CMSTTPT should be used. When CMONGO is used in conjunction with another time point, the SDTM variables CMENRTPT and CMENTPT should be used. Note: CMSTRTPT must refer to the time point anchor described in CMSTTPT. | (NY) | N/A | Sponsors may collect this information rather than start dates. See Section 3.7, Mapping Relative Times from Collection to Submissions, and SDTMIG v3.2 Section 4.1.4.7 for more information. |
Interventions | CM | N/A | N/A | 25 | CMONGO | Ongoing Concomitant Meds | Indication the concomitant medication/treatme nt/therapy is ongoing when no end date is provided. | Was the (concomitant) [medication/treatme nt/therapy] ongoing (as of [the study- specific time point or period])? | Ongoing (as of [the study-specific time point or period]) | Char | R/C | Record the concomitant [medication/treat ment/therapy] as ongoing if the subject has not stopped taking the concomitant [medication/treat ment/therapy] at [the timepoint defined by the study]. If the concomitant medication is ongoing, the end date should be left blank | CMENRF; CMENRTPT | This does not map directly to an SDTM variable. May be used to populate a value into an SDTMIG relative timing variable such as CMENRF or CMENRTPT. When populating CMENRF, if the value of CMONGO is "Y", the value of "DURING", "AFTER" or "DURING/AFTER" may be used. When populating CMENRTPT, if the value of CMONGO is "Y", the value of "ONGOING" may be used. When CMONGO refers to the Study Reference Period (defined in DM.RFSTDTC to DM.RFENDTC) the SDTM variable CMENRF should be populated. When CMONGO is used in conjunction with another time point, the SDTM variables CMENRTPT and CMENTPT should be used. Note: CMENRTPT must refer to a time point anchor described in CMENTPT. | (NY) | N/A | This box should be checked to indicate that the concomitant medication/treatment has not stopped at the time of data collection. It is expected that every recorded medication/treatment should have either an End Date or be checked as Ongoing, but not both. However, in cases where ongoing concomitant medications/treatments are not permitted, it may not be necessary to include an Ongoing field in the CRF. See Section 3.7, Mapping Relative Times from Collection to Submission, for more information about collecting relative date/time; see SDTMIG v3.2 Section 4.1.4 for information about mapping relative times. |
Interventions | CM | N/A | N/A | 26 | CMENDAT | Concomitant Meds End Date | The date that the subject ended/stopped taking the concomitant medication/treatme nt/therapy represented in an unambiguous date format (e.g., DD- MON-YYYY). | What was the (concomitant) [medication/treatme nt/therapy/dose] end date? | End Date | Char | R/C | Record the date the concomitant [medication/treat ment] was stopped using this format (DD-MON- YYYY). If the subject has not stopped taking the concomitant [medication/treat ment] leave this field blank. | CMENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable CMENDTC in ISO 8601 format. | N/A | N/A | The assumption is that sponsors should either have an End Date or will indicate that the medication or therapy was ongoing at the time of collection or at the end of the study. However, in cases where the End Date can be determined from dates collected elsewhere in the CRF it is not necessary to include an End Date in the CRF. For example, if all concomitant medications/treatments are administered only once during a trial, the End Date will be the same as the Start Date. |
Interventions | CM | N/A | N/A | 27 | CMENTIM | Concomitant Meds End Time | The time when the subject ended/stopped taking the concomitant medication/treatme nt/therapy represented in an unambiguous time format (e.g., hh:mm:ss). | What was the [medication/treatme nt/therapy/dose] end time? | End Time | Char | R/C | Record the time (as complete as possible) that the concomitant medication/treatm ent was stopped. | CMENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable CMENDTC in ISO 8601 format. | N/A | N/A | Recommend collecting the time a concomitant medication or treatment was ended only when a protocol or data collection scenarios require it or the subject records the start time in a diary. Typically, an end time is not collected unless the subject is under the direct care of the site at the time a concomitant medication/treatment is stopped. |
Interventions | CM | N/A | N/A | 28 | CMDECOD | Standardized Medication Name | The dictionary- or sponsor-defined standardized text description of the topic variable, CMTRT, or the modified topic variable (CMMODIFY), if applicable. | N/A | N/A | Char | O | N/A | CMDECOD | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. Equivalent to the generic drug name in WHODrug, or a term in SNOMED, ICD9, or other published or sponsor-defined dictionaries. |
Interventions | CM | N/A | N/A | 29 | CMCLAS | CM Medication Class | The class for the intervention, often obtained from a coding dictionary. | N/A | N/A | Char | O | N/A | CMCLAS | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This would generally be the class used for analysis. |
Interventions | CM | N/A | N/A | 30 | CMCLASCD | CM Medication Class Code | The assigned dictionary code for the class for the intervention. | N/A | N/A | Num | O | N/A | CMCLASCD | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Target". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This would generally be the class code used for analysis. |
Interventions | CM | N/A | N/A | 31 | CMATC1 | ATC Level 1 Description | Dictionary text description of the first level of hierarchy within the Anatomical Therapeutic Chemical Classification System. Indicates the anatomical main group. | N/A | N/A | Char | O | N/A | SUPPCM.Q VAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPCM dataset as the value of SUPPCM.QVAL where SUPPCM.QNAM= "CMATC1" and SUPPCM.QLABE L="ATC Level 1 Description". Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. | N/A | N/A | This field does not typically appear on the CRF. This is populated through the sponsor's coding process. |
Interventions | CM | N/A | N/A | 32 | CMATC1CD | ATC Level 1 Code | Dictionary code denoting the first level of hierarchy within the Anatomical Therapeutic Chemical Classification System. Indicates the anatomical main group. | N/A | N/A | Num | O | N/A | SUPPCM.Q VAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPCM dataset as the value of SUPPCM.QVAL where SUPPCM.QNAM ="MATC1CD" and SUPPCM.QLABE L="ATC Level 1 Code". Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. This is populated through the sponsor's coding process. |
Interventions | CM | N/A | N/A | 33 | CMATC2 | ATC Level 2 Description | Dictionary text description for the second level of hierarchy within the Anatomical Therapeutic Chemical Classification System. Indicates the therapeutic main group. | N/A | N/A | Char | O | N/A | SUPPCM.Q VAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPCM dataset as the value of SUPPCM.QVAL where SUPPCM.QNAM= "CMATC2" and SUPPCM.QLABE L="ATC Level 2 Description". Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. | N/A | N/A | This field does not typically appear on the CRF. This is populated through the sponsor's coding process. |
Interventions | CM | N/A | N/A | 34 | CMATC2CD | ATC Level 2 Code | Dictionary code denoting the second level of hierarchy within the Anatomical Therapeutic Chemical Classification System. Indicates the therapeutic main group. | N/A | N/A | Num | O | N/A | SUPPCM.Q VAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPCM dataset as the value of SUPPCM.QVAL where SUPPCM.QNAM= "CMATC2CD" and SUPPCM.QLABE L="ATC Level 2 Code". Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. This is populated through the sponsor's coding process. |
Interventions | CM | N/A | N/A | 35 | CMATC3 | ATC Level 3 Description | Dictionary text description of the third level of hierarchy within the Anatomical Therapeutic Chemical Classification System. Indicates the therapeutic/pharm acological subgroup. | N/A | N/A | Char | O | N/A | SUPPCM.Q VAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPCM dataset as the value of SUPPCM.QVAL where SUPPCM.QNAM= "CMATC3" and SUPPCM.QLABE L="ATC Level 3 Description". Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains.standard variables in SDTM domains. | N/A | N/A | This field does not typically appear on the CRF. This is populated through the sponsor's coding process. |
Interventions | CM | N/A | N/A | 36 | CMATC3CD | ATC Level 3 Code | Dictionary code denoting the third level of hierarchy within the Anatomical Therapeutic Chemical Classification System. Indicates the therapeutic/pharm acological subgroup. | N/A | N/A | Num | O | N/A | SUPPCM.Q VAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPCM dataset as the value of SUPPCM.QVAL where SUPPCM.QNAM= "CMATC3CD" and SUPPCM.QLABE L="ATC Level 3 Code". Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. This is populated through the sponsor's coding process. |
Interventions | CM | N/A | N/A | 37 | CMATC4 | ATC Level 4 Description | Dictionary text description of the fourth level of hierarchy within the Anatomical Therapeutic Chemical Classification System. Indicates the chemical/therapeut ic/pharmacological subgroup. | N/A | N/A | Char | O | N/A | SUPPCM.Q VAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPCM dataset as the value of SUPPCM.QVAL where SUPPCM.QNAM= "CMATC4" and SUPPCM.QLABE L="ATC Level 4 Description". Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. | N/A | N/A | This field does not typically appear on the CRF. This is populated through the sponsor's coding process. |
Interventions | CM | N/A | N/A | 38 | CMATC4CD | ATC Level 4 Code | Dictionary code denoting the fourth level of hierarchy within the Anatomical Therapeutic Chemical Classification System. Indicates the chemical/therapeut ic/pharmacological subgroup. | N/A | N/A | Num | O | N/A | SUPPCM.Q VAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPCM dataset as the value of SUPPCM.QVAL where SUPPCM.QNAM= "CMATC4CD" and SUPPCM.QLABE L="ATC Level 4 Code". Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. This is populated through the sponsor's coding process. |
Interventions | CM | N/A | N/A | 39 | CMATC5 | ATC Level 5 Description | Dictionary text description of the fifth level of hierarchy within the Anatomical Therapeutic Chemical Classification system. Indicates the chemical substance. | N/A | N/A | Char | O | N/A | SUPPCM.Q VAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPCM dataset as the value of SUPPCM.QVAL where SUPPCM.QNAM= "CMATC5" and SUPPCM.QLABE L="ATC Level 5 Description". Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. | N/A | N/A | This field does not typically appear on the CRF. This is populated through the sponsor's coding process. |
Interventions | CM | N/A | N/A | 40 | CMATC5CD | ATC Level 5 Code | Dictionary code denoting the fifth level of hierarchy within the Anatomical Therapeutic Chemical Classification system. Indicates the chemical substance. | N/A | N/A | Num | O | N/A | SUPPCM.Q VAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPCM dataset as the value of SUPPCM.QVAL where SUPPCM.QNAM= "CMATC5CD" and SUPPCM.QLABE L="ATC Level 5 Code". Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. This is populated through the sponsor's coding process. |
Assumptions for the CDASHIG CM - Prior and Concomitant Medications Domain
1. General medications/treatments are defined as any medications/treatments reported by a subject when asked if they have taken any medications in an open-ended way that does not ask about any specific drug. Additional information might be sourced by referring to a subject’s medical record.
2. Medications of interest are defined as any medications or classes of drugs specifically mentioned in the protocol and were not the primary focus for determining the CDASHIG Core designations for the domain (e.g., excluded medications, drugs requiring a washout period prior to dosing in study, rescue medications).
3. As with all the data collection variables recommended in the CDASH standard, it is assumed that sponsors will add other data variables as needed to meet protocol-specific and other data collection requirements (e.g., therapeutic area-specific data elements and others as required per protocol, business practice, and operating procedures).
4. The CMOCCUR field provides a structure for capturing the occurrence of specific medications of interest.
5. Sponsors wishing to capture non-pharmacological therapies (e.g., surgery procedures, aromatherapy, massage, acupuncture) can use the Procedures (PR) domain.
Example CRF for the CDASHIG CM - Prior and Concomitant Medications Domain
Example 1
Title: Concomitant Medications
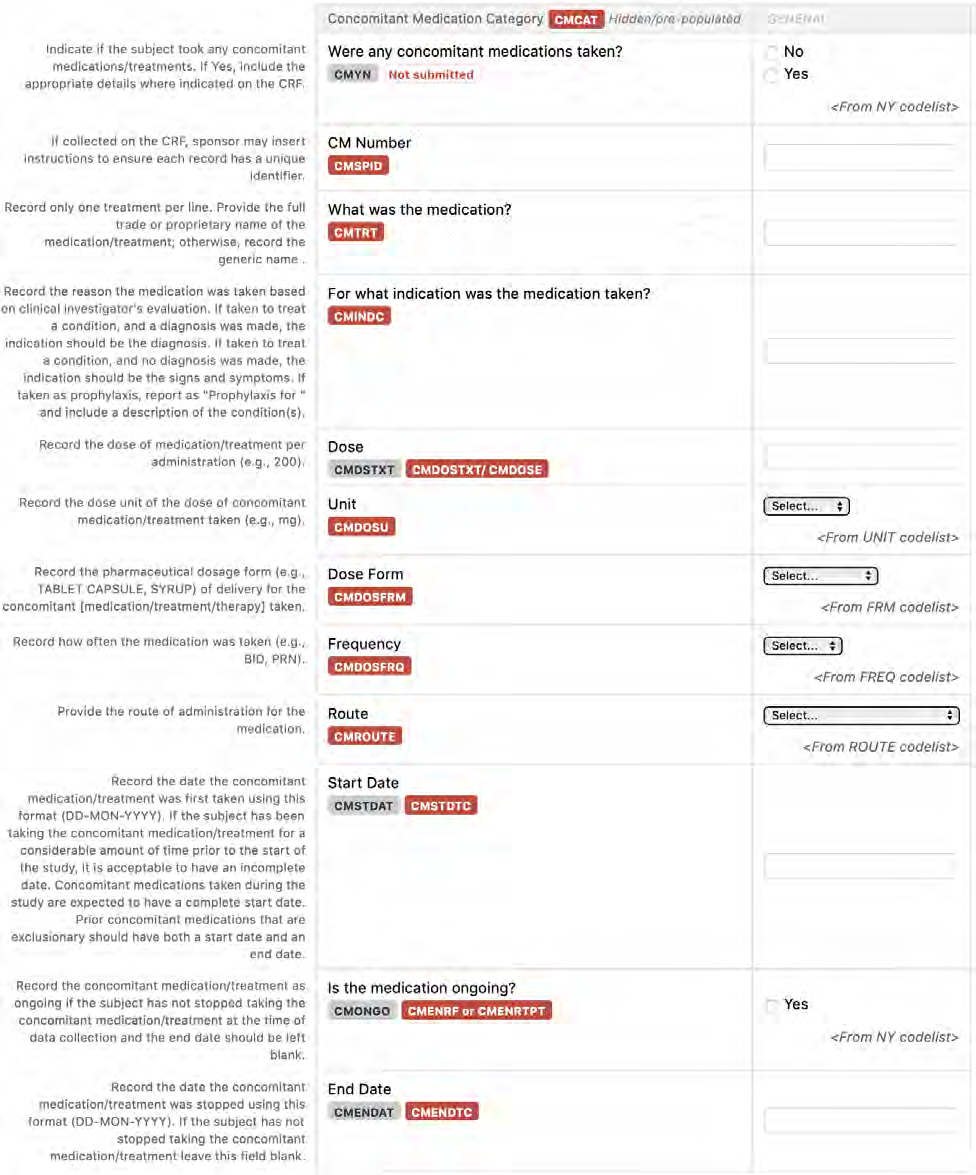
CRF Metadata
| Order | CDASH Variable | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | CMCAT | What is the category for the medication? | Concomitant Medication Category | Record the medication category, if not preprinted on the CRF. | Text | CMCAT |
GENERAL |
Yes | |||||
2 | CMYN | Were any concomitant medications taken? | Any Concomitant Medications | Indicate if the subject took any concomitant medications/treatments. If Yes, include the appropriate details where indicated on the CRF. | Text | N/A |
(NY) | No; Yes |
|||||
3 | CMSPID | What is the medication identifier? | CM Number | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | Text | CMSPID | prompt |
||||||
4 | CMTRT | What was the medication? | Concomitant Medication | Record only one treatment per line. Provide the full trade or proprietary name of the medication/treatment; otherwise, record the generic name . | Text | CMTRT | |||||||
6 | CMINDC | For what indication was the medication taken? | Indication | Record the reason the medication was taken based on clinical investigator's evaluation. If taken to treat a condition, and a diagnosis was made, the indication should be the diagnosis. If taken to treat a condition, and no diagnosis was made, the indication should be the signs and symptoms. If taken as prophylaxis, report as "Prophylaxis for " and include a description of the condition(s). | Text | CMINDC | |||||||
7 | CMDSTXT | What was the individual dose of the medication? | Dose | Record the dose of medication/treatment per administration (e.g., 200). | Text | CMDOSTXT; CMDOSE | CMDOSTXT/ CMDOSE | prompt | |||||
8 | CMDOSU | What is the unit? | Unit | Record the dose unit of the dose of concomitant medication/treatment taken (e.g., mg). | Text | CMDOSU | (UNIT) | CAPSULE; g; IU; mg; mL; PUFF; TABLET; ug | prompt | ||||
9 | CMDOSFRM | What was the dose form of the medication? | Dose Form | Record the pharmaceutical dosage form (e.g., TABLET CAPSULE, SYRUP) of delivery for the concomitant [medication/treatment/therapy] taken. | Text | CMDOSFRM | (FRM) | AEROSOL; CAPSULE; CREAM; GAS; GEL; OINTMENT; PATCH; POWDER; SPRAY; SUPPOSITORY; SUSPENSION; TABLET | prompt | ||||
10 | CMDOSFRQ | What was the frequency of the medication? | Frequency | Record how often the medication was taken (e.g., BID, PRN). | Text | CMDOSFRQ | (FREQ) | BID; PRN; QD; QID; QM; QOD; TID | prompt | ||||
11 | CMROUTE | What was the route of administration of the medication? | Route | Provide the route of administration for the medication. | Text | CMROUTE | (ROUTE) | INTRALESIONAL; INTRAMUSCULAR; INTRAOCULAR; INTRAPERITONEAL; NASAL; ORAL; RECTAL; RESPIRATORY (INHALATION); SUBCUTANEOUS; TOPICAL; TRANSDERMAL; VAGINAL | prompt | ||||
12 | CMSTDAT | What was the start date? | Start Date | Record the date the concomitant medication/treatment was first taken using this format (DD-MON-YYYY). If the subject has been taking the concomitant medication/treatment for a considerable amount of time prior to the start of the study, it is acceptable to have an incomplete date. Concomitant medications taken during the study are expected to have a complete start date. Prior concomitant medications that are exclusionary should have both a start date and an end date. | Date | CMSTDTC | prompt | ||||||
13 | CMONGO | Is the medication ongoing? | Ongoing | Record the concomitant medication/treatment as ongoing if the subject has not stopped taking the concomitant medication/treatment at the time of data collection and the end date should be left blank. | Text | CMENRF; CMENRTPT | CMENRF or CMENRTPT | (NY) | Yes | checkbox | |||
14 | CMENDAT | What was the end date? | End Date | Record the date the concomitant medication/treatment was stopped using this format (DD-MON-YYYY). If the subject has not stopped taking the concomitant medication/treatment leave this field blank. | Date | CMENDTC | prompt |
8.1.3 EC - Exposure as Collected and EX - Exposure
Description/Overview for the CDASHIG EC - Exposure as Collected and CDASHIG EX - Exposure Domains
In the SDTMIG, the Exposure (EX) domain is used to represent exposure to study treatment as described in the protocol. The Exposure as Collected (EC) domain is used to represent the data as collected on the CRF. The CDASHIG EC domain is used in a study when the SDTMIG EX domain cannot be directly populated with the data collected on the CRF.
CDASHIG EC is used when:
1. An alias for the actual treatment name is used (e.g., the study is masked) rather than the actual treatment name (e.g., the study is open label).
2. Exposure data are collected in non-protocol-specified units (e.g., tablets).
3. Scheduled and/or missed doses are collected.
4. Planned doses (e.g., a calculation based on body weight) are collected in addition to actual doses given.
A sponsor may choose to always collect exposure data using the CDASHIG EC domain.
Extensive discussion of the use of the EC and EX domains, including examples of data collection, is available in the SDTMIG.
Specification for the CDASHIG EC - Exposure as Collected and EX - Exposure Domains
Exposure as Collected Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Interventions | EC | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Interventions | EC | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Interventions | EC | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Interventions | EC | N/A | N/A | 4 | EPOCH | Epoch | Name of the Trial Epoch with which this Element of the Arm is associated. | What is the trial epoch? | [Epoch](Period/Phase/ Sponsor-defined phrase) | Char | R/C | [protocol specific] | EPOCH | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EPOCH) | N/A | If the same information is collected more than once in different periods/parts of a study (e.g., Disposition), EPOCH may be needed to differentiate them. Typically, the trial epoch will be preprinted on the CRF as the title of the page. See SDTMIG for further information regarding EPOCH. |
Interventions | EC | N/A | N/A | 5 | ECYN | Any Study Treatment Taken | An indication whether or not the subject took study medication/ treatment. | Were any[study treatment/dose] taken? | Any Study Treatments | Char | O | Indicate if the subject took any study medications. If Yes, include the appropriate details where indicated. | N/A | Does not map to an SDTMIG variable. The SDTM aCRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification that all other fields on the CRF were deliberately left blank. The ECYN is meant to indicate that the exposure as collected form should be completed or inserted into the case book. ECOCCUR would be used when the actual drug name is preprinted on the CRF. While these might be equivalent in a single drug study, there are differences in how they would be used in most trials. Therefore, it does not map into the SDTM variable ECOCCUR, since ECOCCUR indicates whether the subject was actually administered treatment/medication. If actual treatment data is available (ECYN ="Y"), ECOCCUR may be populated based on whether subject was actually administered treatment/medication. |
Interventions | EC | N/A | N/A | 6 | ECCAT | Category of Treatment | A grouping of topic- variable values based on user defined characteristics. | What is the category of the [study treatment/dose]? | [Study Treatment Category]; NULL | Char | O | Record the study treatment category, if not pre-printed on the CRF. | ECCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Interventions | EC | N/A | N/A | 7 | ECSCAT | Subcategory of Treatment | A sub-division of the ECCAT values based on user-defined characteristics. | What is the subcategory of the [study treatment/dose]? | [Study Treatment Subcategory]; NULL | Char | O | Record the study treatment subcategory, if not pre-printed on the CRF. | ECSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. ECSCAT can only be used if there is an ECCAT and it must be a subcategorization of ECCAT. |
Interventions | EC | N/A | N/A | 8 | ECTRT | Treatment | Name of the intervention or treatment known to the subject and/or administrator. | What was the [study treatment/investigational product] name? | [Study Treatment/Investigational Product Name] | Char | R/C | Record the name of study treatment. | ECTRT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | ECTRT is the name of the intervention or treatment known to the subject and/or administrator and it is the SDTM topic variable. It is a equired variable in SDTM and must have a value in CDASH or a plan to populate it in the SDTM submission datasets (i.e., collected or populated from other sources). In a masked study, if treatment is collected and known as Tablet A to the subject or administrator, then ECTRT="TABLET A". If in a masked study the treatment is not known by a synonym and the data are to be exchanged between sponsors, partners and/or regulatory agency(s), then assign ECTRT the value of "MASKED". |
Interventions | EC | N/A | N/A | 9 | ECPRESP | Exposure as Collected Pre- Specified | An indication that a specific intervention or a group of interventions is prespecified on a CRF. | N/A | N/A | Char | O | N/A | ECPRESP | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | For prespecified interventions, a hidden field on a CRF defaulted to "Y", or added during the SDTM dataset creation. If a study collects both prespecified interventions as well as free-text interventions, the value of ECPRESP should be "Y" for all prespecified interventions and null for interventions reported as free-text. |
Interventions | EC | N/A | N/A | 10 | ECOCCUR | Exposure as Collected Occurrence | An indication whether the study treatment was administered when information about the occurrence of a specific intervention is solicited. | Was [study treatment/dose] administered?; Has the subject taken [study treatment/dose]? | [Study Medication/Treatment] | Char | O | Indicate if the subject took study treatment. If Yes, include the appropriate details where indicated. | ECOCCUR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. Not applicable when ECMOOD is "Scheduled". | (NY) | N/A | ECOCCUR is used to indicate whether the subject was actually administered treatment/medication. ECOCCUR should not be used to indicate that the question was not asked or answered. |
Interventions | EC | N/A | N/A | 11 | ECREASOC | Exposure Reason for Occur Value | An explanation of why a scheduled study treatment administration did or did not occur. | What was the reason that the[study treatment/dose] was (not)taken? | Reason (Not) Taken | Char | O | Indicate why the study treatment was or wasnot taken. | SUPPEC.QVAL | This information could be submitted in a SUPPEC dataset as the value of SUPPEC.QVAL where SUPPEC.QNAM="ECREASOC" and SUPPEC.QLABEL ="Reason for Occur Value". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | N/A | N/A | The reason the study treatment was or was not taken may be chosen from a sponsor-defined codelist (e.g., SUBJECT MISTAKE, SUBJECT REFUSED) or entered as free text. When --REASOC is used, --OCCUR must also be populated in the SDTM dataset with a value of "Y" or "N". |
Interventions | EC | N/A | N/A | 12 | ECMOOD | Exposure as Collected Mood | Mode or condition of the record specifying whether the intervention (activity) is intended to happen or has happened. | Does this record describe scheduled [study treatment/dose] or performed [study treatment/dose]? | Scheduled/Performed | Char | O | Indicate if this record has happened or is intended to happen. | ECMOOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. When implemented ECMOOD must be populated for all records. | (BRDGMOOD) | N/A | "SCHEDULED" is for collected subject-level intended dose records. "PERFORMED" is for collected subject-level actual dose records. "Planned" or "Scheduled" can be pre-printed as the CRF name or section header, as applicable. If collecting both the scheduled and performed dosing in the same horizontal record, the sponsor may choose to append "_SCHEDULED" to the ECDOSE/ECDOSTXT variable name to delineate the scheduled dose from the performed dose. The performed dose would just be collected with ECDOSE/ECDOSTXT and ECDOSU. |
Interventions | EC | N/A | N/A | 13 | ECREFID | Exposure as Collected Reference ID | An internal or external identifier such as kit number, bottle label, or vial identifier. | What is the[study treatment/dose] label identifier? | [Study Treatment] Label Identifier | Char | O | Record treatment label identifier. | ECREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This packaging identifier (e.g., kit number, bottle label, vial identifier) may be collected in different ways (e.g., affixing label onto CRF, scanning a bar code). For some study dosing regimens, greater granularity for treatment identifiers may be needed. In this situation, sponsors may need to use additional variables. |
Interventions | EC | N/A | N/A | 14 | ECLOT | Lot Number | Lot Number of the ECTRT product. | What was the lot number of the[study treatment/dose] used? | Lot Number | Char | R/C | Record the lot number that appears on the container holding the study treatment. | ECLOT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | The Lot Number identifies the manufacturing batch of the study treatment. In open-label studies, the reference number on the study treatment container may represent an actual Lot Number and should be submitted using ECLOT. This variable may be populated during the process of creating the SDTM submission datasets. Do not collect other identification variables in this field. |
Interventions | EC | N/A | N/A | 15 | ECFAST | Exposure as Collected Fasting Status | An indication that the subject has abstained from food/water for the specified amount of time. | Was the subject fasting? | Fasting | Char | O | Record whether the subject was fasting prior to the study treatment being taken. | ECFAST | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. Would only be populated when ECMOOD="PERFORMED". | (NY) | N/A | Some study treatments may have a food effect and it is important to know whether the dose was taken while the subject was fasted. |
Interventions | EC | N/A | N/A | 16 | ECDOSFRM | Exposure as Collected Dose Form | The dosage form in which the ECTRT is physically presented | What was the dose form of the [study treatment/dose]? | Dose Form | Char | R/C | Record the dose form (e.g., SOLUTION, TABLET, LOTION) or enter the appropriate code from the code list. | ECDOSFRM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (FRM) | (EXDOSFRM) | This must be collected if it cannot be determined from other sources or if there are multiple options for the same study treatment. |
Interventions | EC | N/A | N/A | 17 | ECSTDAT | Exposure as Collected Start Date | The start date of study treatment, intended or actual, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the ([intended/planned/actual]) ([study treatment/dose]) (start) date? | (Start) Date | Char | HR | Record the start date of the study treatment administration using this format (DD-MON-YYYY). | ECSTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable ECSTDTC in ISO 8601 format. | N/A | N/A | Date when constant dosing interval of the study treatment started or single administration occurred. When collecting the date for an individual dose, the word "start" may be omitted from the Question Text and Prompt. When ECMOOD is collected and ECMOOD is "SCHEDULED", use "intended" in the question text and prompt. When ECMOOD is collected and ECMOOD is "PERFORMED", use "actual" in the question text and prompt. |
Interventions | EC | N/A | N/A | 18 | ECSTTIM | Exposure as Collected Start Time | The start time of study treatment, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the ([intended/planned/actual]) ([study treatment/dose]) (start) time? | (Start) Time | Char | R/C | Record the start time (as complete as possible) when administration of study treatment started. | ECSTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable ECSTDTC in ISO 8601 format. | N/A | N/A | Recommend collecting the time a medication was started only when a protocol or data collection scenarios requires it. When collecting the time for an individual dose, the word "start" may be omitted from the Question Text and Prompt. |
Interventions | EC | N/A | N/A | 19 | ECENDAT | Exposure as Collected End Date | The end date of study treatment, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the ([intended/planned/actual]) ([study treatment/dose]) (end) date? | (End) Date | Char | R/C | Record the end date of the study treatment administration using this format (DD-MON-YYYY). | ECENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable ECENDTC in ISO 8601 format. | N/A | N/A | Date when study treatment period stopped.If start date and end date are not expected to be on the same date, the collection of the end date is required. If the study design indicates that the start and end date are on the same day, the collection of the end date is not required because it can be assigned to be equal to the start date. |
Interventions | EC | N/A | N/A | 20 | ECENTIM | Exposure as Collected End Time | The end time of study treatment, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the ([intended/planned/actual]) ([study treatment/dose]) (end) time? | (End) Time | Char | R/C | Record the time, (as complete as possible) when study treatment administration stopped (e.g., for infusions this is the time when the infusion ended). | ECENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable ECENDTC in ISO 8601 format. | N/A | N/A | Recommend collecting the time a medication was ended when a protocol or data collection scenarios requires it. For infusions, the end time of the infusion is typically needed. |
Interventions | EC | N/A | N/A | 21 | ECDSTXT | Exposure as Collected Dose Description | The dose of study medication taken (per administration). | What was the dose (per administration) (of [study treatment/dose])? | Dose | Char | R/C | Record the dose or amount of study treatment that was administered to/taken by the subject in the period recorded; from the start date/time to the end date/time inclusive. | ECDOSTXT; ECDOSE | This does not map directly to an SDTMIG variable. The data collected in this dose text-format field should be mapped to either ECDOSE if numeric or ECDOSTXT if text. | N/A | N/A | Dose or amount taken for single administration of study treatment or per constant dosing interval recorded. Dose must be collected if it cannot be determined via other methods (e.g., from diary data, drug accountability data, protocol). Care should be taken when mapping ECDSTXT. The data collected in this dose text-format field should be separated or mapped to either ECDOSE if numeric or ECDOSTXT if text. |
Interventions | EC | N/A | N/A | 22 | ECDOSU | Exposure as Collected Dose Units | The unit for intended dose (per administration) for ECDOSE, ECDOSTOT, or ECDOSTXT. | What were the units for the dose? | Units | Char | R/C | Record the unit of dose or amount taken per period recorded (e.g., ng, mg, mg/kg). | ECDOSU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | (EXDOSU) | Unit of dose or amount taken per constant dosing interval recorded. Dose unit must be collected if it cannot be determined via other methods (e.g., determined from protocol, randomization data). The unit should be preprinted on the CRF or a field provided on the CRF to capture it. A CDASH Subset Controlled Terminology Codelist Name is available for dose and volume units. In blinded trials, the collected unit may be tablet, capsule, etc., since the actual unit is also blinded. |
Interventions | EC | N/A | N/A | 23 | ECDOSFRQ | EC Dosing Frequency per Interval | The number of doses given/administered/taken during a specific interval. | What was the frequency of [study treatment/dose] dosing? | Frequency | Char | R/C | Record the frequency the study treatment was administered for a defined period of time (e.g., BID, QID, TID). | ECDOSFRQ | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (FREQ) | (EXDOSFRQ) | This may be collected if it cannot be determined from other sources or if there are multiple options. When possible, the options for dose/amount frequency are pre- printed on the CRF. When collected, the recommendation is to collect dosing information in separate fields (e.g., ECDOSE, ECDOSEU, ECDOSFRQ) for specific and consistent data collection and to enable programmatically utilizing these data. |
Interventions | EC | N/A | N/A | 24 | ECROUTE | EC Route of Administration | The route of administration of the study treatment. | What was the route of administration (of the [study treatment/dose])? | Route | Char | R/C | Record the route of administration (e.g., IV, ORAL, TRANSDERMAL) or enter the appropriate code from the code list. | ECROUTE | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (ROUTE) | (EXROUTE) | This may be collected if it cannot be determined via other methods (e.g., from protocol) or if there are multiple options. |
Interventions | EC | N/A | N/A | 25 | ECDOSRGM | Intended Dose Regimen | The text description of the (intended) schedule or regimen for the Intervention. | What was the intended dose regimen? | Intended Dose Regimen | Char | O | Record the regimen for the study medication. | ECDOSRGM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | The regimen information (e.g., TWO WEEKS ON, TWO WEEKS OFF) may further clarify the dose administration and dose frequency. This may be preprinted or collected. The sponsor may wish to create a codelist to collect this data consistently. |
Interventions | EC | N/A | N/A | 26 | ECDOSADJ | Dose Adjusted | An indication whether or not the dose was adjusted. | Was the dose adjusted? | (Dose) Adjusted | Char | O | Select either Yes or No to indicate whether there was a change in dosing. | N/A | When ECADJ is collected, does not map to an SDTMIG variable. The SDTM Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. When ECADJ is not collected, the sponsor may submit this variable in SUPPEC. | (NY) | N/A | Typically, the intent/purpose of collecting this field is to help with data cleaning and monitoring as it provides a definitive response regarding any dose changes. It provides verification that the associate field on the CRF (ECADJ) was deliberately left blank. However, the sponsor may collect whether the dose was adjusted, without collecting the reason for the change. When using ECMOOD, this field should not be used. |
Interventions | EC | N/A | N/A | 27 | ECADJ | Reason for Dose Adjustment | Description or explanation of why a dose of the study treatment is adjusted. | What was the reason the dose was adjusted? | Reason Adjusted | Char | O | If there was a change in dosing, record the reason for change. | ECADJ | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Captures the reason the dose was changed or modified. The reason may be chosen from a sponsor-defined list (e.g., adverse event, insufficient response) or entered as free text. May be used for variations from protocol-specified doses, or changes from expected doses. Used only when an adjustment is represented in EX dataset. |
Interventions | EC | N/A | N/A | 28 | ECITRPYN | EC Exposure Interrupted | An indication whether of not the exposure was interrupted. | Was the [(study) treatment/dose] interrupted? | [(Study) Treatment / Dose] Interrupted | Char | O | Record if there was an interruption in the study treatment or dosing. | N/A | Does not map to an SDTMIG variable. The SDTMIG Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring when the actual duration of the exposure is collected using the CDASH field ECCINTD. In some situations, if the actual duration of the interruption is not collected, or not derived, this information could be submitted in a SUPPEC.QVAL dataset where SUPPEC.QNAM = "ECITRPYN" and SUPPEC.QLABEL = "Exposure Interrupted". |
Interventions | EC | N/A | N/A | 29 | ECCINTD | EC Interruption Duration | The collected duration of the treatment interruption. | What was the duration of the treatment interruption? | (Interruption) Duration | Char | O | Record the duration of treatment interruption. | SUPPEC.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPEC dataset as the value of SUPPEC.QVAL where SUPPEC.QNAM="ECITRPD" and SUPPEC.QLABEL= "Interruption Duration". Concatenate the collected treatment interruption duration and the duration unit components and create ECITRPD using ISO 8601 Period format. Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | N/A | N/A | This field is used to collect the duration of treatment interruption. In some situations, the duration of the interruption may not be collected but calculated from the treatment start and end times recorded elsewhere in the CRF. |
Interventions | EC | N/A | N/A | 29 | ECCINTDU | EC Interruption Duration Units | The unit for the collected duration of treatment interruption. | What was the interruption duration unit? | (Interruption Duration) Unit | Char | O | Record the unit (e.g., MINUTES, HOURS, DAYS) for the duration of treatment interruption. | SUPPEC.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPEC dataset as the value of SUPPEC.QVAL where SUPPEC.QNAM = "ECITRPD" and SUPPEC.QLABEL= "Interruption Duration". Concatenate the collected treatment interruption duration and the duration unit components and create ECITRPD using ISO 8601 Period format. Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (UNIT) | (EXCINTDU) | The unit should be collected as a qualifier to the number for duration. |
Interventions | EC | N/A | N/A | 30 | ECLOC | EC Location of Dose Administration | A description of the anatomical location of administration. | What was the anatomical location of the ([study treatment/dose]) administration? | Anatomical Location | Char | O | Record the body location where the study treatment was administered (e.g., SHOULDER, HIP, ARM). | ECLOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location where the study treatment was administered. LAT, DIR, PORTOT are used to further describe the anatomical location. |
Interventions | EC | N/A | N/A | 31 | ECLAT | Exposure as Collected Laterality | Qualifier for anatomical location further detailing side of the body for the study treatment administration. | What was the side of the anatomical location of the ([study treatment/dose]) administration? | Side | Char | O | Record the side of the body location where the study treatment was administered (e.g., Left, Right). | ECLAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | Further details the laterality of the location where the study treatment was administered. This may be preprinted or collected. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Interventions | EC | N/A | N/A | 32 | ECDIR | Exposure as Collected Directionality | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What was the directionality of the anatomical location of the ([study treatment/dose]) administration? | Directionality | Char | O | Record the directionality of the body location where the study treatment was administered (e.g., Anterior, Lower, Proximal, Upper). | ECDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Interventions | EC | N/A | N/A | 33 | ECVAMT | EC Vehicle Amount | The amount of the prepared product (treatment + vehicle) administered or given. | What was the total amount (Drug + Vehicle) (of [study treatment/dose]) administered? | Total Amount (Drug + Vehicle) | Num | O | Record the total amount (treatment +vehicle) that was administered/given to the subject. | ECVAMT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTM variable ECTRTV may also be populated during the process of creating the SDTM submission datasets. | N/A | N/A | Administration amount that was given to the subject. Note: should not be the diluent amount alone. The ECTRTV field may be collected if it cannot be determined from other sources. |
Interventions | EC | N/A | N/A | 34 | ECVAMTU | EC Vehicle Amount Units | The unit of measurement for the prepared product (treatment + vehicle). | What was the unit for the amount (of [study treatment/dose]) administered? | Unit | Char | O | Record the unit of total amount (treatment +vehicle) administered/given to the subject (e.g., mL). | ECVAMTU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | N/A | Unit of the administration amount. |
Interventions | EC | N/A | N/A | 35 | ECFLRT | Exposure as Collected Infusion Rate | The flow rate for the total amount of drug + vehicle administered to the subject. | What was the [study treatment/dose] infusion rate? | Infusion Rate | Num | O | Record the Rate of Infusion (e.g., if rate is 10 mL/min. Record 10 as the infusion rate). | SUPPEC.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPEC dataset as the value of SUPPEC.QVAL where SUPPEC.QNAM="ECFLRT" and SUPPEC.QLABEL= "Infusion Rate". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | N/A | N/A | Infusion rate can be used to derive dose. |
Interventions | EC | N/A | N/A | 36 | ECFLRTU | Exposure as Collected Infusion Rate Unit | The unit of measure for the flow rate for the total amount of drug + vehicle administered to the subject. | What was the unit for the ([study treatment/dose]) infusion rate? | Infusion Rate Unit | Char | O | Record the unit for the infusion rate (e.g., mL/min). | SUPPEC.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPEC dataset as the value of SUPPEC.QVAL where SUPPEC.QNAM="ECFLRTU" and SUPPEC.QLABEL= "Infusion Rate Unit". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (UNIT) | (EXFLRTU) | Unit of the infusion rate. |
Interventions | EC | N/A | N/A | 37 | ECTPT | EC Planned Time Point Name | A text description of planned time point when measurements should be taken as defined in the protocol. | What was the planned time point for [study treatment/dose]? | [Planned Time Point Name] | Char | R/C | Record the planned time point of study treatment administration if not pre-printed on the CRF. | ECTPT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. See SDTMIG for additional information on representing time points. The SDTMIG time point anchors ECTPTREF (text description) and ECRFTDTC (date/time) may be needed, as well as SDTMIG variables ECTPTNUM, ECELTM. | N/A | N/A | Planned time points are needed to differentiate multiple sequential assessments. It is recommended that time points should be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as Planned Time Point can be included as the column header. |
Interventions | EC | N/A | N/A | 38 | ECTRTCMP | Completed Treatment | An indication whether the subject completed the intended regimen. | Did the subject complete the full course of [study treatment/dose]? | Completed Treatment | Char | O | Select either Yes or No to indicate whether subject has completed the full course of treatment. | SUPPEC.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPEC dataset as the value of SUPPEC.QVAL where SUPPEC.QNAM = "ECTRTCMP" and SUPPEC.QLABEL ="Completed Treatment". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | Depending on how the study treatment details are collected via the CRF/eCRF, it may be possible to derive those data if the regimen data are collected. |
Exposure Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Interventions | EX | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Interventions | EX | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Interventions | EX | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Interventions | EX | N/A | N/A | 4 | EPOCH | Epoch | Name of the Trial Epoch with which this Element of the Arm is associated. | What is the trial epoch? | [Epoch](Period/Phase/Sponsor-defined phrase) | Char | R/C | [protocol specific] | EPOCH | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EPOCH) | N/A | If the same information is collected more than once in different periods/parts of a study (e.g., Disposition), EPOCH may be needed to differentiate them. Typically, the trial epoch will be preprinted on the CRF as the title of the page. See SDTMIG for further information regarding EPOCH. |
Interventions | EX | N/A | N/A | 5 | EXYN | Any Study Treatment Taken | An indication whether or not the subject took study medication/treatment. | Were any[study treatment/dose] taken? | Any Study Treatments | Char | O | Indicate if the subject took any study medications. If Yes, include the appropriate details where indicated. all other fields on the | N/A | Does not map to an SDTMIG variable. The SDTM aCRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification that all other fields on the CRF were deliberately left blank. The EXYN variable is a cleaning or EDC convention meant to indicate that the exposure form should be completed or inserted into the case book. |
Interventions | EX | N/A | N/A | 6 | EXCAT | Category of Treatment | A grouping of topic- variable values based on user-defined characteristics. | What is the category of the [study treatment/dose] ? | [Study Treatment Category]; NULL | Char | O | Record the study treatment category, if not preprinted on the CRF. | EXCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Interventions | EX | N/A | N/A | 7 | EXSCAT | Subcategory of Treatment | A sub-division of the EXCAT values based on user-defined characteristics. | What is the subcategory of the [study treatment/dose] ? | [Study Treatment Subcategory]; NULL | Char | O | Record the study treatment subcategory, if not preprinted on the CRF. | EXSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. EXSCAT can only be used if there is an EXCAT and it must be a subcategorization of EXCAT. |
Interventions | EX | N/A | N/A | 8 | EXTRT | Name of Treatment | Name of the study treatment or intervention given per single administration or during the "constant dosing interval" for the observation. | What was the [study treatment/investigational product] name? | [Study Treatment/Investigational Product Name] | Char | R/C | Record the name of study treatment. | EXTRT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | EXTRT captures the name of the investigational treatment. This is typically collected for open label studies and populated for blinded studies during the SDTM-based dataset creation. Since EXTRT is the SDTMIG topic variable, it is a required variable in SDTM and must have a value in CDASH or a plan to populate it in the SDTM submission datasets (i.e., collected or populated from other sources). |
Interventions | EX | N/A | N/A | 9 | EXREFID | Exposure Reference ID | An internal or external identifier such as kit number, bottle label, vial identifier. | What is the [study treatment/dose] label identifier? | Treatment Label Identifier | Char | R/C | Record treatment label identifier. | EXREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This packaging identifier (e.g., kit number, bottle label, vial identifier) may be collected in different ways (e.g., affixing label onto CRF, scanning a bar code). For some study dosing regimens, greater granularity for treatment identifiers may be needed. |
Interventions | EX | N/A | N/A | 10 | EXLOT | Lot Number | Lot Number of the EXTRT product. | What was the lot number of the[study treatment/dose] used? | Lot Number | Char | R/C | Record the lot number that appears on the container holding the study treatment. | EXLOT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | The Lot Number identifies the manufacturing batch of the study treatment. In open label studies, the reference number on the study treatment container may represent an actual Lot Number and is submitted using EXLOT. This variable may be populated during the process of creating the SDTM submission datasets. Do not collect other identification variables in this field. |
Interventions | EX | N/A | N/A | 11 | EXFAST | Exposure Fasting Status | An indication that the subject has abstained from food/water for the specified amount of time. | Was the subject fasting? | Fasting | Char | O | Record whether the subject was fasting prior to the study treatment being taken. | EXFAST | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | Some study treatments may have a food effect and it is important to know whether the dose was taken while the subject was fasted. |
Interventions | EX | N/A | N/A | 12 | EXDOSFRM | Exposure Dose Form | The dosage form in which the EXTRT is physically presented. | What was the dose form of the [study treatment/dose] ? | Dose Form | Char | R/C | Record the dose form (e.g., SOLUTION, TABLET, LOTION) or enter the appropriate code from the code list. | EXDOSFRM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (FRM) | (EXDOSFRM) | This must be collected if it cannot be determined from other sources or if there are multiple options. |
Interventions | EX | N/A | N/A | 13 | EXSTDAT | Exposure Start Date | The [start] date of study treatment, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the ([intended/planned/actual]) ([study treatment/dose]) (start) date? | (Start) Date | Char | HR | Record the start date of the study treatment administration using this format (DD-MON-YYYY). | EXSTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable EXSTDTC in ISO 8601 format. | N/A | N/A | Date when constant dosing interval of the study treatment started or single administration occurred. When collecting the date for an individual dose, the word "start" may be omitted from the Question Text and Prompt. |
Interventions | EX | N/A | N/A | 14 | EXSTTIM | Exposure Start Time | The [start] time of the study treatment, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the ([intended/planned/actual]) ([study treatment/dose]) (start) time? | (Start) Time | Char | R/C | Record the start time (as complete as possible) when administration of study treatment started. | EXSTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable EXSTDTC in ISO 8601 format. | N/A | N/A | Recommend collecting the time a medication was started only when a protocol or data collection scenarios requires it. |
Interventions | EX | N/A | N/A | 15 | EXENDAT | Exposure End Date | The end date of study treatment, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the ([intended/planned/actual]) ([study treatment/dose]) (end) date? | (End) Date | Char | R/C | Record the end date or last date of administration of study treatment using this format (DD-MON-YYYY). | EXENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable EXENDTC in ISO 8601 format. | N/A | N/A | If start date and end date are not expected to be on the same date, the end date is required. If the study design indicates that the start and end date are on the same day, the end date is not required since it can be assigned to be equal to the start date. |
Interventions | EX | N/A | N/A | 16 | EXENTIM | Exposure End Time | The end time of treatment, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the ([intended/planned/actual]) ([study treatment/dose]) (end) time? | (End) Time | Char | R/C | Record the time, (as complete as possible) when study treatment administration stopped (e.g., for infusions this is the time when the infusion ended). | EXENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable EXENDTC in ISO 8601 format. | N/A | N/A | Recommend collecting the time a medication was ended when a protocol or data collection scenarios requires it. For infusions, the end time of the infusion is typically needed. |
Interventions | EX | N/A | N/A | 17 | EXDSTXT | Exposure Dose Description | Dose [per administration]. | What was the dose [per administration] (of [study treatment/dose]) ? | Dose | Char | R/C | Record the dose or amount of study treatment that was administered to/taken by the subject in the period recorded; from the start date/time to the end date/time inclusive. | EXDOSTXT;EXDOSE | This does not map directly to an SDTMIG variable. Numeric values map to EXDOSE in SDTM. Non- numeric values (e.g., 200- 400) map to EXDOSTXT in SDTM. | N/A | N/A | Dose or amount taken for single administration of study treatment or per constant dosing interval recorded. Dose must be collected if it cannot be determined via other methods (e.g., from diary data, drug accountability data, protocol). The data collected in this dose text-format field should be mapped to either SDTMIG variable EXDOSE (if numeric) or EXDOSTXT (if text). |
Interventions | EX | N/A | N/A | 18 | EXDOSU | Exposure Dose Unit | The unit for intended dose [per administration] for EXDOSE, EXDOSTOT, or EXDOSTXT. | What was the unit for the dose? | Unit | Char | R/C | Record the unit of dose or amount taken per period recorded (e.g., ng, mg, mg/kg). | EXDOSU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | N/A | Unit of dose or amount taken per constant dosing interval recorded. Dose unit must be collected if it cannot be determined via other methods (e.g., from protocol, randomization data). The unit should be preprinted on the CRF or a field provided on the CRF to capture it. A CDASH Subset Controlled Terminology Codelist Name is available for general dose and volume units. |
Interventions | EX | N/A | N/A | 19 | EXDOSFRQ | Exposure Dosing Frequency per Interval | The number of doses given/administered/taken during a specific interval. | What was the frequency of[study treatment/dose] dosing? | Frequency | Char | R/C | Record the frequency the study treatment was administered for a defined period of time. | EXDOSFRQ | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (FREQ) | N/A | This may be collected if it cannot be determined from other sources or if there are multiple options. When possible, the options for dose/amount frequency are preprinted on the CRF. When collected, the recommendation is to collect dosing information in separate fields (e.g., ECDOSE, ECDOSEU, ECDOSFRQ) for specific and consistent data collection and to enable programmatically utilizing these data. |
Interventions | EX | N/A | N/A | 20 | EXROUTE | Exposure Route of Administration | The route of administration of the study treatment. | What was the route of administration (of the [study treatment/dose] )? | Route | Char | R/C | Record the route of administration (e.g., IV, ORAL, TRANSDERMAL) or enter the appropriate code from the code list. | EXROUTE | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (ROUTE) | (EXROUTE) | This may be collected if it cannot be determined via other methods (e.g., from protocol) or if there are multiple options. |
Interventions | EX | N/A | N/A | 21 | EXDOSRGM | Intended Dose Regimen | The text description of the (intended) schedule or regimen for the Intervention. | What was the intended dose regimen (of the [study treatment/dose] )? | Intended Dose Regimen | Char | O | Record the regimen for the study medication. | EXDOSRGM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | The regimen information may further clarify the dose administration and dose frequency (e.g., TWO WEEKS ON, TWO WEEKS OFF). This may be preprinted or collected. The sponsor may wish to create a codelist to collect this data consistently. |
Interventions | EX | N/A | N/A | 22 | EXDOSADJ | Dose Adjusted | An indication whether or not the dose was adjusted. | Was the dose adjusted? | (Dose) Adjusted | Char | O | Select either Yes or No to indicate whether there was a change in dosing. | N/A | When EXADJ is collected, does not map to an SDTMIG variable. The SDTM Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. When EXADJ is not collected, the sponsor may submit this variable as a SUPPQ. | (NY) | N/A | Typically, the intent/purpose of collecting this field is to help with data cleaning and monitoring as it provides a definitive response regarding any dose changes. It provides verification that the associate field on the CRF (EXADJ) was deliberately left blank. However, the sponsor may collect whether the dose was adjusted, without collecting the reason for the change. |
Interventions | EX | N/A | N/A | 23 | EXADJ | Reason for Dose Adjustment | Description or explanation of why a dose of the study treatment is adjusted. | What was the reason the dose was adjusted (from planned)? | Reason Adjusted | Char | O | If there was a change in dosing, record the reason for change. | EXADJ | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Captures the reason the dose was changed or modified. The reason may be chosen from a sponsor-defined list (e.g., adverse event, insufficient response) or entered as free text. May be used for variations from protocol-specified doses, or changes from expected doses. |
Interventions | EX | N/A | N/A | 24 | EXITRPYN | EX Exposure Interrupted | An indication whether of not the exposure was interrupted. | Was the [(study) treatment/dose] interrupted? | [(Study) Treatment / Dose] Interrupted | Char | O | Record if there was an interruption in the study treatment or dosing. | N/A | Does not map to an SDTMIG variable. The SDTMIG Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring when the actual duration of the exposure is collected using the CDASH field EXCINTD. In some situations, if the actual duration of the interruption is not collected, or not derived, this information could be submitted in a SUPPEX.QVAL dataset where SUPPEX.QNAM = "EXITRPYN" and SUPPEX.QLABEL = "Exposure Interrupted". |
Interventions | EX | N/A | N/A | 25 | EXCINTD | Exposure Interruption Duration | The collected duration of the treatment interruption. | If the dose was interrupted, how long was the interruption? | (Interruption) Duration | Char | O | Record the duration of treatment interruption. | SUPPEX.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPEX dataset as the value of SUPPEX.QVAL where SUPPEX.QNAM="EXITRPD" and SUPPEX.QLABEL= "Interruption Duration". Concatenate the collected treatment interruption duration and the duration unit components and create EXITRPD using ISO 8601 Period format. Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | N/A | N/A | In some situations, the duration of the interruption may be calculated from the administration start and end times recorded elsewhere in the CRF. |
Interventions | EX | N/A | N/A | 26 | EXCINTDU | Exposure Interruption Duration Units | The unit for the collected duration of treatment interruption. | If the dose was interrupted, what were the units for the interruption duration? | (Interruption Duration) Unit | Char | O | Record the unit (e.g., MINUTES, HOURS, DAYS) for the duration of treatment interruption. | SUPPEX.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPEX dataset as the value of SUPPEX.QVAL where SUPPEX.QNAM = "EXITRPD" and SUPPEX.QLABEL= "Interruption Duration". Concatenate the collected treatment interruption duration and the duration unit components and create EXITRPDusing ISO 8601 Period format. Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (UNIT) | (EXCINTDU) | The unit should be collected and converted into ISO 8601 period format. |
Interventions | EX | N/A | N/A | 27 | EXLOC | Exposure Location of Dose Administration | A description of the anatomical location of administration. | What was the anatomical location of the ([study treatment/dose] ) administration? | Anatomical Location | Char | O | Record the body location where the study treatment was administered (e.g., SHOULDER, HIP, ARM). | EXLOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location where the study treatment was administered. LAT, DIR, PORTOT are used to further describe the anatomical location. |
Interventions | EX | N/A | N/A | 28 | EXVAMT | Exposure Vehicle Amount | The amount of the prepared product (treatment + vehicle) administered or given. | What was the total amount (Drug + Vehicle)(of [study treatment/dose] ) administered? | Total Amount | Num | O | Record the total amount (treatment +vehicle) that was administered/given to the subject. | EXVAMT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTM variable ECTRTV may also be populated during the process of creating the SDTM submission datasets. | N/A | N/A | Administration amount that was given to the subject. Note: should not be the diluent amount alone. The ECTRTV field may be collected if it cannot be determined from other sources. |
Interventions | EX | N/A | N/A | 29 | EXVAMTU | Exposure Vehicle Amount Units | The unit of measure for the prepared product (treatment + vehicle). | What was the unit for the amount (of [study treatment/dose] ) administered? | Unit | Char | O | Record the unit of total amount (treatment +vehicle) administered/given to the subject (e.g., mL). | EXVAMTU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | (EXVOLTU) | Unit of the administration amount. A CDASH Subset Controlled Terminology Codelist Name is available for dose and volume units. |
Interventions | EX | N/A | N/A | 30 | EXFLRT | Exposure Infusion Rate | The flow rate for the total amount of drug + vehicle administered to the subject. | What was the [study treatment/dose] infusion rate? | Infusion Rate | Num | O | Record the Rate of Infusion (e.g., if rate is 10 mL/min. Record 10 as the infusion rate). | SUPPEX.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPEX dataset as the value of SUPPEX.QVAL where SUPPEX.QNAM = "EXFLRT" and SUPPEX.QLABEL= "Infusion Rate". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | N/A | N/A | Infusion rate can be used to derive dose. |
Interventions | EX | N/A | N/A | 31 | EXFLRTU | Exposure Infusion Rate Unit | The unit of measure for the flow rate for the total amount of drug + vehicle administered to the subject. | What were the units for the [study treatment/dose] infusion rate? | (Infusion Rate) Unit | Char | O | Record the unit for the infusion rate (e.g., mL/min). | SUPPEX.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPEX dataset as the value of SUPPEX.QVAL where SUPPEX.QNAM ="EXFLRTU" and SUPPEX.QLABEL="Infusion Rate Unit". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (UNIT) | (EXFLRTU) | Unit of the infusion rate. |
Interventions | EX | N/A | N/A | 32 | EXTPT | Exposure Planned Time Point Name | A text description of planned time point when measurements should be taken as defined in the protocol. | What was the planned time point for [study treatment/dose] ? | [Planned Time Point Name] | Char | R/C | Record the planned time point of study treatment administration if not preprinted on the CRF. | EXTPT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. See SDTMIG for additional information on representing time points. The SDTMIG time point anchors EXTPTREF (text description) and EXRFTDTC (date/time) may be needed, as well as SDTMIG variables EXTPTNUM, EXELTM. | N/A | N/A | Planned time points are needed to differentiate multiple sequential assessments. It is recommended that time points should be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as Planned Time Point can be included as the column header. |
Interventions | EX | N/A | N/A | 33 | EXTRTCMP | Completed Treatment | Subject did not did not complete the intended regimen. | Did the subject complete the full course of [study treatment/dose] ? | Completed Treatment | Char | O | Select either Yes or No to indicate whether subject has completed the full course of treatment. | SUPPEX.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPEX dataset as the value of SUPPEX.QVAL where SUPPEX.QNAM = "EXTRTCMP" and SUPPEX.QLABEL=" Completed Treatment". Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. | (NY) | N/A | Depending on how the study treatment details are collected via the CRF/eCRF, it may be possible to derive those data if the regimen data are collected. |
Interventions | EX | N/A | N/A | 34 | EXLAT | Exposure Laterality | Qualifier for anatomical location further detailing side of the body for the study treatment administration. | What was the side of the anatomical location of the ([study treatment/dose] ) administration? | Side | Char | O | Record the side of the body location where the study treatment was administered (e.g., Left, Right). | EXLAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | Further details the laterality of the location where the study treatment was administered. This may be preprinted or collected. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Interventions | EX | N/A | N/A | 35 | EXDIR | Exposure Directionality | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What was the directionality of the anatomical location of the ([study treatment/dose] ) administration? | Directionality | Char | O | Record the directionality of the body location where the study treatment was administered (e.g., Anterior, Lower, Proximal, Upper). | EXDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Assumptions for the CDASHIG EC - Exposure as Collected and EX - Exposure Domains
1. If the SDTMIG EC dataset would be an exact duplicate of the SDTMIG EX dataset, then the sponsor may choose to collect data using either the CDASHIG EC or EX domain. (See the SDTMIG for additional information on submission.)
2. The collected --TRT value is the name of the study treatment that is known to the subject or investigative site (e.g., ECTRT="TABLET A" when EXTRT=<'treatment name'>).
3. If dosing data are collected in non-protocol-specified units, the data are collected in CDASHIG EC dataset, then programmatically converted to the protocol-specified units when the data are mapped to the SDTMIG EX domain.
| Dosing units specified in the protocol | Dosing information as collected on the CRF and represented in EC | Representation in EX |
|---|---|---|
200 mg tablet |
2 tablets |
400 mg |
100 mg capsule |
1 capsule |
100 mg |
5 mg/kg |
99 mL |
5 mg/kg |
4. If a treatment is such that start and stop times are not required, and only 1 dosing date is collected, then the collected dosing date will map to both the start date (--STDTC) and end date (--ENDTC) in the submitted exposure dataset(s).
5. If scheduled dosing is collected, the ECMOOD variable may be used to distinguish between "SCHEDULED" and "PERFORMED" dosing records. The sponsor may choose to use this variable to capture multiple scheduled dosing records, if needed.
6. ECOCCUR:
a. ECOCCUR value of "N" indicates a dose was not taken, not given, or missed.
b. ECOCCUR is generally not applicable for Scheduled records.
c. ECOCCUR = "N" is the standard representation of the collected doses not taken, not given, or missed.
7. Dose amount variables (e.g., ECDOSE, ECDOSTXT) must not be set to zero (0) as an alternative method for indicating doses not taken, not given, or missed.
Example CRFs for the CDASHIG EC- Exposure as Collected and CDASHIG EX - Exposure Domains
Example 1
Title: Exposure as Collected
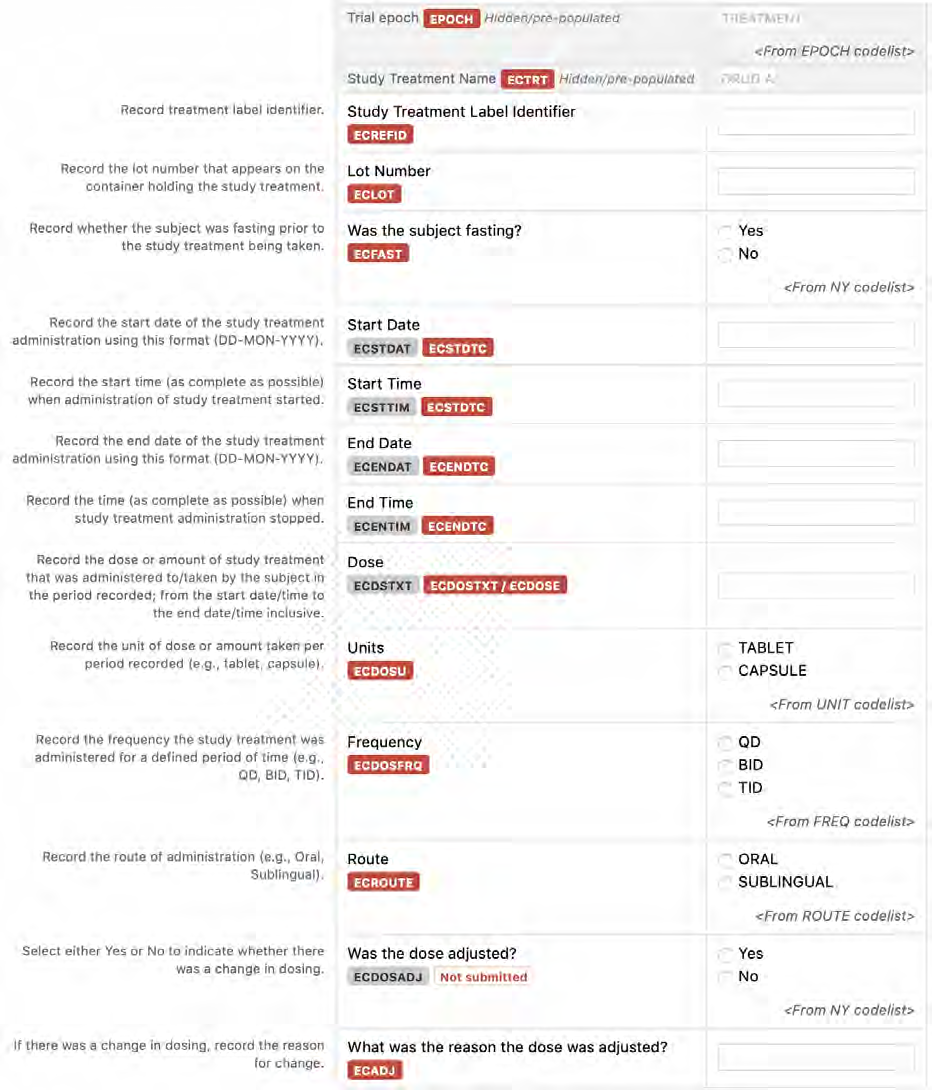
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
EPOCH |
1 |
What is the trial epoch? |
Trial epoch |
[protocol specific] |
Text |
EPOCH |
(EPOCH) | TREATMENT |
Prompt | Y | |||
ECTRT |
2 |
What was the study treatment name? |
Study Treatment Name |
Record the name of study treatment. |
Text |
ECTRT | DRUG A |
Prompt | Y | ||||
ECREFID |
3 |
What is the study treatment label identifier? |
Study Treatment Label Identifier |
Record treatment label identifier. |
Text |
ECREFID | Prompt | ||||||
ECLOT |
4 |
What was the lot number of the study treatment used? |
Lot Number |
Record the lot number that appears on the container holding the study treatment. |
Text |
ECLOT | Prompt | ||||||
ECFAST |
5 |
Was the subject fasting? |
Fasting |
Record whether the subject was fasting prior to the study treatment being taken. |
Text |
ECFAST | (NY) |
Yes; No | Radio | ||||
ECSTDAT |
6 |
What was the study treatment start date? |
Start Date |
Record the start date of the study treatment administration using this format (DD-MON-YYYY). |
Date |
ECSTDTC | Prompt | ||||||
ECSTTIM |
7 |
What was the study treatment start time? |
Start Time |
Record the start time (as complete as possible) when administration of study treatment started. |
Time |
ECSTDTC | Prompt | ||||||
ECENDAT |
8 |
What was the study treatment end date? |
End Date |
Record the end date of the study treatment administration using this format (DD-MON-YYYY). |
Date |
ECENDTC | Prompt | ||||||
ECENTIM |
9 |
What was the study treatment end time? |
End Time |
Record the time (as complete as possible) when study treatment administration stopped. |
Time |
ECENDTC | Prompt | ||||||
ECDSTXT |
10 |
What was the dose per administration of study treatment? |
Dose |
Record the dose or amount of study treatment that was administered to/taken by the subject in the period recorded; from the start date/time to the end date/time inclusive. |
Text |
ECDOSTXT; ECDOSE |
ECDOSTXT / ECDOSE | Prompt | |||||
ECDOSU |
11 |
What were the units for the dose? |
Units |
Record the unit of dose or amount taken per period recorded (e.g., tablet, capsule). |
Text |
ECDOSU | (UNIT) |
TABLET; CAPSULE | Prompt |
Radio | |||
ECDOSFRQ |
12 |
What was the frequency of study treatment dosing? |
Frequency |
Record the frequency the study treatment was administered for a defined period of time (e.g., QD, BID, TID). |
Text |
ECDOSFRQ | (FREQ) |
QD; BID; TID | Prompt |
Radio | |||
ECROUTE |
13 |
What was the route of administration of the study treatment? |
Route |
Record the route of administration (e.g., Oral, Sublingual). |
Text |
ECROUTE | (ROUTE) |
ORAL; SUBLINGUAL | Prompt |
Radio | |||
ECDOSADJ |
14 |
Was the dose adjusted? |
Dose Adjusted |
Select either Yes or No to indicate whether there was a change in dosing. |
Text |
N/A | (NY) |
Yes; No | Radio | ||||
ECADJ |
15 |
What was the reason the dose was adjusted? |
Reason Adjusted |
If there was a change in dosing, record the reason for change. |
Text |
ECADJ |
Example 2
Title: Exposure as Collected – Missed Dose
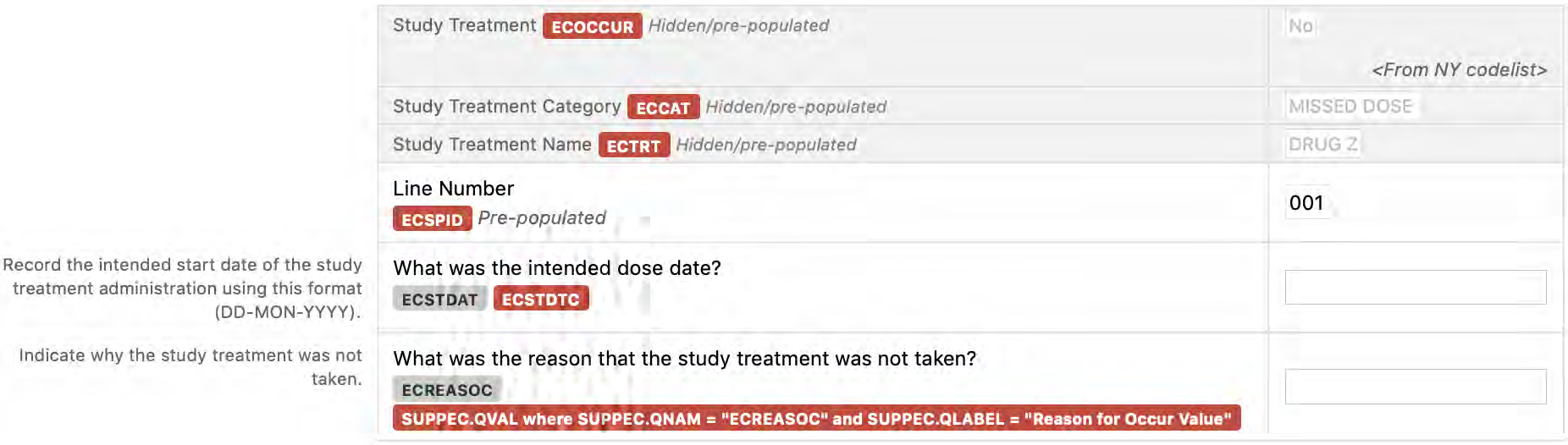
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
ECOCCUR |
1 |
Was study treatment administered? |
Study Treatment |
Indicate if the subject took study treatment. If Yes, include the appropriate details where indicated. |
Text |
ECOCCUR | (NY) | No | Y |
||||
ECCAT |
2 |
What is the category of the study treatment? |
Study Treatment Category |
Record the study treatment category, if not pre-printed on the CRF. |
Text |
ECCAT | MISSED DOSE |
Prompt | Y |
||||
ECTRT |
3 |
What was the study treatment name? |
Study Treatment Name |
Record the name of study treatment. |
Text |
ECTRT | DRUG Z |
Prompt | Y |
||||
ECSPID |
4 |
What is the observation identifier? |
Line Number |
If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. |
Text |
ECSPID | 001 |
Prompt | |||||
ECSTDAT |
5 |
What was the intended dose date? |
Date |
Record the intended start date of the study treatment administration using this format (DD-MON- YYYY). |
Date |
ECSTDTC | QText | ||||||
ECREASOC |
6 |
What was the reason that the study treatment was not taken? |
Reason Not Taken |
Indicate why the study treatment was not taken. |
Text |
SUPPEC.QVAL |
SUPPEC.QVAL where SUPPEC.QNAM = "ECREASOC" and SUPPEC.QLABEL = "Reason for Occur Value" |
Example 3
Title: Exposure as Collected - Scheduled vs Performed
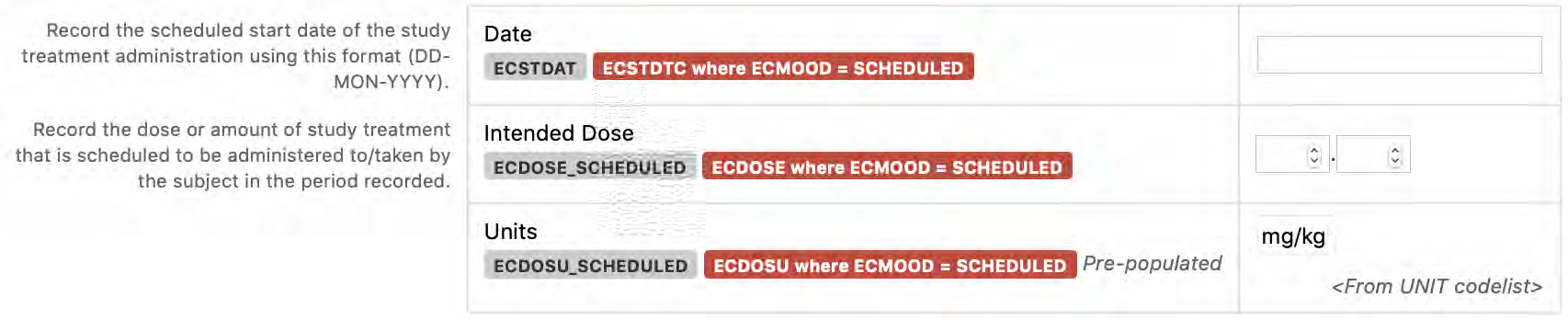
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target | SDTM Variable Target | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
ECSTDAT | 1 | What was the intended dose date? | Date | Record the scheduled start date of the study treatment administration using this format (DD- MON-YYYY). | Date | ECSTDTC | ECSTDTC where ECMOOD = SCHEDULED | ||||||
ECDOSE_SCHEDULED | 2 | What was the intended dose per administration of study treatment? | Intended Dose | Record the dose or amount of study treatment that is scheduled to be administered to/taken by the subject in the period recorded. | Float | ECDOSE | ECDOSE where ECMOOD = SCHEDULED | prompt | |||||
ECDOSU_SCHEDULED | 3 | What were the units for the dose? | Units | Record the unit of dose or amount taken per period recorded (e.g., ng, mg, mg/kg). | Text | ECDOSU | ECDOSU where ECMOOD = SCHEDULED | (UNIT) | mg/kg | prompt |
Title: Exposure as Collected
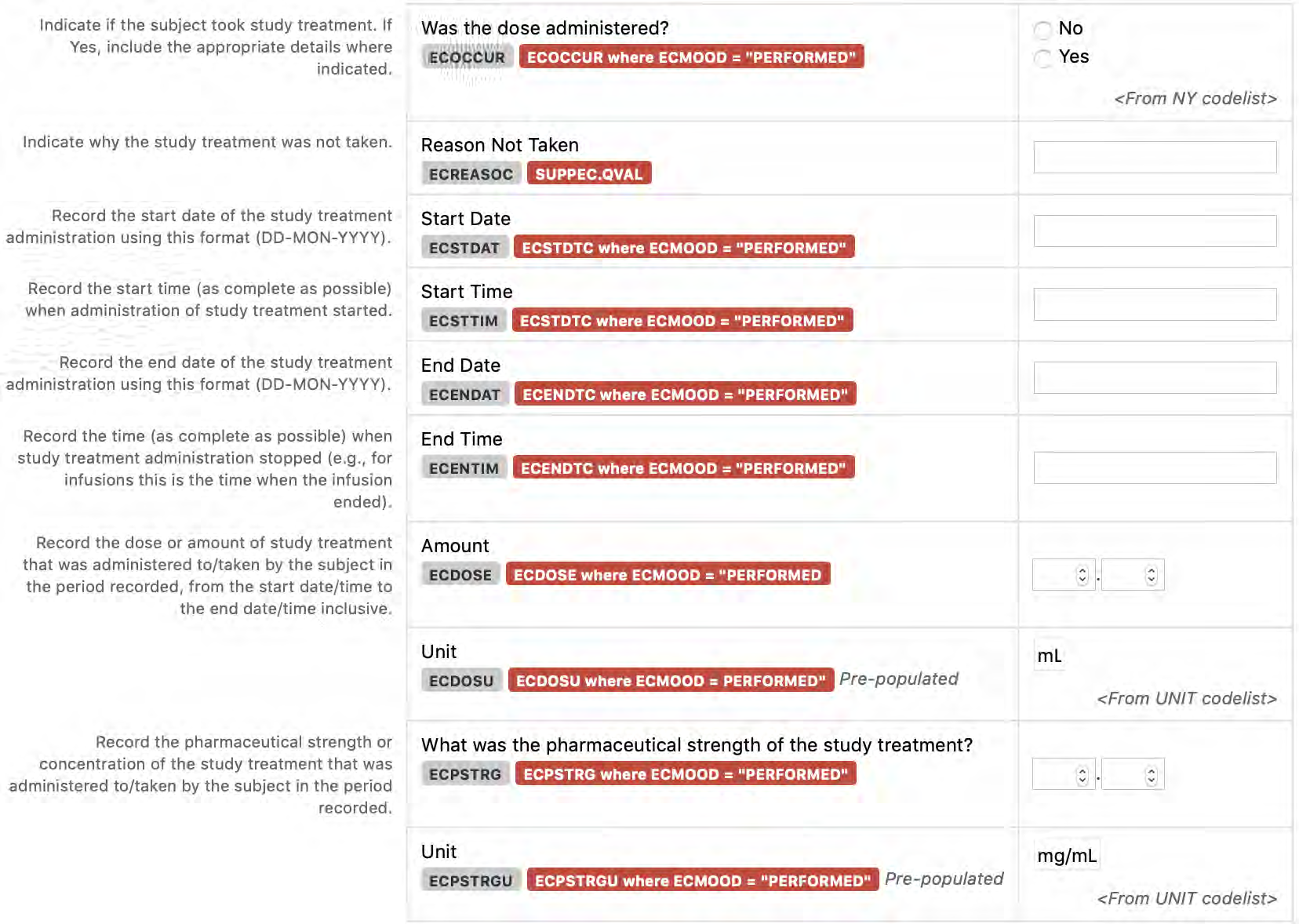
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTM Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
ECOCCUR | 3 | Was the dose administered? | Dose Aministered | Indicate if the subject took study treatment. If Yes, include the appropriate details where indicated. | Text | ECOCCUR | ECOCCUR where ECMOOD = "PERFORMED" | (NY) | No; Yes | qtext | |||
ECREASOC | 4 | What was the reason that the study treatment was not taken? | Reason Not Taken | Indicate why the study treatment was not taken. | Text | SUPPEC.QVAL | prompt | ||||||
ECSTDAT | 5 | What was the start date? | Start Date | Record the start date of the study treatment administration using this format (DD- MON-YYYY). | Date | ECSTDTC | ECSTDTC where ECMOOD = "PERFORMED" | prompt | |||||
ECSTTIM | 6 | What was the start time? | Start Time | Record the start time (as complete as possible) when administration of study treatment started. | Time | ECSTDTC | ECSTDTC where ECMOOD = "PERFORMED" |
prompt | |||||
ECENDAT | 7 | What was the end date? | End Date | Record the end date of the study treatment administration using this format (DD- MON-YYYY). | Date | ECENDTC | ECENDTC where ECMOOD = "PERFORMED" | prompt | |||||
ECENTIM | 8 | What was the end time? | End Time | Record the time (as complete as possible) when study treatment administration stopped (e.g., for infusions this is the time when the infusion ended). | Time | ECENDTC | ECENDTC where ECMOOD = "PERFORMED" | prompt | |||||
ECDOSE | 11 | What was the amount of study treatment administered? | Amount | Record the dose or amount of study treatment that was administered to/taken by the subject in the period recorded, from the start date/time to the end date/time inclusive. | Float | ECDOSE | ECDOSE where ECMOOD = "PERFORMED | prompt | |||||
ECDOSU | 12 | What was the unit for the dose? | Unit | Record the unit of dose or amount taken per period recorded (e.g., ng, mg, mg/kg). | Text | ECDOSU | ECDOSU where ECMOOD = PERFORMED" | (UNIT) |
mL | prompt |
|||
ECPSTRG | 13 | What was the pharmaceutical strength of the study treatment? | Pharmaceutical Strength | Record the pharmaceutical strength or concentration of the study treatment that was administered to/taken by the subject in the period recorded. | Float | ECPSTRG | ECPSTRG where ECMOOD = "PERFORMED" |
||||||
ECPSTRGU | 14 | What was the unit of the pharmaceutical strength of the study treatment? | Unit | Record the unit of concentration or strength of the study treatment per period recorded (e.g., ng, mg, mg/kg). | Text | ECPSTRGU | ECPSTRGU where ECMOOD = "PERFORMED" | (UNIT) |
mg/mL |
Example 4
Title: Exposure Start and Stop Dates
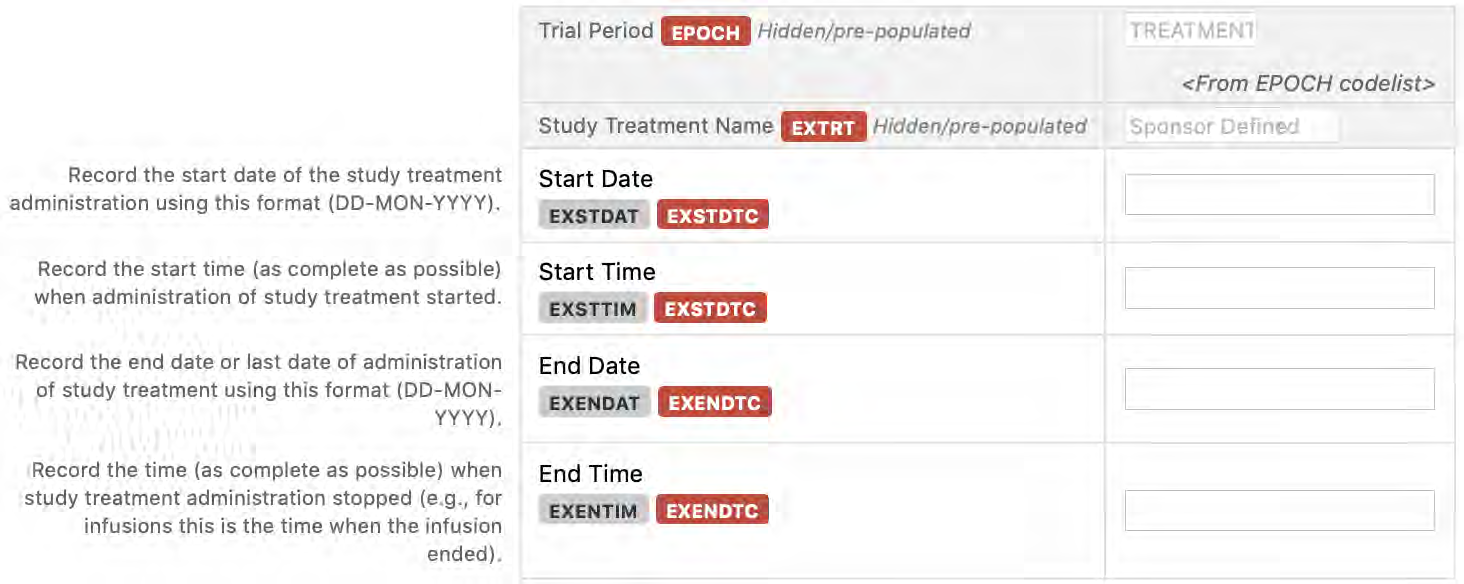
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Value | SDTM Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
EPOCH | 1 | What is the trial period? | Trial Period | [protocol specific] | Text | EPOCH | (EPOCH) | TREATMENT |
Prompt | Y | |||
EXTRT |
2 | What was the study treatment? | Study Treatment Name | Record the name of study treatment. | Text | EXTRT | Sponsor Defined | Prompt | Y | ||||
EXSTDAT | 3 | What was the study treatment start date? | Start Date | Record the start date of the study treatment administration using this format (DD-MON-YYYY). | Date | EXSTDTC | Prompt | ||||||
EXSTTIM | 4 | What was the study treatment start time? | Start Time | Record the start time (as complete as possible) when administration of study treatment started. | Time | EXSTDTC | Prompt | ||||||
EXENDAT | 5 | What was the study treatment end date? | End Date | Record the end date or last date of administration of study treatment using this format (DD-MON-YYYY). | Date | EXENDTC | Prompt | ||||||
EXENTIM | 6 | What was the study treatment end time? | End Time | Record the time (as complete as possible) when study treatment administration stopped (e.g., for infusions this is the time when the infusion ended). | Time | EXENDTC | Prompt |
8.1.4 PR - Procedures
Description/Overview for the CDASHIG PR - Procedures DomainThe Procedures (PR) domain should be used to collect details describing a subject’s therapeutic and diagnostic procedures conducted before, during, and/or after the study. Measurements obtained from procedures are to be represented in the appropriate Findings domain(s). For example, the details of an endoscopy (e.g., date and time of start and stop) are represented in the PR domain; microscopic results would be represented in the Microscopic (MI) domain.
Specification for the CDASHIG PR - Procedures Domain
Procedures Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variablev | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Interventions |
PR |
N/A |
N/A |
1 |
STUDYID |
Study Identifier |
A unique identifier for a study. |
What is the study identifier? |
[Protocol/Study] |
Char |
HR |
N/A |
STUDYID |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or created during SDTM-based dataset creation before submission. |
Interventions |
PR |
N/A |
N/A |
2 |
SITEID |
Study Site Identifier |
A unique identifier for a site within a study. |
What is the site identifier? |
Site (Identifier) |
Char |
HR |
N/A |
DM.SITEID |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Interventions |
PR |
N/A |
N/A |
3 |
SUBJID |
Subject Identifier for the Study |
A unique subject identifier within a site and a study. |
What [is/was] the (study) [subject/participant] identifier? |
[Subject/Participant] (Identifier) |
Char |
HR |
Record the identifier for the subject. |
DM.SUBJID |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Interventions |
PR |
N/A |
N/A |
4 |
PRYN |
Any Procedures Performed |
An indication whether or not the subject had any procedures performed. |
Were any surgical, therapeutic, or diagnostic procedures performed? |
Any Procedures |
Char |
O |
Indicate if the subject had any surgical, therapeutic or diagnostic procedures. If "Yes", include the appropriate details where indicated on the CRF. |
N/A |
Does not map to an SDTMIG variable. The SDTM aCRF is annotated to indicate that this field is NOT SUBMITTED. |
(NY) |
N/A |
The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification that all other fields on the CRF were deliberately left blank. |
Interventions |
PR |
N/A |
N/A |
5 |
PRCAT |
Procedure Category |
A grouping of topic- variable values based on user-defined characteristics. |
What was the category of the procedure? |
[Procedure Category]; NULL |
Char |
O |
Record the procedure category, if not preprinted on the CRF. |
PRCAT |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Interventions |
PR |
N/A |
N/A |
6 |
PRSCAT |
Procedure Subcategory |
A sub-division of the PRCAT values based on user-defined characteristics. |
What was the subcategory of the procedure? |
[Procedure Subcategory]; NULL |
Char |
O |
Record the procedure subcategory, if not preprinted on the CRF. |
PRSCAT |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
Sponsor-defined controlled terminology. This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. PRSCAT can only be used if there is a PRCAT and it must be a subcategorization of PRCAT. |
Interventions |
PR |
N/A |
N/A |
7 |
PRSPID |
Procedure Sponsor- Defined Identifier |
A sponsor-defined identifier. In CDASH, This is typically used for preprinted or auto- generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. |
[Sponsor defined question] |
[Sponsor defined] |
Char |
O |
If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. |
PRSPID |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. |
N/A |
N/A |
Because SPID is a sponsor- defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile procedure records with medical history and/or with AEs. May be used to record preprinted number (e.g., line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Interventions |
PR |
N/A |
N/A |
8 |
PRTRT |
Reported Name of Procedure |
The verbatim surgical, therapeutic, or diagnostic procedure's name. |
What was the procedure name? |
[Procedure Name]; (Specify) Other |
Char |
HR |
Record only one procedure per line. |
PRTRT |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
In most cases, the verbatim procedure names or therapy will be coded to a standard dictionary (e.g., MedDRA, SNOMED) after the data have been collected on the CRF. |
Interventions |
PR |
N/A |
N/A |
9 |
PRDECOD |
Standardized Procedure Name |
The dictionary- or sponsor-defined standardized text description of PRTRT, or the modified topic variable (PRMODIFY), if applicable. |
N/A |
[Standardized Procedure Name] |
Char |
O |
N/A |
PRDECOD |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
(PROCEDUR) |
N/A |
This is typically not a data collection field that will appear on the CRF. If the sponsor chooses to code the procedure, the sponsor will populate this through the coding process. If PRPRESP is used, and the information about a specific standardized procedure name is being solicited, the data from PRTRT may map directly to the SDTMIG PRDECOD variable. |
Interventions |
PR |
N/A |
N/A |
10 |
PRMODIFY |
Modified Procedure Name |
If the value for PRTERM is modified for coding purposes, then the modified text is placed here. |
N/A |
N/A |
Char |
O |
N/A |
PRMODIFY |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
This is not a data collection field that will appear on the CRF. If the sponsor chooses to code the procedure, the sponsor will populate this through the coding process. |
Interventions |
PR |
N/A |
N/A |
11 |
PRPRESP |
Procedure prespecified |
An indication that a specific intervention or a group of interventions is prespecified on a CRF. |
N/A |
N/A |
Char |
O |
N/A |
PRPRESP |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
(NY) |
N/A |
For prespecified interventions, a hidden field on a CRF defaulted to "Y", or added during the SDTM dataset creation. If a study collects both prespecified interventions as well as free-text interventions, the value of PRPRESP should be "Y" for all prespecified interventions and null for interventions reported as free text. |
Interventions |
PR |
N/A |
N/A |
12 |
PROCCUR |
Procedure Occurrence |
An indication whether a prespecified procedure (PRTRT) happened when information about the occurrence of a specific intervention is solicited. |
Was [PRDECOD/PRTRT] performed?; Has the subject had [PRDECOD/PRTRT]? |
[PRDECOD/PRTRT] Performed |
Char |
O |
Indicate if [specific procedure] was performed by checking Yes or No. |
PROCCUR |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. If the response was not asked or answered, populate the SDTMIG variable PRSTAT with "NOT DONE". |
(NY) |
N/A |
PROCCUR is used to report the occurrence of a prespecified procedure or a group of procedures. PROCCUR is not used for spontaneously free text- reported procedures. The site should be able to indicate that the response was not asked or answered. |
Interventions |
PR |
N/A |
N/A |
13 |
PRREASOC |
Procedure Reason for Occur Value |
An explanation of why a scheduled procedure did or did not occur. |
What was the reason that the procedure was (not) performed? |
Reason (Not) Performed |
Char |
O |
Indicate why the procedure was or wasnot performed. |
SUPPPR.QVAL |
This information could be submitted in a SUPPPR dataset as the value of SUPPPR.QVAL where SUPPPR.QNAM = "PRREASOC" and SUPPPR.QLABEL="Reason for Occur Value". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. |
N/A |
N/A |
The reason the scheduled procedure was or was not performed may be chosen from a sponsor-defined codelist (e.g., SUBJECT REFUSED) or entered as free text. When --REASOC is used, --OCCUR must also be populated in the SDTM dataset with a value of "Y" or "N". |
Interventions |
PR |
N/A |
N/A |
14 |
PRREASND |
Procedure Reason Not Done |
An explanation of why the data are not available. |
What was the reason not done? |
Reason not done |
Char |
O |
Provide the reason why the procedure was not done. |
PRREASND |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
The reason the data are not available may be chosen from a sponsor-defined list (e.g., broken equipment, subject refused) or entered as free text. When PRREASND is used, the SDTMIG variable PRSTAT should also be populated in the SDTM-based dataset. The value "NOT DONE" here indicates that the subject was not questioned/data was not collected. It does not mean that the subject did not have the procedure. |
Interventions |
PR |
N/A |
N/A |
15 |
PRPRIOR |
Prior Procedure |
Indication the procedure occurred prior to [PRSTTPT] or prior to the date in DM.RFSTDTC. |
Was the procedure performed prior to [PRSTTPT]?; Was the procedure performed prior to study start? |
Prior to [PRSTTPT]; Prior to study |
Char |
O |
Check if the procedure was started before the specified point in time. |
PRSTRTPT; PRSTRF |
This does not map directly to an SDTMIG variable. May be used to populate a value into an SDTM relative timing variable such as PRSTRF or PRSTRTPT. When populating PRSTRF, or PRSTRTPT, if the value of the CDASH field PRPRIOR is "Y" a value from the CDISC CT (STENRF) may be used. When PRPRIOR refers to the Study Reference Period (defined in DM.RFSTDTC to DM.RFENDTC) the SDTM variable PRSTRF should be populated. When PRPRIOR is compared to another time point, the SDTM variables PRSTRTPT and PRSTTPT should be used. Note: PRSTRTPT must refer to the time point anchor described in PRSTTPT. |
(NY) |
N/A |
See Section 3.7, Mapping Relative Times from Collection to Submissions, and and SDTMIG v3.2 Section 4.1.4.7 for more information. for information about mapping relative times. |
Interventions |
PR |
N/A |
N/A |
16 |
PRSTDAT |
Procedure Start Date |
The date or start date of when the procedure started or was performed, represented in an unambiguous date format (e.g., DD-MON-YYYY). |
What was the procedure (start) date ? |
(Start) Date |
Char |
R/C |
Record the date the procedure was started or performed using this format (DD- MON-YYYY). Procedures performed during the study are expected to have a complete start date. Prior procedures that are exclusionary should have both a start and end date. |
PRSTDTC |
This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable PRSTDTC in ISO 8601 format. |
N/A |
N/A |
The preferred method is to collect a complete Start Date. Partial dates (e.g., providing year only) for procedures started a considerable amount of time prior to the start of study are acceptable. |
Interventions |
PR |
N/A |
N/A |
17 |
PRONGO |
Ongoing Procedure |
Indication the procedure is ongoing when no end date is provided. |
Was the procedure ongoing (as of the [study-specific timepoint or period])? |
Ongoing (as of the [study-specific timepoint or period]) |
Char |
O |
Indicate if the procedure has not ended at the time of data collection and the end date should be left blank. |
PRENRTPT; PRENRF |
This does not map directly to an SDTM variable. May be used to populate a value into an SDTMIG relative timing variable such as PRENRF or PRENRTPT. When populating PRENRF, if the value of PRONGO is "Y", the value of "DURING", "AFTER" or "DURING/AFTER" may be used. When populating PRENRTPT, if the value of PRONGO is "Y", the value of "ONGOING" may be used. When PRONGO refers to the Study Reference Period (defined in DM.RFSTDTC to DM.RFENDTC) the SDTM variable PRENRF should be populated. When PRONGO is used in conjunction with another time point, the SDTM variables PRENRTPT and PRENTPT should be used. Note: PRENRTPT must refer to a time point anchor described in PRENTPT. |
(NY) |
N/A |
Completed to indicate that the procedure has not stopped at the time point defined by the study. The purpose of collecting this field is to help with data cleaning and monitoring; this field provides further confirmation that the End Date was deliberately left blank. See Section 3.7, Mapping Relative Times from Collection to Submissions, and and SDTMIG v3.2 Section 4.1.4.7 for more information. for information about mapping relative times. |
Interventions |
PR |
N/A |
N/A |
18 |
PRENDAT |
Procedure End Date |
The end date of the procedure, represented in an unambiguous date format (e.g., DD-MON- YYYY). |
What was the procedure (end) date? |
(End) Date |
Char |
R/C |
Record the end date of the procedure using this format (DD- MON-YYYY). If the procedure has not ended, leave this field blank and populate PRONGO as "Y". |
PRENDTC |
This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTM variable PRENDTC in ISO 8601 format. |
N/A |
N/A |
The assumption is that sponsors should either have an End Date or will indicate that the procedure was ongoing at the time of collection or at the end of the study. However, in cases where the End Date can be determined from dates collected elsewhere in the CRF it is not necessary to include an End Date in the CRF. For example, if the procedure is started and stopped within the same day, the End Date will be the same as the Start Date. |
Interventions |
PR |
N/A |
N/A |
19 |
PRINDC |
Procedure Indication |
The condition, disease, symptom, or disorder that the procedure was used to address or investigate (e.g., why the therapy was taken or administered, why the procedure was performed). |
For what indication was the [PRTRT] performed? |
Indication |
Char |
O |
Record the reason the procedure was performed based on clinical investigator's evaluation. If performed to treat a condition, and a diagnosis was made, the indication should be the diagnosis. If performed to treat a condition, and no diagnosis was made, the indication should be the signs and symptoms. If taken as prophylaxis, report as "Prophylaxis for " and include a description of the condition(s). |
PRINDC |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
This additional information is collected on the CRF when the sponsor wants to capture the reason(s) why a procedure was performed. This information can then be used as deemed appropriate for coding, analysis (e.g., in the classification of procedures), for reconciling the procedures performed on a subject with their provided medical history, and/or AEs/SAEs as part of the data clean-up and monitoring process. |
Interventions |
PR |
N/A |
N/A |
20 |
PRAENO |
Related Adverse Event ID |
Identifier for the adverse event that is the indication for this procedure. |
What was the identifier for the adverse event(s) for which the procedure was performed? |
Related Adverse Event Identifier |
Char |
O |
Record the identifier of the adverse event for which this procedure was performed. |
N/A |
This does not map directly to an SDTMIG variable. For the SDTM submission datasets, may be used to create RELREC to link this record with a record in the Adverse Events domain. |
N/A |
N/A |
The intent is to establish a link between the adverse event and the procedure performed for the adverse event. PRAENO can be used to identify a relationship between records in the PR dataset and records in the AE dataset. See SDTMIG v3.2 Section 8.2 for information on RELREC. |
Interventions |
PR |
N/A |
N/A |
21 |
PRMHNO |
Related Medical History Event ID |
Identifier for the medical history event that is the indication for this procedure. |
What was the identifier for the medical history event(s) for which the procedure was performed? |
Related Medical History Event Identifier |
Char |
O |
Record the identifier of the medical history event for which this procedure was performed. |
N/A |
This does not map directly to an SDTMIG variable. For the SDTM submission datasets, may be used to create RELREC to link this record with a record in the Medical History domain. | N/A |
N/A |
The intent is to establish a link between the medical history condition and the procedure undergone for the medical history condition. PRMHNO can be used to identify a relationship between records in the PR dataset and records in the MH dataset. See SDTMIG v3.2 Section 8.2 for information on RELREC. |
Interventions |
PR |
N/A |
N/A |
22 |
PRDSTXT |
Procedure Dose Description |
The dose/amount administered during the procedure. |
What was the [dose/amount] of [PRTRT] (per administration/for the procedure)? |
[Dose/Amount] |
Char |
O |
Record the [dose/amount] of [PRTRT] per administered. |
PRDOSE; PRDOSTXT |
This does not map directly to an SDTMIG variable. Numeric values map to PRDOSE in SDTM. Non-numeric values (e.g., 200-400) map to PRDOSTXT in SDTM. |
N/A |
N/A |
Defining this data collection field as a dose text field allows for flexibility in capturing dose entries as numbers, text or ranges. The data collected in this dose text-format field should be separated or mapped to either SDTMIG PRDOSE if numeric or PRDOSTXT if text. |
Interventions |
PR |
N/A |
N/A |
23 |
PRDOSU |
Procedure Dose Unit |
The unit for intended dose/amount for PRDOSE, PRDOSTOT, or PRDOSTXT. |
What was the unit? |
Unit |
Char |
O |
Record the unit for the amount of [PRTRT] performed or administered. |
PRDOSU |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
(UNIT) |
N/A |
When sponsors collect data for amount of [PRTRT] performed or administered, Unit should be collected as well (if applicable). |
Interventions |
PR |
N/A |
N/A |
24 |
PRDOSFRQ |
Procedure Frequency per Interval |
The number/amount of the procedure that was given/administered/taken during a specific interval. |
What was the frequency of [PRTRT]? |
Frequency |
Char |
O |
Record how often the procedure was performed. |
PRDOSFRQ |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
(FREQ) |
N/A |
This may be collected if it cannot be determined from other sources or if there are multiple options. Usually expressed as the number of procedures given per a specific interval. |
Interventions |
PR |
N/A |
N/A |
25 |
PRROUTE |
Procedure Route of Administration |
The route of administration of the procedure. |
What was the route of administration of the procedure? |
Route |
Char |
O |
Provide the route of administration for the procedure. |
PRROUTE |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
(ROUTE) |
N/A |
This additional information may be important to collect on the CRF when the sponsor would want to capture a procedure's route of administration for comparative analysis purposes. |
Interventions |
PR |
N/A |
N/A |
26 |
PRLOC |
Location of Procedure |
A description of the anatomical location of an procedure, such as location of a biopsy. |
What was the anatomical location where the procedure was performed? |
Anatomical Location |
Char |
O |
Record the body location where the procedure was performed. |
PRLOC |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
(LOC) |
N/A |
Collected when the sponsor needs to identify the specific anatomical location (e.g., Liver for the Biopsy). LAT, DIR, PORTOT are used to further describe the anatomical location. |
Interventions |
PR |
N/A |
N/A |
27 |
PRLAT |
Procedure Laterality |
Qualifier for anatomical location further detailing side of the body for the procedure administration. |
What was the side of the anatomical location of the administration? |
Side |
Char |
O |
Record the side of the anatomical location where the procedure was administered. |
PRLAT |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
(LAT) |
N/A |
Further details the laterality of the location where the procedure was administered/taken. This may be preprinted or collected. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Interventions |
PR |
N/A |
N/A |
28 |
PRDIR |
Procedure Directionality |
Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. |
What was the directionality of the anatomical location of the procedure? |
Directionality |
Char |
O |
Record the direction of the anatomical location where the procedure was administered. |
PRDIR |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
(DIR) |
N/A |
May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Interventions |
PR |
N/A |
N/A |
29 |
PRPORTOT |
Procedure Portion or Totality |
Qualifier for anatomical location further detailing the distribution (i.e., arrangement of, apportioning of). |
What was the portion or totality of the anatomical location that was treated? |
Portion or Totality |
Char |
O |
Record the portion of the body location that was treated. |
PRPORTOT |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
(PORTOT) |
N/A |
Collected when the sponsor needs to identify the specific portionality for the anatomical locations. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Interventions |
PR |
N/A |
N/A |
30 |
PRFAST |
Procedure Fasting Status |
An indication that the subject has abstained from food/water for the specified amount of time. |
Was the subject fasting? |
Fasting |
Char |
O |
Record whether the subject was fasting prior to the procedure being performed. |
PRFAST |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
(NY) |
N/A |
This information is collected when the procedure may be affected by whether the subject was fasting. This may not be relevant for all procedures. |
Interventions |
PR |
N/A |
N/A |
31 |
PRDOSRGM |
Procedure Intended Dose Regimen |
The text description of the (intended) schedule or regimen for the procedure. |
What was the intended procedure regimen? |
Intended Procedure Regimen |
Char |
O |
Record the intended regimen for the procedure to be performed. |
PRDOSRGM |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
The regimen information may further clarify the dose administration and dose frequency (e.g., TWO WEEKS ON, TWO WEEKS OFF). This may be prespecified or collected. The sponsor may wish to create a codelist to collect this data consistently. |
Interventions |
PR |
N/A |
N/A |
32 |
PRDOSADJ |
Procedure Adjusted |
An indication whether or not the procedure dose/amount was adjusted. |
Was the procedure dose adjusted? |
(Dose) Adjusted |
Char |
O |
Record if the procedure was adjusted from planned. |
N/A |
When PRADJ is collected, does not map to an SDTMIG variable. The SDTM Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. When PRADJ is not collected, the sponsor may submit this variable as a SUPPQ. |
(NY) |
N/A |
Typically, the intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification that the associate field on the CRF (PRADJ) was deliberately left blank. However, the sponsor may collect whether the procedure dose/amount was adjusted, without collecting the reason for the change. |
Interventions |
PR |
N/A |
N/A |
33 |
PRADJ |
Reason for Procedure Adjustment |
Description or explanation of why a procedure dose/amount was adjusted. |
What was the reason the procedure dose was adjusted? |
Reason Adjusted |
Char |
O |
Record why the procedure dose was adjusted from planned. |
PRADJ |
Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. |
N/A |
N/A |
Captures reason [PRTRT] dose was changed or modified. The reason may be chosen from a sponsor- defined list (e.g., adverse event, insufficient response) or entered as free text. |
Interventions |
PR |
N/A |
N/A |
34 |
PRTRTCMP |
Completed Procedure |
Indication whether the subject completed the intended regimen. |
Did the subject complete the full course of the [PRTRT]? |
Completed [PR Intervention Topic] |
Char |
O |
Record if the subject completed the intended regimen. |
SUPPPR.QVAL |
This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPPR dataset as the value of SUPPPR.QVAL where SUPPPR.QNAM= "PRTRTCMP" and SUPPPR.QLABEL="Treatment Completed". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. |
(NY) |
N/A |
Depending on how the [PTTRT] dose details are collected via the CRF/eCRF, it may be possible to derive those data if the regimen data are collected. |
Interventions |
PR |
N/A |
N/A |
35 |
PRITRPYN |
Procedure Interrupted |
An indication whether the procedure was interrupted. |
Was the procedure interrupted? |
Procedure Interrupted |
Char |
O |
Record if the procedure was interrupted. |
N/A |
Does not map to an SDTMIG variable. The SDTM aCRF is annotated to indicate that this field is NOT SUBMITTED. |
(NY) |
N/A |
Provides a definitive response regarding any procedure interruption. The intent/purpose of collecting this field is to help with data cleaning and monitoring when the duration of the interruption is collected. In some situations, if the actual duration of the interruption is not collected, or not derived, this information could be submitted in a SUPPPR dataset. |
Interventions |
PR |
N/A |
N/A |
36 |
PRITRPRS |
Reason Procedure Interrupted |
An indication of why the intervention was interrupted. |
Why was the procedure interrupted? |
Reason Procedure Interrupted |
Char |
O |
Record the reason the procedure was interrupted. |
SUPPPR.QVAL |
This information could be submitted in a SUPPPR dataset as the value of SUPPPR.QVAL where SUPPPR.QNAM="PRITRPRS" and SUPPPR.QLABEL ="Reason Intervention Interrupted". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. |
N/A |
N/A |
This CDASH field is use to collected the reason why an intervention was interrupted. The sponsor may define controlled terminology. |
Interventions |
PR |
N/A |
N/A |
37 |
PRCINTD |
Procedure Interruption Duration |
The collected duration of the procedure interruption. |
What was the duration of the procedure interruption? |
(Interruption) Duration |
Char |
O |
Record how long the procedure was interrupted before it resumed. |
SUPPPR.QVAL |
This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPPR dataset as the value of SUPPPR.QVAL where SUPPPR.QNAM="PRITRPD" and SUPPPR.QLABEL= "Interruption Duration". Concatenate the collected procedure interruption duration and the duration unit components and create PRITRPD using ISO 8601 Period format. Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. |
N/A |
N/A |
This field is used to collect the duration of procedure interruption. In some situations, the duration of the interruption may not be collected but calculated from the procedure start and end times recorded elsewhere in the CRF. |
Interventions |
PR |
N/A |
N/A |
38 |
PRCINTDU |
Procedure Interruption Duration Units |
The unit for the collected duration of the procedure interruption. |
What was the interruption duration unit? |
(Interruption Duration) Unit |
Char |
O |
Record the unit for the duration of interruption of the procedure. |
SUPPPR.QVAL |
This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPPR dataset as the value of SUPPPR.QVAL where SUPPPR.QNAM="PRITRPD" and SUPPPR.QLABEL= "Interruption Duration". Concatenate the collected procedure interruption duration and the duration unit components and create PRITRPD using ISO 8601 Period format. Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. |
(UNIT) |
N/A |
The unit should be collected as a qualifier to the number for duration. |
Interventions |
PR |
N/A |
N/A |
39 |
PRLLT |
Procedure Lowest Level Term |
MedDRA text description of the Lowest Level Term. |
N/A |
N/A |
Char |
O |
N/A |
SUPPPR.QVAL |
This information could be submitted in a SUPPPR dataset as the value of SUPPPR.QVAL where SUPPPR.QNAM="PRLLT" and SUPPPR.QLABEL="Lower Level Term". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". |
N/A |
N/A |
This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using the MedDRA coding dictionary. Rationale for adding coding variables to CDASH is to provide a more complete Data Management package. This variable could be used in the PR domain for coding. Another dictionary can be used and the appropriate coding elements added, if appropriate, in the SUPPPR.QVAL dataset. |
Interventions |
PR |
N/A |
N/A |
40 |
PRLLTCD |
Procedure Lowest Level Term Code |
MedDRA Lowest Level Term code. |
N/A |
N/A |
Num |
O |
N/A |
SUPPPR.QVAL |
This information could be submitted in a SUPPPR dataset as the value of SUPPPR.QVAL where SUPPPR.QNAM="PRLLTCD" and SUPPPR.QLABEL="Lower Level Term Code". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". |
N/A |
N/A |
This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using the MedDRA coding dictionary. Rationale for adding coding variables to CDASH is to provide a more complete data management package. This variable could be used in the PR domain for MedDRA coding. Other dictionaries can be used and the appropriate coding elements added, if appropriate, in the SUPPPR.QVAL dataset. |
Interventions |
PR |
N/A |
N/A |
41 |
PRPTCD |
Procedure Preferred Term Code |
MedDRA code for the Preferred Term. |
N/A |
N/A |
Num |
O |
N/A |
SUPPPR.QVAL | This information could be submitted in a SUPPPR.QVAL dataset where SUPPPR.QNAM="PRPTCD" and SUPPPR.QLABEL= "Preferred Term Code Lower Level Term Code". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using the MedDRA coding dictionary. Rationale for adding coding variables to CDASH is to provide a more complete data management package. This variable could be used in the PR domain for MedDRA coding. Other dictionaries can be used and the appropriate coding elements added, if appropriate, in the SUPPPR.QVAL dataset. |
Interventions | PR | N/A | N/A | 42 | PRHLT | Procedure High Level Term | MedDRA text description of High Level Term from the primary path. | N/A | N/A | Char | O | N/A | SUPPPR.QVAL | This information could be submitted in a SUPPPR dataset as the value of SUPPPR.QVAL where SUPPPR.QNAM="PRHLT" and SUPPPR.QLABEL="High Level Term". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". | N/A | N/A | This is not a data collection field that would appear on the CRF. Sponsors populate this through the coding process. This is applicable to items using the MedDRA coding dictionary. Rationale for adding coding variables to CDASH is to provide a more complete data management package. This variable could be used in the PR domain for MedDRA coding. Other dictionaries can be used and the appropriate coding elements added, if appropriate, in the SUPPPR.QVAL dataset. |
Interventions | PR | N/A | N/A | 43 | PRHLTCD | Procedure High Level Term Code | MedDRA High Level Term code from the primary path. | N/A | N/A | Num | O | N/A | SUPPPR.QVAL | This information could be submitted in a SUPPPR.QVAL dataset where SUPPPR.QNAM="PRHLTCD" and SUPPPR.QLABEL="High Level Term Code". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This is not a data collection field that would appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using the MedDRA coding dictionary. Rationale for adding coding variables to CDASH is to provide a more complete data management package. This variable could be used in the PR domain for MedDRA coding. Other dictionaries can be used and the appropriate coding elements added, if appropriate, in the SUPPPR.QVAL dataset. |
Interventions | PR | N/A | N/A | 44 | PRHLGT | Procedure High Level Group Term | MedDRA text description of the High Level Group Term from the primary path. | N/A | N/A | Char | O | N/A | SUPPPR.QVAL | This information could be submitted in a SUPPPR dataset as the value of SUPPPR.QVAL where SUPPPR.QNAM="PRHLTGT" and SUPPPR.QLABEL="High Level Group Term". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using the MedDRA coding dictionary. Rationale for adding coding variables to CDASH is to provide a more complete data management package. This variable could be used in the PR domain for MedDRA coding. Other dictionaries can be used and the appropriate coding elements added, if appropriate, in the SUPPPR.QVAL dataset. |
Interventions | PR | N/A | N/A | 45 | PRHLGTCD | Procedure High Level Group Term Code | MedDRA High Level Group Term code from the primary path. | N/A | N/A | Num | O | N/A | SUPPPR.QVAL | This information could be submitted in a SUPPPR dataset as the value of SUPPPR.QVAL where SUPPPR.QNAM= "PRHLTGTCD" and SUPPPR.QLABEL="High Level Group Term Code". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". | N/A | N/A | This is not a data collection field that would appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using the MedDRA coding dictionary. Rationale for adding coding variables to CDASH is to provide a more complete data management package. This variable could be used in the PR domain for MedDRA coding. Other dictionaries can be used and the appropriate coding elements added, if appropriate, in the SUPPPR.QVAL dataset. |
Interventions | PR | N/A | N/A | 46 | PRSOC | PR Primary System Organ Class | MedDRA Primary System Organ Class description associated with the intervention. | N/A | N/A | Char | O | N/A | SUPPPR.QVAL | This information could be submitted in a SUPPPR.QVAL dataset where SUPPPR.QNAM="PRSOC" and SUPPPR.QLABEL="Primary System Organ Class". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This is not a data collection field that would appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using the MedDRA coding dictionary. Rationale for adding coding variables to CDASH is to provide a more complete data management package. This variable could be used in the PR domain for MedDRA coding. Other dictionaries can be used and the appropriate coding elements added, if appropriate, in the SUPPPR.QVAL dataset. |
Interventions | PR | N/A | N/A | 47 | PRSOCCD | PR Primary System Organ Class Code | MedDRA Primary System Organ Class code. | N/A | N/A | Num | O | N/A | SUPPPR.QVAL | This information could be submitted in a SUPPPR dataset as the value of SUPPPR.QVAL where SUPPPR.QNAM= "PRSOCCD" and SUPPPR.QLABEL="Primary System Organ Class Code". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. Sponsors should include an Origin column in the SUPPQ dataset to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using the MedDRA coding dictionary. Rationale for adding coding variables to CDASH is to provide a more complete data management package. This variable could be used in the PR domain for MedDRA coding. Other dictionaries can be used and the appropriate coding elements added, if appropriate, in the SUPPPR.QVAL dataset. |
Assumptions for the CDASHIG PR - Domain
1. Information on procedures is generally collected either by recording free text or using a prespecified list of terms.
2. Because the solicitation of information on specific therapeutic and diagnostic procedures may affect the frequency at which they are reported, the fact that a specific procedure was solicited may be of interest to reviewers. PROCCUR is used to indicate whether a prespecified procedure occurred. A value of "Y" indicates that the procedure occurred; "N" indicates that it did not. If a procedure was not prespecified, the value of PROCCUR should not be collected.
Example CRFs for the CDASHIG PR - Procedures Domain
Example 1
Title: Prespecified Procedures

CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre-Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
PULMANGI_PRCAT |
1 | What was the category of the procedure? | Procedure Category | Record the procedure category, if not preprinted on the CRF. | Text | PRCAT | PRCAT where PRTRT = "PULMONARY ANGIOGRAM" | OUTPATIENT PROCEDURES | Prompt | ||||
PULMANGI_PRTRT |
2 | What was the procedure name? | Procedure Name | Record only one procedure per line. | Text | PRTRT | PULMONARY ANGIOGRAM | Prompt | |||||
PULMANGI_PRPRESP |
3 | N/A | N/A | N/A | Text | PRPRESP | PRPRESP where PRTRT = "PULMONARY ANGIOGRAM" | (NY) | Yes | Yes | Radio | Y | |
PULMANGI_PROCCUR |
4 | Was a pulmonary angiogram performed? | Pulmonary Angiogram Performed | Indicate if a pulmonary angiogram was performed by checking Yes or No. | Text | PROCCUR | PROCCUR where PRTRT = "PULMONARY ANGIOGRAM" | (NY) | Yes; No | Radio | |||
PULMANGI_PRSTDAT |
5 | What was the procedure Start date? | Start Date | Record the date the procedure was started or performed using this format (DD-MON-YYYY). | Date | PRSTDTC | PRSTDTC where PRTRT = "PULMONARY ANGIOGRAM" | Prompt | |||||
PULMANGI_PRINDC |
6 | For what indication was the pulmonary angiogram performed? | Indication | Record the reason the procedure was performed based on clinical investigator's evaluation. If performed to treat a condition, and a diagnosis was made, the indication should be the diagnosis. If performed to treat a condition, and no diagnosis was made, the indication should be the signs and symptoms. If taken as prophylaxis, report as "Prophylaxis for" and include a description of the condition(s). | Text | PRINDC | PRINDC where PRTRT = "PULMONARY ANGIOGRAM" | ||||||
PULMANGI_PRAENO |
7 | What was the identifier for the adverse event(s) for which the procedure was performed? | Adverse Event Identifier | Record the identifier of the adverse event for which this procedure was performed. | Text | N/A | Associate with related AE record in RELREC where PRTRT = "PULMONARY ANGIOGRAM" | Prompt | |||||
CARDCATH_PRCAT |
8 | What was the category of the procedure? | Procedure Category | Record the procedure category, if not preprinted on the CRF. | Text | PRCAT | PRCAT where PRTRT = "CARDIAC CATHETERIZATION" | INPATIENT PROCEDURES | Prompt | ||||
CARDCATH_PRTRT |
9 | What was the procedure name? | Procedure Name | Record only one procedure per line. | Text | PRTRT | CARDIAC CATHETERIZATION | Prompt | |||||
CARDCATH_PRPRESP |
10 | N/A | N/A | N/A | Text | PRPRESP | PRPRESP where PRTRT = "CARDIAC CATHETERIZATION" | (NY) | Yes | Yes | Radio | Y | |
CARDCATH_PROCCUR |
11 | Was cardiac catheterization performed? | Cardiac Catheterization Performed | Indicate if cardiac catheterization was performed by checking Yes or No. | Text | PROCCUR | PROCCUR where PRTRT = "CARDIAC CATHETERIZATION" | (NY) | Yes; No | Radio | |||
CARDCATH_PRSTDAT |
12 | What was the procedure start date? | Start Date | Record the date the procedure was started or performed using this format (DD-MON-YYYY). | Date | PRSTDTC | PRSTDTC where PRTRT = "CARDIAC CATHETERIZATION" | ||||||
CARDCATH_PRITRPYN |
13 | Was the procedure interrupted? | Procedure Interrupted | Record if the procedure was interrupted. | Text | N/A | (NY) | Yes; No | Radio | ||||
CARDCATH_PRCINTD |
14 | What was the duration of the procedure interruption? | Collected Interruption Duration | Record how long the procedure was interrupted before it resumed. | Text | SUPPPR.QVAL | SUPPPR.QVAL where SUPPPR.QNAM="PRITRPD" and SUPPPR.QLABEL= "Interruption Duration" and PRTRT = "CARDIAC CATHETERIZATION" | ||||||
CARDCATH_PRCINTDU |
15 | What was the interruption duration unit? | Interruption Duration Unit | Record the unit for the duration of interruption of the procedure. | Text | N/A | SUPPPR.QVAL where SUPPPR.QNAM="PRITRPD" and SUPPPR.QLABEL= "Interruption Duration" and PRTRT = "CARDIAC CATHETERIZATION" | (UNIT) | HOURS; min | Prompt | Radio | ||
CARDCATH_PRINDC |
16 | For what indication, was the cardiac catheterization performed? | Indication | Record the reason the procedure was performed based on clinical investigator's evaluation. If performed to treat a condition, and a diagnosis was made, the indication should be the diagnosis. If performed to treat a condition, and no diagnosis was made, the indication should be the signs and symptoms. If taken as prophylaxis, report as "Prophylaxis for" and include a description of the condition(s). | Text | PRINDC | PRINDC where PRTRT = "CARDIAC CATHETERIZATION" | ||||||
CARDCATH_PRAENO |
17 | What was the identifier for the adverse event(s) for which the procedure was performed? | Adverse Event Identifier | Record the identifier of the adverse event for which this procedure was performed. | Text | N/A | Associate with related AE record in RELREC where PRTRT = "CARDIAC CATHETERIZATION" | Prompt |
Example 2
Title: Spontaneously Reported Procedures
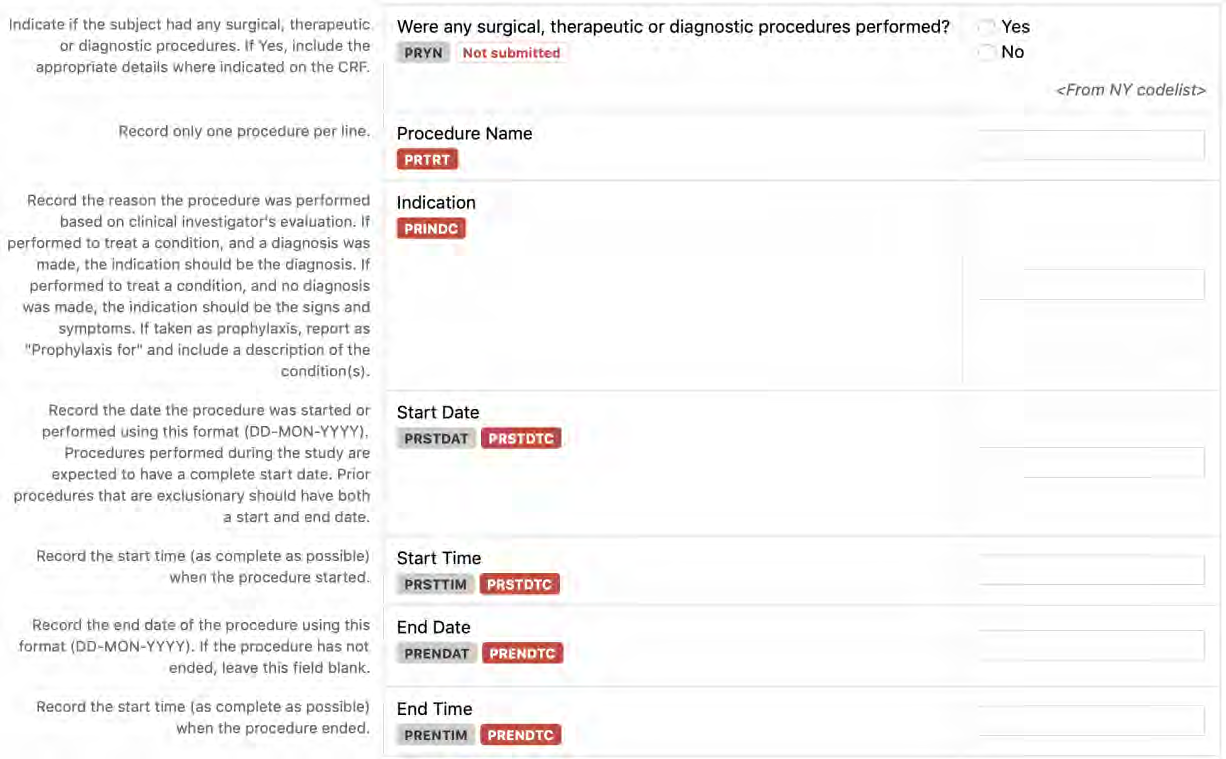
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
PRYN |
1 | Were any surgical, therapeutic or diagnostic procedures performed? | Any Procedures | Indicate if the subject had any surgical, therapeutic or diagnostic procedures. If Yes, include the appropriate details where indicated on the CRF. | Text | N/A | (NY) | Yes; No | Radio | ||||
PRTRT |
2 | What was the procedure name? | Procedure Name | Record only one procedure per line. | Text | PRTRT | Prompt | ||||||
PRINDC |
3 | For what indication, was the procedure performed? | Indication | Record the reason the procedure was performed based on clinical investigator's evaluation. If performed to treat a condition, and a diagnosis was made, the indication should be the diagnosis. If performed to treat a condition, and no diagnosis was made, the indication should be the signs and symptoms. If taken as prophylaxis, report as "Prophylaxis for" and include a description of the condition(s). | Text | PRINDC | Prompt | ||||||
PRSTDAT |
4 | What was the procedure start date? | Start Date | Record the date the procedure was started or performed using this format (DD-MON- YYYY). Procedures performed during the study are expected to have a complete start date. Prior procedures that are exclusionary should have both a start and end date. | Date | PRSTDTC | Prompt | ||||||
PRSTTIM |
5 | What was the procedure start time? | Start Time | Record the start time (as complete as possible) when the procedure started. | Time | PRSTDTC | Prompt | ||||||
PRENDAT |
6 | What was the procedure end date? | End Date | Record the end date of the procedure using this format (DD-MON-YYYY). If the procedure has not ended, leave this field blank. | Date | PRENDTC | Prompt | ||||||
PRENTIM |
7 | What was the procedure end time? | End Time | Record the start time (as complete as possible) when the procedure ended. | Time | PRENDTC | Prompt |
8.1.5 SU - Substance Use
Description/Overview for the CDASHIG SU - Substance Use Domain
This domain is used to collect information on substance use when this information is relevant to the assessment of the efficacy and safety of therapies. The amount of information collected for this domain depends upon the sponsor's protocol. In many protocols, only information on the use of the substance is required. In this case, many of the variables in this domain (e.g., duration, amount) would not be collected.
CDASH recommends the use of the more descriptive CDASHIG variable SUNCF with responses of “Never", "Current", or "Former” for each substance use type, rather than a simple "Yes/No" response. Based on the wide variability of protocol definitions of use, the specific definitions and timeframes for the SUNCF responses would be sponsor/protocol-defined. By using the SUNCF response categories for usage, a number of questions about use and frequency can be collapsed, in turn decreasing the number of data points required in the SU domain. More detailed information about duration, amount, and start and end dates are optionally captured.
Specification for the CDASHIG SU - Substance Use Domain
Substance Use Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Interventions | SU | N/A | N/A |
1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM- based dataset creation before submission. |
Interventions | SU | N/A | N/A |
2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Interventions | SU | N/A | N/A |
3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Interventions | SU | N/A | N/A |
4 | SUTRT | Reported Name of Substance | The type of substance (e.g., TOBACCO, ALCOHOL, CAFFEINE or CIGARETTES, CIGARS, COFFEE). | What [is/was] the [name/type] of (the) substance used? | [Type of Substance] | Char | HR | N/A | SUTRT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsors may require different types of substance use data (e.g., illicit drug use, cigarettes); the value for category may be preprinted on the CRF as a label for the prompt for Substance Use. If a more detailed type of substance appears on the CRF (e.g., CIGARETTES, CIGARS rather than TOBACCO), SUCAT should be TOBACCO and SUTRT should be CIGARETTES. |
Interventions | SU | N/A | N/A |
5 | SUCAT | Category for Substance Use | A grouping of topic-variable values based on user-defined characteristics. | What is/was the category of the substance (used)? | [Substance (Used) Category]; NULL | Char | R/C | Record the Substance Used category, if not preprinted on the CRF. | SUCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology (e.g., TOBACCO, ALCOHOL, CAFFEINE). Sponsors may require different types of substance use data (e.g., illicit drug use, cigarettes); the value for category may be preprinted on the CRF. If a more detailed type of substance appears on the CRF (e.g., CIGARETTES, CIGARS, rather than TOBACCO), SUCAT is TOBACCO and SUTRT is CIGARETTES. If the sponsor does not specify a type of tobacco on the CRF, SUTRT is TOBACCO. If SUCAT is not collected (e.g. it is self- evident from the protocol design), it could be populated during the SDTM-based dataset creation process. |
Interventions | SU | N/A | N/A |
6 | SUSCAT | Subcategory for Substance Use | A sub-division of the SUCAT values based on user-defined characteristics. | What was the subcategory of the substance (used)? | [Substance (Used) Subcategory]; NULL | Char | O | Record the Substance Use subcategory, if not preprinted on the CRF. | SUSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. The value for subcategory may be preprinted on the CRF or hidden. SUSCAT can only be used if there is a SUCAT and it must be a subcategorization of SUCAT. |
Interventions | SU | N/A | N/A |
7 | SUPRESP | SU prespecified | An indication that a specific intervention or a group of interventions is prespecified on a CRF. | N/A | N/A | Char | O | N/A | SUPRESP | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | For prespecified interventions, a hidden field on a CRF defaulted to Y, or added during the SDTM dataset creation. If a study collects both prespecified interventions as well as free- text interventions, the value of SUPRESP should be Y for all prespecified interventions and null for interventions reported as free-text. |
Interventions | SU | N/A | N/A |
8 | SUYN | Any Substance Used | An indication whether or not any data was collected for the intervention topic. | Were any [sponsor- phrase/substance name/recreational drugs] used? | Any [Substance Name (Used)] | Char | O | Indicate if the subject had used any (sponsor-defined phrase/recreational drugs/alcohol/substance name). | N/A | Does not map to an SDTMIG variable. The SDTM Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | General prompt question to aid in monitoring and data cleaning. This provides verification that all other fields on the CRF were deliberately left blank. This is a field that can be used on any interventions CRF to indicate whether or not there is data to record. |
Interventions | SU | N/A | N/A |
9 | SUNCF | Never Current Former Usage | Indication the prespecified substance was used. | Has the subject ever [used/consumed] [SUTRT/SUCAT]? | ([Substance]) Usage | Char | R/C | Check the appropriate box to indicate if the subject has ever used/consumed tobacco/alcohol/caffeine, currently consumes tobacco/alcohol/caffeine, or formerly used/consumed tobacco/alcohol/caffeine. | SUOCCUR; SUSTRTPT; SUSTRF; SUENRTPT; SUENRF; SUPPSU.QVAL | This does not map directly to an SDTMIG variable. May be used to populate SUOCCUR and relative timing variables. | (NCF) | (SUNCF) | The 3 options (NEVER, CURRENT, FORMER) are sponsor-defined in relation to the protocol. If the sponsor has specific definitions, these definitions are detailed in the instructions to the site. As this type of response does not correspond exactly to an SDTM variable, CDASH recommends using the CDASHIG variable SUNCF. Sponsors must decide how to populate the appropriate relative timing variables when the SDTM-based datasets are created. For example, If SUNCF ="Never", the value of SUOCCUR will be "N" and all relative timing variables will be null. If the sponsor chooses to populate the relative start references (SUSTRTPT, SUSTRF) the value will be "BEFORE" when SUNCF= "CURRENT" and "FORMER". If the sponsor also chooses to use relative end references (SUENRF, SUENRTPT) , the SUENRTPT value will be "ONGOING" when SUNCF="CURREN" while the value of SUENRF will be "DURING/AFTER". Note: When using SUSTRTPT and/or SUENRTPT, these must refer to a "time point anchor" such as SCREENING in SUSTTPT/SUENTPT. |
Interventions | SU | N/A | N/A |
10 | SUSPID | Substance Use Sponsor- Defined Identifier | A sponsor-defined identifier. In CDASH, This is typically used for preprinted or auto-generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor-defined question] | [Sponsor defined] | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | SUSPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Since SPID is a sponsor- defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile data. May be used to record preprinted number (e.g., line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Interventions | SU | N/A | N/A |
11 | SUREASND | Reason Substance Use Not Collected | An explanation of why the data are not available. | What was the reason the data was not collected? | Reason Not Collected | Char | O | Provide the reason why the substance used data were not collected. | SUREASND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | The reason the data are not available may be chosen from a sponsor defined list (e.g., subject refused) or entered as free text. When PRREASND is used, the SDTMIG variable PRSTAT should also be populated in the SDTM-based dataset. |
Interventions | SU | N/A | N/A |
12 | SUDSTXT | Substance Dose Description | The amount of substance used (e.g., 1-2 packs, 8 ounces). | What is/was the amount of [SUTRT] used/consumed? | Amount | Char | O | Check the appropriate box to indicate the amount of tobacco/alcohol/caffeine the subject consumes on a regular basis. | SUDOSE; SUDOSU; SUDOSTXT | This does not map directly to an SDTMIG variable. Numeric values map to SUDOSE in SDTM. Non- numeric values (e.g., 200-400) map to SUDOSTXT in SDTM. | N/A | N/A | Where possible, the options for dose/amount are preprinted on the CRF. In the example given in the Definition, (packs) and (ounces) are included as a point of reference. They would, of course, be submitted as SUDOSU. Care should be taken to map each record to the appropriate SDTM variable SUDOSTXT (text results that cannot be represented in a numeric field) and SUDOSE (numeric results). |
Interventions | SU | N/A | N/A |
13 | SUDOSFRQ | Substance Use Frequency per Interval | The number/amount of the of substance consumed per a specific interval. | What [is/was] the frequency of [SUTRT] [use/consumption]? | Frequency | Char | O | Record how often the subject regularly [uses / consumes] (the) [substance]. | SUDOSFRQ | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (FREQ) | N/A | When possible, the options for dose/amount frequency are preprinted on the CRF. (e.g., PER DAY, PER WEEK, OCCASIONAL). |
Interventions | SU | N/A | N/A |
14 | SUSTDAT | Substance Use Start Date | The date substance use started, represented in an unambiguous date format (e.g., DD-MON-YYYY). | What was the start date of [SUTRT/SUCAT] use/consumption? | Start Date | Char | O | Record the start date of the substance use using this format (DD-MON-YYYY). | SUSTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable SUSTDTC in ISO 8601 format. | N/A | N/A | The sponsor may choose to capture a complete date or any variation thereof (e.g., month and year, year). |
Interventions | SU | N/A | N/A |
15 | SUENDAT | Substance Use End Date | The date substance use ended, represented in an unambiguous date format (e.g., DD-MON-YYYY). | What was the end date of [SUTRT/SUCAT] use/consumption? | End Date | Char | O | Record the end date of the substance use using this format (DD-MON-YYYY). | SUENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable SUENDTC in ISO 8601 format. | N/A | N/A | The sponsor may choose to capture a complete date or any variation thereof (e.g., month and year, year). |
Interventions | SU | N/A | N/A |
16 | SUCDUR | Substance Use Collected Duration | Collected duration of the substance use. | What was the duration of [SUTRT/SUCAT] use/consumption? | Duration | Char | O | Provide the duration of the substance use (e.g., Record how long the subject has smoked). | SUDUR | This does not map directly to an SDTMIG variable. For the SDTM- based dataset, concatenating the CDASH collected duration and collected duration unit and populate the SDTMIG variable SUDUR in ISO 8601 format. Example: P1DT2H (for 1 day, 2 hours). | N/A | N/A | This is only collected on the CRF if this level of detail is needed and if SUSTDAT and SUENDAT are not collected on the CRF. |
Interventions | SU | N/A | N/A |
17 | SUCDURU | Substance Use Collected Duration Unit | Unit of the collected duration of the substance use. Used only if duration was collected on the CRF. | What was the unit of duration of [SUTRT/SUCAT] use/consumption? | (Duration) Unit | Char | O | Select the appropriate duration unit of the substance use. | SUDUR | This does not map directly to an SDTMIG variable. For the SDTM- based dataset, concatenating the CDASH collected duration and collected duration unit and populate the SDTMIG variable SUDUR in ISO 8601 format. Example: P1DT2H (for 1 day, 2 hours). | (UNIT) | N/A | The sponsor-defined options should be preprinted on the CRF to avoid making this a free text field. This will allow the response to be translated into ISO 8601 format. |
Interventions | SU | N/A | N/A | 18 | SUMODIFY | Modified Substance Name | If the value for SUTERM is modified for coding purposes, then the modified text is placed here. | N/A | N/A | Char | O | N/A | SUMODIFY | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This is not a data collection field that would appear on the CRF. If the sponsor chooses to code the substance use, the sponsor will populate this through the coding process. |
Interventions | SU | N/A | N/A | 19 | SUDECOD | Standardized Substance Name | The dictionary or sponsor-defined standardized text description of SUTRT, or the modified topic variable (SUMODIFY), if applicable. | N/A | N/A | Char | O | N/A | SUDECOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata to indicate that the data was "ASSIGNED". | N/A | N/A | This is typically not a data collection field that will appear on the CRF. If the sponsor chooses to code the substance use, the sponsor will populate this through the coding process. Equivalent to the generic drug name in WHODrug, or a term in SNOMED, ICD9, or other published or sponsor-defined dictionaries. If SUPRESP is used, and the information about a specific standardized substance name is being solicited, the data from SUTRT may map directly to the SDTMIG SUDECOD variable. |
Assumptions for the CDASHIG SU - Substance Use Domain
1. Categories SUCAT and SUSCAT
a. Sponsors may require different types of substance use data (e.g., illicit drug use, cigarettes) to be collected; the value for category may be preprinted on the CRF.
b. SUCAT and SUSCAT should not be redundant with SUTRT. For example, if a more detailed type of substance usage is collected on the CRF (e.g., “CIGARETTES”, “CIGARS”), SUCAT should be “TOBACCO” and SUTRT could be “CIGARETTES”, "CIGARS". If the sponsor does not solicit responses about specific types of substance used, on the CRF (e.g., “CIGAR”, “CIGARETTE”), the value of SUTRT is the more general description of the substance (e.g., “TOBACCO") and SUCAT is generally null. This practice avoids assigning the same value to both SUTRT and SUCAT. However, for consistency across studies, the sponsor may elect to repeat the values of SUTRT in SUCAT.
2. The SDTMIG variable SUPRESP should be prepopulated to the value of "Y" when information about the use of a specific substance is solicited on the CRF.
3. Relative Timing Variables
a. Relative timing variables are used to represent collected data in the SDTM tabulations in those cases where a Start Date or an End Date has not been collected, but some indication of when/if the intervention or event started or ended has been collected. In the CDASHIG SU domain, if the CDASHIG variable SUNCF is used (with the possible responses of "Never", "Current", or "Former"), the collected values may be used to derive a value into an SDTMIG relative timing variable to represent when the subject started or stopped using the substance relative to either a time point or to a period of time in the study. For example, if the value collected in SUNCF is "Current", the value of "ONGOING" may be represented in the SDTMIG Variable SUENRTPT to indicate that the subject was still using cigarettes as of the time point described in SUENTPT. It is recommended that the sponsor collect either a date or a description of a time point that will be used in conjunction with relative timing variables. See SDTMIG v3.2 Section 4.1.4.7 for more information about relative timing variables.
b. If the actual, complete start date or end date of the substance use has been collected, there is no need to use relative timing variables.
4. Start and End Dates
a. Start and end dates can be collected if this level of detail is required by the protocol. Partial dates may be collected when the subject does not remember the complete date of when substance use started or ended. The sponsor may choose to capture a complete date or any variation thereof (e.g., month and year, year).
b. Sponsors may elect to capture only a start date, or only an end date, and use the associated SDTMIG relative timing variables to represent information about the date not collected.
c. If the sponsor is only interested in collecting whether or not the subject is consuming a particular substance, start and end dates are optional and may be omitted, and SUNCF may be collected as described above.
5. Coding
a. Coding may be performed if deemed necessary by the sponsor. The SDTMIG variable SUDECOD is a permissible variable in the SDTMIG SU domain.
b. Coding variables are not usually displayed on CRFs. If a sponsor chooses to display coding on the form, CDASH does not advocate using that as a field for entry by site personnel.
Example CRFs for the CDASHIG SU - Substance Use Domain
Example 1
Title: Recreational Drug Substance Use
|
Example CRF Completion Instructions Record the substance(s) used by the subject. |
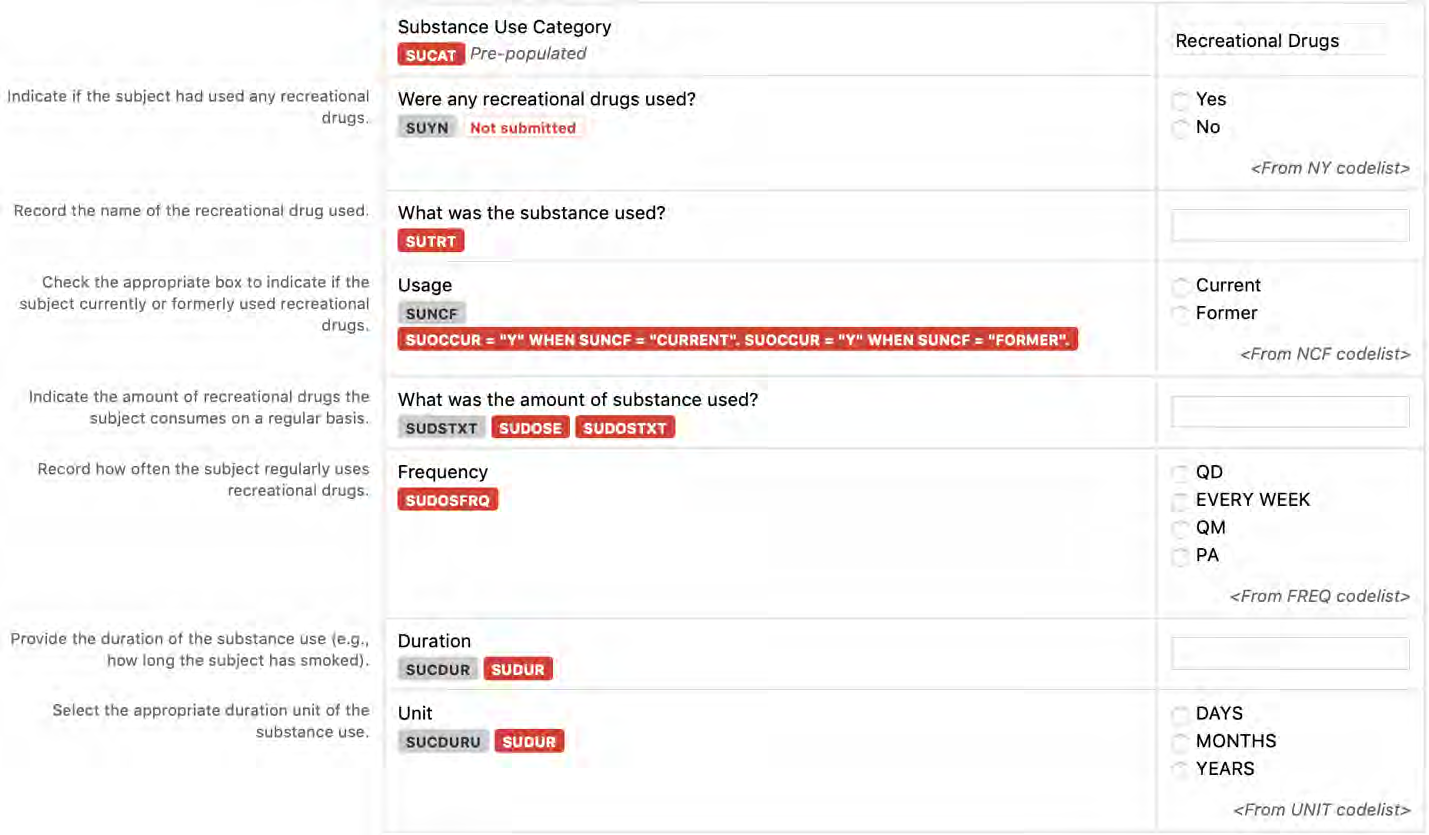
CRF Metatadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
SUCAT | 1 | What is the category of the substance used? | Substance Use Category | Record the Substance Used category, if not pre-printed on the CRF. | Text | SUCAT | Recreational Drugs | ||||||
SUYN | 2 | Were any recreational drugs used? | Any Recreational Drugs Used | Indicate if the subject had used any recreational drugs. | Text | N/A | (NY) | Yes; No | |||||
SUTRT | 3 | What was the substance used? | Type of Recreational Drug Used | Record the name of the recreational drug used. | Text | SUTRT | |||||||
SUNCF | 4 | Does subject use recreational drugs? | Usage | Check the appropriate box to indicate if the subject currently or formerly used recreational drugs. | Text | SUOCCUR; SUSTRTPT; SUSTRF; SUENRTPT; SUENRF | SUOCCUR = "Y" WHEN SUNCF = "CURRENT". SUOCCUR = "Y" WHEN SUNCF = "FORMER". | (NCF) | Current; Former | Prompt | |||
SUDSTXT | 5 | What was the amount of substance used? | Amount | Indicate the amount of recreational drugs the subject consumes on a regular basis. | Text | SUDOSE; SUDOSTXT | |||||||
SUDOSFRQ | 6 | What was the frequency of recreational drug use? | Frequency | Record how often the subject regularly uses recreational drugs. | Text | SUDOSFRQ | (FREQ) | QD; EVERY WEEK; QM; PA | Prompt | ||||
SUCDUR | 7 | What was the duration of recreational drug use consumption? | Duration | Provide the duration of the substance use (e.g., how long the subject has smoked). | Text | SUDUR | Prompt | ||||||
SUCDURU | 8 | What was the unit of duration of recreational drug use? | Unit | Select the appropriate duration unit of the substance use. | Text | SUDUR | (UNIT) | DAYS; MONTHS; YEARS | Prompt |
Example 2
Title: Alcohol Substance Use
|
Example CRF Completion Instructions The amount of each type of alcohol consumed should be an integer. The following description should be used in determining the amount consumed: 1 Beer Unit = 12 oz or 360 ml 1 Wine Unit = 5 oz or 150 ml 1 Spirits Unit = 1.5 oz or 45 ml |
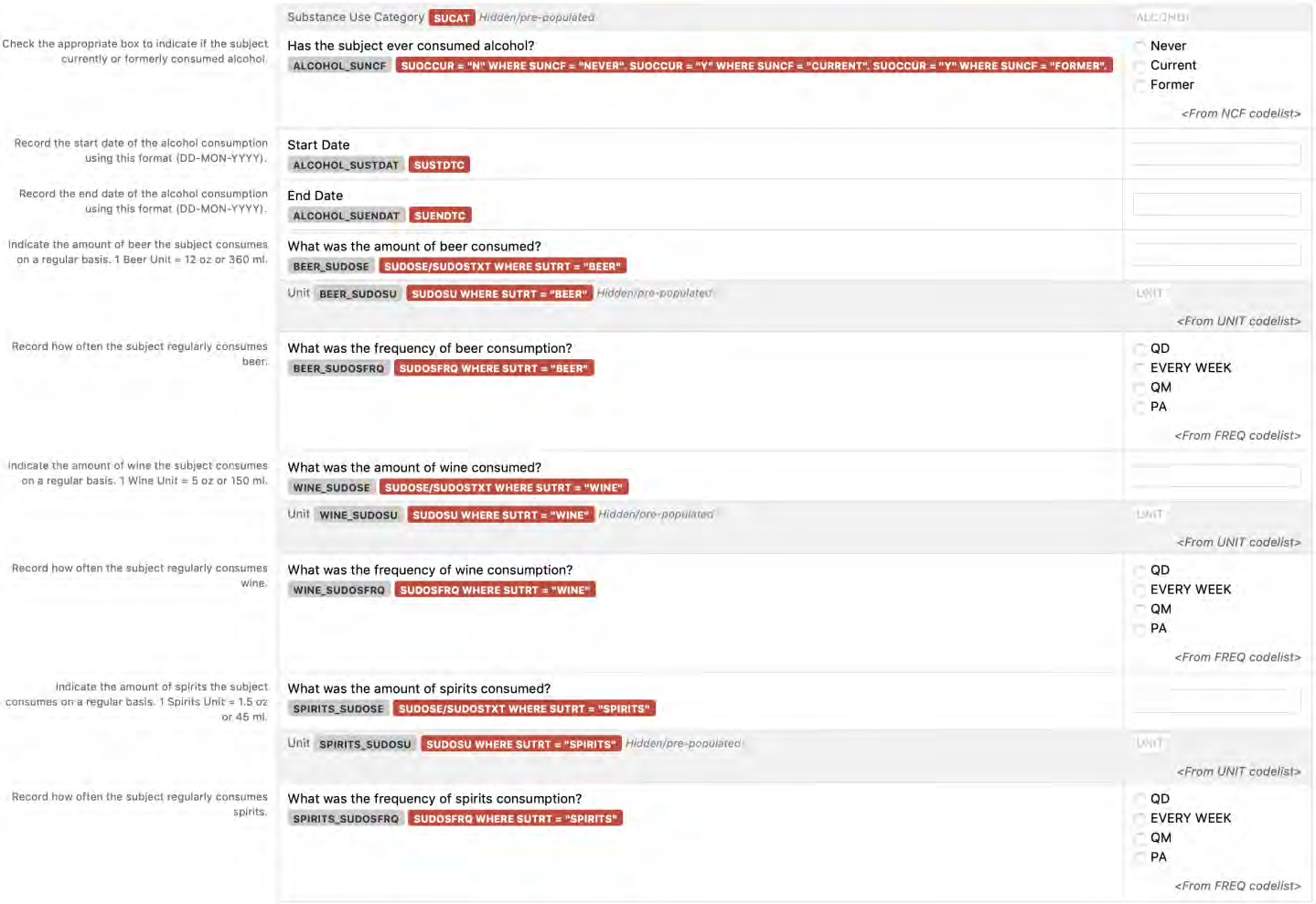
CRF Metatadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
SUCAT | 1 | What is the category of the substance used? | Substance Use Category | The amount of each type of alcohol consumed should be an integer. The following description should be used in determining the amount consumed: 1 Beer Unit = 12 oz or 360 ml. 1 Wine Unit = 5 oz or 150 ml. 1 Spirits Unit = 1.5 oz or 45 ml. | Text | SUCAT | ALCOHOL | Prompt | Yes | ||||
ALCOHOL_SUNCF | 2 | Has the subject ever consumed alcohol? | Usage | Check the appropriate box to indicate if the subject currently or formerly consumed alcohol. | Text | SUOCCUR | SUOCCUR = "N" WHERE SUNCF = "NEVER". SUOCCUR = "Y" WHERE SUNCF = "CURRENT". SUOCCUR = "Y" WHERE SUNCF = "FORMER". | (NCF) | Never; Current; Former | ||||
ALCOHOL_SUSTDAT | 3 | What was the start date of alcohol consumption? | Start Date | Record the start date of the alcohol consumption using this format (DD-MON-YYYY). | Date | SUSTDTC | Prompt | ||||||
ALCOHOL_SUENDAT | 4 | What was the end date of alcohol consumption? | End Date | Record the end date of the alcohol consumption using this format (DD-MON-YYYY). | Date | SUENDTC | Prompt | ||||||
BEER_SUDOSE | 5 | What was the amount of beer consumed? | Amount | Indicate the amount of beer the subject consumes on a regular basis. 1 Beer Unit = 12 oz or 360 ml. | Text | SUDOSE; SUDOSTXT | SUDOSE/SUDOSTXT WHERE SUTRT = "BEER" | ||||||
BEER_SUDOSU | 6 | What was the unit for the amount of beer consumption? | Unit | N/A | Text | SUDOSU | SUDOSU WHERE SUTRT = "BEER" | (UNIT) | UNIT | Yes | |||
BEER_SUDOSFRQ | 7 | What was the frequency of beer consumption? | Frequency | Record how often the subject regularly consumes beer. | Text | SUDOSFRQ | SUDOSFRQ WHERE SUTRT = "BEER" | (FREQ) | QD; EVERY WEEK; QM; PA | Prompt | |||
WINE_SUDOSE | 8 | What was the amount of wine consumed? | Amount | Indicate the amount of wine the subject consumes on a regular basis. 1 Wine Unit = 5 oz or 150 ml. | Text | SUDOSE; SUDOSTXT | SUDOSE/SUDOSTXT WHERE SUTRT = "WINE" | ||||||
WINE_SUDOSU | 9 | What was the unit for the amount of wine consumption? | Unit | N/A | Text | SUDOSU | SUDOSU WHERE SUTRT = "WINE" | (UNIT) | UNIT | Yes | |||
WINE_SUDOSFRQ | 10 | What was the frequency of wine consumption? | Frequency | Record how often the subject regularly consumes wine. | Text | SUDOSFRQ | SUDOSFRQ WHERE SUTRT = "WINE" | (FREQ) | QD; EVERY WEEK; QM; PA | Prompt | |||
SPIRITS_SUDOSE | 11 | What was the amount of spirits consumed? | Amount | Indicate the amount of spirits the subject consumes on a regular basis. 1 Spirits Unit = 1.5 oz or 45 ml. | Text | SUDOSE; SUDOSTXT | SUDOSE/SUDOSTXT WHERE SUTRT = "SPIRITS" | ||||||
SPIRITS_SUDOSU | 12 | What was the unit for the amount of spirits consumption? | Unit | N/A | Text | SUDOSU | SUDOSU WHERE SUTRT = "SPIRITS" | (UNIT) | UNIT | Yes | |||
SPIRITS_SUDOSFRQ | 13 | What was the frequency of spirits consumption? | Frequency | Record how often the subject regularly consumes spirits. | Text | SUDOSFRQ | SUDOSFRQ WHERE SUTRT = "SPIRITS" | (FREQ) | QD; EVERY WEEK; QM; PA | Prompt |
8.2 Events Class Domains
The Events class includes CDASH domains that define standards for the collection of occurrences or incidents occurring during a trial (e.g., adverse events, disposition) or prior to a trial (e.g., medical history).
8.2.1 General CDASH Assumptions for Events Domains
1. CDASH --YN variables with the question text "Were there any <'events'>?" (e.g., “Were there any adverse events?”, “Were there any healthcare encounters?”) are intended to assist in the cleaning of data and in confirming there are no missing values. These questions can be added to any CRF in order to capture this information.
2. CDASH --CAT and/or -SCAT are generally not entered on the CRF by sites. Implementers may prepopulate and display these category values to help sites understand what data should be recorded on the CRF. Implementers may also prepopulate hidden variables with the values assigned within their operational database. Categories and subcategories are typically self-evident from the protocol design, and could be populated during SDTM dataset creation.
3. Date and Time Variables
a. CDASH date variables (e.g., --DAT, --STDAT,--ENDAT) are concatenated with the CDASH time variables (e.g., --TIM, --STTIM, --ENTIM, if time is collected) into the appropriate SDTM --DTC variables (e.g., --DTC, – STDTC, --ENDTC) using the ISO 8601 format.
b. Collecting the time of an event is only appropriate if it can be easily obtained and if there is a scientific reason, such as the need to know the order of events (e.g., the adverse event started after dosing). An example of this would be a study where the subject is confined to a phase 1 unit and under the direct care of the unit staff at the time that the event started or using time to tie together dosing and pharmacokinetic (PK) sample collection.
4. CDASH --COCCUR variable is used only if a specific event is solicited (preprinted) on the CRF and the CRF elicits a response from the CDASH Codelist --COCCUR. As of the time of publication, CDASH Controlled Terminology includes values that indicate that the data was not collected (Not Done). Whereas the SDTM Controlled Terminology for --OCCUR only includes "N"," Y", and "UNKNOWN" responses, if the CDASH variable --OCCUR is used, the CRF would require a second question to indicate that the data was not collected. In STDM, the CDASH --COCCUR variable will be mapped to the SDTM variable --OCCUR and --STAT variables. Responses of "Y" or "N" map directly to SDTM -- OCCUR. Responses of "NOT DONE" or "NOT COLLECTED" map to SDTM --STAT as "NOT DONE". - -COCCUR is not used if the events are not prespecified.
5. The CDASH variable --REASND is used in conjunction with SDTM variable --STAT. The value "NOT DONE" in --STAT indicates that the subject was not questioned about the event or that data was not collected; it does not mean that the subject had no events.
6. The CDASH --SPID variable may be populated by the sponsor's data collection system. If collected, it can be beneficial to use an identifier in a data query to communicate clearly to the site the specific record in question. This field may be populated by the sponsor's data collection system.
7. Coding
a. The CDASH variables used for coding are not data collection fields that will appear on the CRF itself. Sponsors will populate these through the coding process.
b. When free-text event terms are entered, the location may be included in --TERM to facilitate coding and further clarify the event. This location information does not need to be removed from the verbatim term when the SDTM submission datasets are created.
c. The CDASH variables --LLT, --LLTCD, --PTCD, --HLT, --HLTCD, --HLGT, --HLGTCD, --SOC, and --SOCCD are only applicable to events coded in MedDRA.
8. Location (--LOC, --LAT, --DIR, --PORTOT)
a. Location is collected when the sponsor needs to identify the specific anatomical location of the event.
b. Implementers may collect the location information using a subset list of controlled terminology on the CRF. Location variables can be pre-populated as needed. There is currently some overlap across the LOC, LAT, and DIR variables for controlled terminology. While the overlap exists, ensure that this overlap for these variables is not part of database design.
8.2.2 AE - Adverse Events
Description/Overview for the CDASHIG AE - Adverse Events Domain
The Adverse Events (AE) domain includes clinical data describing "any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have to have a causal relationship with this treatment" (ICH E2A). In consultation with regulatory authorities, sponsors may extend or limit the scope of adverse event collection (e.g., collecting pre-treatment events related to trial conduct, not collecting events that are assessed as efficacy endpoints). The events included in the AE domain should be consistent with the protocol requirements. Adverse event terms may be captured either as free text or via a prespecified list of terms. The structure of the SDTMIG AE domain is 1 record per adverse event per subject. It is the sponsor's responsibility to define an event. This definition may vary based on the sponsor's requirements for characterizing and reporting product safety and is usually described in the protocol.
As with all the data collection variables recommended in CDASH, it is assumed that sponsors will add other data variables as needed to meet protocol-specific and other data collection requirements (e.g., therapeutic area-specific data elements and others as required per protocol, business practice, or operating procedures). Sponsors should define the appropriate collection period for adverse events.
Specification for the CDASHIG AE - Adverse Events Domain
Adverse Events Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Events | AE | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Events | AE | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted for the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Events | AE | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Events | AE | N/A | N/A | 4 | AEYN | Any Adverse Event | An indication whether or not any AEs were experienced during the study. | Were any adverse events experienced? | Any Adverse Events | Char | O | Indicate if the subject experienced any adverse events. If yes, include the appropriate details where indicated on the CRF. | N/A | Does not map to an SDTMIG variable. The SDTM aCRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification that all other fields on the CRF were deliberately left blank. |
Events | AE | N/A | N/A | 5 | AECAT | Category for Adverse Event | A grouping of topic- variable values based on user-defined characteristics. | What is the category of the adverse event? | [Adverse Event Category]; NULL | Char | O | Record the adverse event category, if not preprinted on the CRF. | AECAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Events | AE | N/A | N/A | 6 | AESCAT | Subcategory for Adverse Event | A sub-division of the AECAT values based on user-defined characteristics. | What is the subcategory of the adverse event? | [Adverse Event Subcategory]; NULL | Char | O | Record the adverse event subcategory, if not preprinted on the CRF. | AESCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. AESCAT can only be used if there is an AECAT and it must be a subcategorization of AECAT. |
Events | AE | N/A | N/A | 7 | AESPID | AE Sponsor- Defined Identifier | A sponsor-defined identifier. In CDASH, This is typically used for preprinted or auto- generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor-defined question] | Sponsor defined | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | AESPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Because SPID is a sponsor-defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile concomitant medications, procedures and/or medical history records with AEs. If CMAENO or PRAENO is used, this is the identifier to which CMAENO or PRAENO refers. May be used to record preprinted number (e.g., line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Events | AE | N/A | N/A | 8 | AETERM | Reported Term for the Adverse Event | The reported or prespecified name of the adverse event. | What is the adverse event term? | Adverse Event | Char | HR | Record only 1 diagnosis, sign or symptom per line (e.g., nausea and vomiting should not be recorded in the same entry, but as 2 separate entries). Using accepted medical terminology, enter the diagnosis (if known); otherwise enter a sign or symptom. | AETERM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Can be represented either as an open- entry field to capture verbatim terms reported by subjects or could be preprinted in the situation where solicited AEs of interest are captured. In most cases, the verbatim term (i.e., investigator-reported term) will be coded to a standard medical dictionary such as MedDRA or WHO ART, after the data have been collected on the CRF. |
Events | AE | N/A | N/A | 9 | AEOCCUR | Adverse Event Occurrence | An indication whether a prespecified adverse event or a group of adverse events occurred when information about the occurrence of a specific event is solicited. | Did the subject have [prespecified adverse event/group of adverse events]? | [Specific Adverse Event] | Char | O | Indicate if [specific adverse event] has occurred/is occurring by checking Yes or No. | FAORRES | This does not map directly to an SDTMIG variable. Because the SDTM AE domain is intended to hold only adverse events that actually happen, all values collected in AEOCCUR for prespecified AEs should be submitted in a Findings About Adverse Events data set (FAAE) where FAORRES=the value of AEOCCUR where FATESTCD="OCCUR". In addition, where AEOCCUR="Y", there should be a corresponding record in the AE domain. | (NY) | N/A | The CDASH variable AEOCCUR is used to indicate the occurrence of prespecified adverse events (e.g., "Did the subject have high blood pressure?"). AEOCCUR should not be used for spontaneously reported adverse events. The site should be able to indicate that the response was not asked or answered. |
Events | AE | N/A | N/A | 10 | AEPRESP | prespecified Adverse Event | An indication that a specific event, or group of events, are prespecified on a CRF. | N/A | N/A | Char | O | N/A | AEPRESP | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | A hidden field on a CRF defaulted to "Y", or added during SDTM-based dataset creation, when the AE is prespecified. Null for spontaneously reported events. If a study collects both prespecified adverse events as well as free-text events, the value of AEPRESP should be "Y" for all prespecified events and null for events reported as free- text. AEPRESP is a permissible field in SDTM and may be omitted from the SDTM-based dataset if all events were collected as free text. |
Events | AE | N/A | N/A | 11 | AESTDAT | Adverse Event Start Date | The start date of the adverse event, represented in an unambiguous date format (e.g., DD- MON-YYYY). | What is the adverse event start date? | Start Date | Char | HR | Record the start date of the AE using this format (DD-MON- YYYY). | AESTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable AESTDTC in ISO 8601 format. | N/A | N/A | N/A |
Events | AE | N/A | N/A | 12 | AESTTIM | Start Time of Adverse Event | The start time of the adverse event, represented in an unambiguous time format (e.g., hh:mm:ss) | What is the adverse event start time? | Start Time | Char | R/C | Record the start time (as complete as possible) of the AE. | AESTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable AESTDTC in ISO 8601 format. | N/A | N/A | Collecting the time an AE started is only appropriate if it can be realistically determined and if there is a scientific reason for needing to know this level of detail. An example is where the subject is under the direct care of the site at the time the event started and the study design is such that it is important to know the AE start time with respect to dosing. |
Events | AE | N/A | N/A | 13 | AELOC | AE Location of Event | A description of the anatomical location relevant for the adverse event. | What is the anatomical location of the adverse event? | Anatomical Location | Char | O | Indicate the anatomical location of the adverse event. | AELOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. LAT, DIR, PORTOT are used to further describe the anatomical location. |
Events | AE | N/A | N/A | 14 | AELAT | Adverse Event Laterality | Qualifier for anatomical location further detailing the side of the body relevant for the event. | What is the side of the anatomical location of the adverse event? | Side | Char | O | Record the side of the anatomical location of the adverse event. | AELAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Events | AE | N/A | N/A | 15 | AEDIR | Adverse Event Directionality | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What is the directionality of the anatomical location of the adverse event? | Directionality | Char | O | Record the directionality of the anatomical location of the adverse events. | AEDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Events | AE | N/A | N/A | 16 | AEPORTOT | AE Location Portion or Totality | Qualifier for anatomical location further detailing the distribution (i.e., arrangement of, apportioning of). | What is the portion or totality of the anatomical location of the adverse event? | Portion or Totality | Char | O | Indicate the portion or totality anatomical location of the adverse event. | AEPORTOT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (PORTOT) | N/A | Collected when the sponsor needs to identify the specific portionality for the anatomical locations. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Events | AE | N/A | N/A | 17 | AEONGO | Ongoing Adverse Event | Indication that an adverse event is ongoing when no End Date is provided. | Is the adverse event ongoing (as of [the study- specific time point or period])? | Ongoing (as of [the study-specific time point or period]) | Char | O | Indicate if the adverse event has not resolved at the time of data collection; leave the End Date blank. | AEENRTPT; AEENRF | This does not map directly to an SDTMIG variable. May be used to populate a value into an SDTMIG relative timing variable such as AEENRF or AEENRTPT. When populating AEENRF, if the value of AEONGO is "Y", the value of "DURING", "AFTER" or "DURING/AFTER" may be used. When populating AEENRTPT, if the value of AEONGO is "Y", the value of "ONGOING" may be used. When AEONGO refers to the Study Reference Period (defined in DM.RFSTDTC to DM.RFENDTC) the SDTMIG variable AEENRF should be populated. When | AEONGO is compared to another time point, the SDTMIG variables AEENRTPT and AEENTPT should be used. Note: AEENRTPT must refer to a time point anchor described in AEENTPT. (NY) | N/A | Completed to indicate that the AE has not resolved at the time of data collection, when no End Date is collected. In some cases the ongoing status may be determined from AE Outcome. The purpose of collecting this field is to help with data cleaning and monitoring; this field provides further confirmation that the End Date was deliberately left blank. Often used as a tick/checkbox. |
Events | AE | N/A | N/A | 18 | AEENDAT | Adverse Event End Date | The date when the adverse event resolved/ended, represented in an unambiguous date format (e.g., DD- MON-YYYY). | What was the adverse event end date? | End Date | Char | R/C | Record the date that the AE resolved using this format (DD-MON-YYYY). If the AE is ongoing, leave the field blank. | AEENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable AEENDTC in ISO 8601 format. | N/A | N/A | The definition of resolved is sponsor- specific. The preferred method is to collect a complete end date (if applicable). Partial dates (e.g., providing year only, month and year only) may be acceptable. |
Events | AE | N/A | N/A | 19 | AEENTIM | End Time of Adverse Event | The time when the adverse event ended/resolved, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the adverse event end time? | End Time | Char | R/C | Record the time (as complete as possible) that the AE resolved. | AEENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable AEENDTC in ISO 8601 format. | N/A | N/A | Collecting the time an AE resolved is only appropriate if it can be realistically determined and if there is a scientific reason for needing to know this level of detail. An example is where the subject is under the direct care of the site at the time the event resolved and the study design is such that it is important to know the AE end time with respect to dosing. |
Events | AE | N/A | N/A | 20 | AESEV | AE Severity/Intensity | The severity or intensity of the event. | What is the severity of the adverse event? | Severity | Char | R/C | The reporting physician/healthcare professional will assess the severity of the event using the sponsor-defined categories. This assessment is subjective and the reporting physician/ healthcare professional should use medical judgment to compare the reported AE to similar type events observed in clinical practice. Severity is not equivalent to seriousness. | AESEV | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (AESEV) | N/A | Either AESEV or AETOXGR must appear on the CRF. Some studies may mandate the collection of both. Refer to ICH E3 Section 12.2.4 guidelines for Clinical Study Reports. |
Events | AE | N/A | N/A | 21 | AETOXGR | AE Standard Toxicity Grade | The grade of the severity of the event using a standard "toxicity" scale (e.g., NCI CTCAE). | What is the [NCI CTCAE/Name of scale (toxicity) grade] of the adverse event? | [NCI CTCAE/ Name of the scale] (Toxicity) Grade | Char | R/C | The reporting physician/healthcare professional will assess the severity of the adverse event using the specified grades scale. | AETOXGR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the toxicity scale name and version used to map the terms utilizing the Define-XML external code list attributes. | N/A | N/A | Either AESEV or AETOXGR must appear on the CRF. Some studies may mandate the collection of both. Refer to ICH E3 Section 12.2.4 guidelines for CSR. CTCAE grade is commonly used in oncology studies, although it can also be used elsewhere. Other published "toxicity- like" scales can also be used. |
Events | AE | N/A | N/A | 22 | AESER | AE Serious Event | An indication whether or not the adverse event is determined to be "serious" based on what is defined in the protocol. | Was the adverse event serious? | Serious | Char | R/C | Assess if an adverse event should be classified as serious based on the serious criteria defined in the protocol. | AESER | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | This field is related to the individual serious adverse event type fields, which may or may not be collected on the CRF. Either AESER or all the Adverse Serious type fields must be present on the CRF. The sponsor should check if the regulatory agencies to which the data will be submitted require the collection of this data. |
Events | AE | N/A | N/A | 23 | AESDTH | Results in Death | An indication the serious adverse event resulted in death. | Did the adverse event result in death? | Death | Char | R/C | Record whether the serious adverse event resulted in death. | AESDTH | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | If the details regarding Serious AEs are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Sponsors may only collect the AESER field because the individual serious adverse event types might be collected in a separate pharmacovigilance database and therefore are not collected in the clinical database. The sponsor should check if the regulatory agencies to which the data will be submitted require the collection of this data. |
Events | AE | N/A | N/A | 24 | DTHDAT | Death Date | Date of death for any subject who died. | What [is/was] the subject's date of death? | Death Date | Char | O | Record the date of death. | DM.DTHDTC | This field does not map directly to an SDTMIG variable. For the SDTM dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTM variable DTHDTC in ISO 8601 format. | N/A | N/A | The CDASH model defines Death Date as a timing variable; this is not included as a timing variable in the SDTMIG. It may be collected on any CRF deemed appropriate by the sponsor, but should only be collected once. The SDTMIG variable DTHDTC is mapped to the DM domain during the SDTM submission dataset creation process. The SDTMIG variable DM.DTHFLG is not a CDASH variable, but it is typically populated during the SDTM submission dataset creation process. Death Date may be mapped to other SDTM domains, as deemed appropriate by the sponsor (e.g., DS). |
Events | AE | N/A | N/A | 25 | AESLIFE | Is Life Threatening | An indication the serious adverse event was life threatening. | Was the adverse event life threatening? | Life Threatening | Char | R/C | Record whether the serious adverse event is life threatening. | AESLIFE | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | If the details regarding Serious AEs are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Sponsors may only collect the AESER field because the individual serious adverse event types might be collected in a separate pharmacovigilance database and therefore are not collected in the clinical database. The sponsor should check if the regulatory agencies to which the data will be submitted require the collection of this data. |
Events | AE | N/A | N/A | 26 | AESHOSP | Requires or Prolongs Hospitalization | An indication the serious adverse event resulted in an initial or prolonged hospitalization. | Did the adverse event result in initial or prolonged hospitalization for the subject? | Hospitalization (initial or prolonged) | Char | R/C | Record whether the serious adverse event resulted in an initial or prolonged hospitalization. | AESHOSP | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | If the details regarding Serious AEs are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Sponsors may only collect the AESER field because the individual serious adverse event types might be collected in a separate pharmacovigilance database and therefore are not collected in the clinical database. The sponsor should check if the regulatory agencies to which the data will be submitted require the collection of this data. |
Events | AE | N/A | N/A | 27 | AESDISAB | Persist or Signif Disability/Incapacity | An indication the serious adverse event was associated with a persistent or significant disability or incapacity. | Did the adverse event result in disability or permanent damage? | Disability or Permanent Damage | Char | R/C | Record whether the serious adverse event resulted in a persistent or significant disability or incapacity. | AESDISAB | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | If the details regarding Serious AEs are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Sponsors may only collect the AESER field because the individual serious adverse event types might be collected in a separate pharmacovigilance database and therefore are not collected in the clinical database. The sponsor should check if the regulatory agencies to which the data will be submitted require the collection of this data. |
Events | AE | N/A | N/A | 28 | AESCONG | Congenital Anomaly or Birth Defect | An indication the serious adverse event was associated with a congenital anomaly or birth defect. | Was the adverse event associated with a congenital anomaly or birth defect? | Congenital Anomaly or Birth Defect | Char | R/C | Record whether the serious adverse event was associated with congenital anomaly or birth defect. | AESCONG | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | If the details regarding Serious AEs are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Sponsors may only collect the AESER field because the individual serious adverse event types might be collected in a separate pharmacovigilance database and therefore are not collected in the clinical database. The sponsor should check if the regulatory agencies to which the data will be submitted require the collection of this data. |
Events | AE | N/A | N/A | 29 | AESINTV | Needs Intervention to Prevent Impairment | An indication an adverse event required medical or surgical intervention to preclude permanent impairment of a body function, or prevent permanent damage to a body structure, due to the use of a medical product. | Did the adverse event require intervention to prevent permanent impairment or damage resulting from the use of a medical product? | Needs Intervention to Prevent Impairment | Char | O | Record whether the serious adverse event required intervention to prevent permanent impairment or damage due to the use of a medical product. | SUPPAE.QVAL | This does not map directly to an SDTMIG variable. Sponsors should see requirements for the reporting of adverse events involving medical devices. Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. | (NY) | N/A | If the details regarding Serious AEs are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Sponsors may only collect the AESER field because the individual serious adverse event types might be collected in a separate pharmacovigilance database and therefore are not collected in the clinical database. The sponsor should check if the regulatory agencies to which the data will be submitted require the collection of this data. |
Events | AE | N/A | N/A | 30 | AESMIE | Other Medically Important Serious Event | An indication additional categories for seriousness apply. | Was the adverse event a medically important event not covered by other serious criteria? | Other Serious (Important Medical Events) | Char | R/C | Record whether the serious adverse event is an important medical event, which may be defined in the protocol or in the Investigator Brochure. | AESMIE | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | If the details regarding Serious AEs are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Sponsors may only collect the AESER field because the individual serious adverse event types might be collected in a separate pharmacovigilance database and therefore are not collected in the clinical database. The sponsor should check if the regulatory agencies to which the data will be submitted require the collection of this data. |
Events | AE | N/A | N/A | 31 | AESCAN | Involves Cancer | An indication the serious event was associated with the development of cancer. | Was the adverse event associated with the development of cancer? | Cancer | Char | O | Record whether the serious adverse event was associated with development of cancer. | AESCAN | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | If the details regarding Serious AEs are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Sponsors may only collect the AESER field because the individual serious adverse event types might be collected in a separate pharmacovigilance database and therefore are not collected in the clinical database. The sponsor should check if the regulatory agencies to which the data will be submitted require the collection of this data. "Involves cancer" (AESCAN) and "Occurred with overdose" (AESOD) are not part of the ICH definition of a serious adverse event, but these categories are available for use in studies conducted under guidelines that existed prior to the FDA's adoption of the ICH definition. |
Events | AE | N/A | N/A | 32 | AESOD | Occurred with Overdose | An indication the serious event occurred with an overdose. | Did the adverse event occur with an overdose? | Overdose | Char | O | Record whether the serious adverse event occurs with an overdose. | AESOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | If the details regarding Serious AEs are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Sponsors may only collect the AESER field because the individual serious adverse event types might be collected in a separate pharmacovigilance database and therefore are not collected in the clinical database. The sponsor should check if the regulatory agencies to which the data will be submitted require the collection of this data. "Involves cancer" (AESCAN) and "Occurred with overdose" (AESOD) are not part of the ICH definition of a serious adverse event, but these categories are available for use in studies conducted under guidelines that existed prior to the FDA's adoption of the ICH definition. |
Events | AE | N/A | N/A | 33 | AEREL | AE Causality | An indication the study treatment had a causal effect on the adverse event, as determined by the clinician/investigator. | Was this adverse event related to study treatment? | Relationship to Study Treatment | Char | HR | Indicate if the cause of the adverse event is related to the study treatment and cannot be reasonably explained by other factors (e.g., subject's clinical state, concomitant therapy, other interventions). | AEREL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsored-defined controlled terminology is used to indicate the relationship between the AE and the study treatment (e.g., ICH E2B examples include "Not Related", "Unlikely Related", "Possibly Related", "Related"). Another possibility is the use of "Y" and "N". CDISC Controlled Terminology may be defined in the future. It is recommended that sponsors check with the appropriate regulatory authority for population of this variable to ensure it meets expectations for submission. |
Events | AE | N/A | N/A | 34 | AEACN | Action Taken with Study Treatment | A description of the action taken with study treatment as a result of the event. | What action was taken with study treatment? | Action Taken with Study Treatment | Char | R/C | Record changes made to the study treatment resulting from the adverse event. | AEACN | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (ACN) | N/A | CDISC Controlled Terminology is used to indicate the action taken with the study treatment in response to the AE. How to handle multiple actions taken is a sponsor- specific decision. If this information is collected elsewhere (e.g., on the Exposure CRF), then it is not required to be collected on the AE CRF. This variable is not to be used for actions taken with devices. See the SDTMIG for Medical Devices for information on reporting multiple actions, or actions with multiple devices. |
Events | AE | N/A | N/A | 35 | AEACNDEV | Actions Taken with Device | A description of the action taken, with respect to a device used in a study (which may or may not be the device under study), as a result of the event. | What action was taken with a device used in the study? | Action Taken with Device | Char | O | Record the action taken resulting from the adverse event that are related to a study or non-study device. | SUPPAE.QVAL | This does not map directly an SDTMIG variable. The sponsor may submit this data in a SUPPAE dataset where SUPPAE.QNAM = "AEACNDEV" and SUPPAE.QLABEL = "Actions Taken with Device". Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. | N/A | N/A | Sponsor-controlled terminology for actions that are related to the device (e.g., Device Removed, Primary Care Physician Notified). See the SDTMIG-MD for information on reporting multiple actions, or actions with multiple devices. |
Events | AE | N/A | N/A | 36 | AEACNOTH | Other Action Taken | A description of other action taken as a result of the event that is unrelated to dose adjustments of the study treatment. | What other action was taken? | Other Action Taken | Char | O | Record all other action(s) taken resulting from the adverse event that are unrelated to study treatments given because of this adverse event. | AEACNOTH | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This field is usually collected as a free text field. If possible/desired, the sponsor can create controlled terminology (e.g., Treatment Unblinded, Primary Care Physician Notified). |
Events | AE | N/A | N/A | 37 | AEOUT | Outcome of Adverse Event | A description of the outcome of an event. | What is the outcome of this adverse event? | Outcome | Char | R/C | Record the appropriate outcome of the event in relation to the subject's status. | AEOUT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (OUT) | N/A | CDISC Controlled Terminology is used to indicate the outcome of the event as it relates to the subject's status. The Outcome controlled terminology includes ICH E2B values. The use of this field is the recommended way to describe whether and how the AE resolved. Since the outcome of an AE may be "Death", if this field is NOT used, be sure to provide another form, such as Disposition, with clear instructions to record deaths there. |
Events | AE | N/A | N/A | 38 | AEDIS | AE Caused Study Discontinuation | An indication whether the event caused the subject to discontinue from the study. | Did the adverse event cause the subject to be discontinued from the study? | Caused Study Discontinuation | Char | O | Record if the AE caused the subject to discontinue from the study. | SUPPAE.QVAL | This does not map directly an SDTMIG variable. May be used to create a RELREC to link the Adverse Event to the Disposition record (see SDTMIG v3.2 Section 8.2). The sponsor may also submit this data in a SUPPAE dataset where SUPPAE.QNAM = "AEDIS" and SUPPAE.QLABEL = "Caused Study Discontinuation", if appropriate. Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. | (NY) | N/A | Because the Action Taken field was defined to only collect the changes made to the study treatment due to the AE, an additional field was created to identify the AE(s) that caused the subject to discontinue from the study. Some sponsors opt to capture this information only on the Subject Disposition CRF, whereas others choose to collect this data on both the Subject Disposition and AE CRFs, so the specific AE term(s) and related data can be identified. If the CRF is designed to link the DS and AE records, then RELREC can be used to identify that relationship. |
Events | AE | N/A | N/A | 39 | AERLNSYN | AE Related to Non- Study Treatment | An indication whether in the investigator's opinion the event may have been due to a treatment other than study treatment. | Was this adverse event due to treatment other than study treatment? | Related to Non- Study Treatment | Char | O | Indicate if this adverse event was due to treatment other than study treatment. If yes, briefly describe this non-study treatment relationship. | N/A | Does not map to an SDTMIG variable. The SDTM aCRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification that the CDASH AERELNST field on the CRF was deliberately left blank. |
Events | AE | N/A | N/A | 40 | AERELNST | AE Relationship to Non-Study Treatment | Description of the investigator's opinion as to whether the adverse event may have been due to a treatment other than study treatment. | What is the relationship to non- study treatment? | Relationship to Non-Study Treatment | Char | O | Record the investigator's opinion as to whether the event may have been due to a treatment other than study drug. | AERELNST | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | May be reported as free text (e.g., "MORE LIKELY RELATED TO ASPIRIN USE"). If possible/desired, sponsors can create controlled terminology. |
Events | AE | N/A | N/A | 41 | AESI | Adverse Event of Special Interest | An adverse event of special interest (serious or non- serious) is one of scientific and medical concern specific to the sponsor's product or program, for which ongoing monitoring and rapid communication by the investigator to the sponsor can be appropriate. Such an event might warrant further investigation in order to characterize and understand it. Depending on the nature of the event, rapid communication by the trial sponsor to other parties (e.g., regulators) might also be warranted. | Is this event of special interest? | Adverse Event of Special Interest | Char | O | Record the investigator's opinion as to whether the event is an adverse event of special interest by the sponsor. | N/A | Does not map to an SDTM variable. The SDTM aCRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | This CDASH field may be used just to trigger other CRF pages, or populate a value in AECAT or AESCAT. If submitted, this information could be submitted in a SUPPAE dataset where SUPPAE.QNAM = "AESI" and SUPPAE.QLABEL = "Adverse Event of Special Interest. Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. |
Events | AE | N/A | N/A | 42 | AEPATT | Pattern of Adverse Event | Used to indicate the pattern of the event over time. | What is the adverse event pattern? | Pattern | Char | O | For each AE, check the pattern of the AE. If a single event, choose single. | AEPATT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Used to report the pattern of the AE (e.g., INTERMITTENT, CONTINUOUS, SINGLE EVENT). For crossover trials, it is NOT recommended to capture this field for intermittent AEs. Instead, the AE should have corresponding Start and Stop dates to capture when the AE started and stopped. |
Events | AE | N/A | N/A | 43 | AECONTRT | Concomitant or Additional Trtmnt Given | An indication whether a concomitant or additional treatment given because of the occurrence of the event. | Was a concomitant or additional treatment given due to this adverse event? | Concomitant or Additional Trtmnt Given | Char | O | Indicate if any non- study treatments were received because of this adverse event. If "Yes" is answered for this question, medications should be recorded on the ConMed CRF and procedures recorded on the Procedures CRF. | AECONTRT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | If medication data are reported, the CMAENO variable (on the ConMed CRF) may be used to collect the associated AE Identifier in order to populate RELREC. If procedures are reported, the PRAENO variable (on the Procedures CRF) may be used to collect the associated AE Identifier in order to populate RELREC. |
Events | AE | N/A | N/A | 44 | AEMODIFY | AE Modified Reported Term | If the value for AETERM is modified to facilitate coding, then AEMODIFY will contain the modified text. | N/A | N/A | Char | R/C | N/A | AEMODIFY | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This is not a data collection field that would appear on the CRF. Sponsors will populate this through the coding process. |
Events | AE | N/A | N/A | 45 | AEDECOD | AE Dictionary- Derived Term | The dictionary or standardized text description of AETERM or the modified topic variable (AEMODIFY), if applicable. | N/A | N/A | Char | O | N/A | AEDECOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target.The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the Define- XML external codelist attributes. | N/A | N/A | This is typically not a data collection field that would appear on the CRF. Sponsors will populate this through the coding process. Equivalent to the Preferred Term (PT in MedDRA). |
Events | AE | N/A | N/A | 46 | AELLT | AE Lowest Level Term Code | MedDRA text description of the Lowest Level Term. | N/A | N/A | Char | R/C | N/A | AELLT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | AE | N/A | N/A | 47 | AELLTCD | AE Lowest Level Term Code | MedDRA Lowest Level Term code. | N/A | N/A | Num | R/C | N/A | AELLTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | AE | N/A | N/A | 48 | AEPTCD | AE Preferred Term Code | MedDRA code for the Preferred Term. | N/A | N/A | Num | R/C | N/A | AEPTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | AE | N/A | N/A | 49 | AEHLT | AE High Level Term | MedDRA text description of the High Level Term from the primary path. | N/A | N/A | Char | R/C | N/A | AEHLT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | AE | N/A | N/A | 50 | AEHLTCD | AE High Level Term Code | MedDRA High Level Term code from the primary path. | N/A | N/A | Num | R/C | N/A | AEHLTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | AE | N/A | N/A | 51 | AEHLGT | AE High Level Group Term | MedDRA text description of the High Level Group Term from the primary path. | N/A | N/A | Char | R/C | N/A | AEHLGT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | AE | N/A | N/A | 52 | AEHLGTCD | AE High Level Group Term Code | MedDRA High Level Group Term code from the primary path. | N/A | N/A | Num | R/C | N/A | AEHLGTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | AE | N/A | N/A | 53 | AESOC | AE Primary System Organ Class | MedDRA Primary System Organ Class text description associated with the event. | N/A | N/A | Char | R/C | N/A | AESOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | AE | N/A | N/A | 54 | AESOCCD | AE Primary System Organ Class Code | MedDRA code for the primary System Organ Class. | N/A | N/A | Num | R/C | N/A | AESOCCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | AE | N/A | N/A | 55 | AEACNOYN | Any Other Actions Taken | An indication whether any other actions were taken in response to the adverse event that were unrelated to study treatment dose changes or other non- study treatments given because of this adverse event. | Were any other actions taken in response to this adverse event? | Any Other Action(s) Taken | Char | O | Indicate whether any other action(s) were taken in response to the adverse event that are unrelated to study treatment dose changes or other non-study treatments given because of this adverse event. If yes, briefly describe these actions. | N/A | Does not map to an SDTMIG variable. The SDTM aCRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification that the AEACNOTH field on the CRF was deliberately left blank. |
Assumptions for the CDASHIG AE - Adverse Events Domain
1. The CDASHIG AEYN variable with the question text “Were any adverse events experienced?” is intended to assist in the cleaning of data and in confirming that there are no missing values. This CDASHIG variable is not included as part of the SDTMIG AE domain for submission. This question is annotated as "NOT SUBMITTED" on the CRF.
2. Categories AECAT and AESCAT
a. AECAT and AESCAT should not be redundant with the dictionary coding provided by AEDECOD and AESOC (i.e., they should provide a different means of defining or classifying AE records). b. AECAT and AESCAT are intended for categorizations that are defined in advance. Forexample, a sponsor may have a separate CRF page for AEs of special interest and then another page for all other AEs. In cases where a category of AEs of special interest resembles a part of the dictionary hierarchy (e.g., "CARDIAC EVENTS"), the categorization represented by AECAT and AESCAT may differ from the categorization derived from the coding dictionary.
3. Presence or Absence of Events
a. AEs are most often collected as free-text, spontaneously reported adverse events. There may be cases where the occurrences of specific adverse events are solicited, per protocol requirements. In that case, the prespecified adverse events would be listed on the CRF with a "Yes/No" question (AEOCCUR) asking about the occurrence of each. b. The CDASHIG variable AEOCCUR does not map directly to an SDTM variable. Because the SDTM AE domain is intended to hold only adverse events that actually happen, all values collected in AEOCCUR for prespecified AEs should be submitted in a Findings About Adverse Events domain (FAAE), where FAORRES = the value of AEOCCUR where FATESTCD = "OCCUR". In addition, where AEOCCUR = "Y", there should be a corresponding record in the AE domain. c. It is important to reiterate the distinction between adverse events and clinical events, especially if there may be prespecified terms. In consulting with regulatory authorities, certain events (e.g., events associated with protocol endpoints) may simply be clinical events. (See Section 8.2.3, Clinical Events, for more details.)
4. Coding
a. AEDECOD is the preferred term derived by the sponsor from the coding dictionary. It is a required SDTMIG variable and must have a value. It is expected that the reported term (AETERM) will be coded using a standard dictionary such as MedDRA. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the define.xml external codelist attributes. b. AEMODIFY is a permissible SDTMIG variable and should be included if the sponsor’s coding procedure permits modification of a verbatim term. The modified term is listed in AEMODIFY. The variable should be populated as per the sponsor’s coding procedure. c. The CDASHIG elements AELLT, AELLTCD, AEPTCD, AEHLT, AEHLTCD, AEHLGT, AEHLGTCD, AEBDSYCD, AESOC, and AESOCCD are only applicable to events coded in MedDRA. These items are not expected for non-MedDRA coding.
5. Relative Timing Variables
a. The AEONGO field does not map directly to an SDTMIG variable but it may be used to derive a value into an SDTMIG relative timing variable such as AEENRF or AEENRTPT. When populating AEENRF, if the AEONGO field is checked, a value of "DURING", "AFTER", or "DURING/AFTER" may be derived, as is appropriate to the study. When populating AEENRTPT, if the AEONGO field is checked, the value of "ONGOING" may be derived. Note: AEENRTPT must refer to a "time point anchor" as described in AEENTPT. b. Note that AEONGO is a special use case of "Yes/No" where the question is usually presented as a single possible response of "Yes" when there is no applicable end date at the time of collection. In this case, if the box is checked and the end date is blank, the desired SDTMIG relative timing variable can be derived according to note 5a above. If the box is not checked (AEONGO is NULL) and an end date is present, no SDTMIG relative timing variable would be derived. In some cases, unique to AE, the ongoing status may be determined from AE Outcome. AEONGO is only used to derive an appropriate SDTMIG relative timing variable and should not be submitted on its own in the AE or SUPPAE dataset. The purpose of collecting this field is to help with data cleaning and monitoring; this field provides further confirmation that the end date was deliberately left blank.
6. CDASHIG Action Taken Variables
a. CDASHIG variables AEACN, AECONTRT, AEACNDEV, AEACNOYN, and AEACNOTH are used to collect the action taken as the result of an AE. b. AEACN describes action taken with study treatment as a result of the event. It is expected that a response would be provided for this question for all AEs. If multiple treatments are administered, then a corresponding variable should be created to capture the action taken for each treatment. c. AEACNOTH describes Other Action(s) taken in response to the adverse event that are unrelated to study treatment dose changes or other non-study treatment given because of this adverse event. This field is usually collected as a free-text field (e.g., Treatment Unblinded, Primary Care Physician Notified). If possible/desired, the sponsor can create sponsor-defined controlled terminology. The CDASHIG variable AEACNOYN is used in conjunction with AEACNOTH to assist in the cleaning of data and in confirming that AEACNOTH is not missing. AEACNOYN is not included as part of the SDTMIG AE domain for submission and is annotated as NOT SUBMITTED on the CRF. The CDASHIG variable AEACNOYN should only be used on the AE CRF. d. AECONTRT indicates if any non-study treatments were received because of this adverse event. If "Yes" is answered for this question, any drugs used are recorded on the Concomitant Medications CRF and any procedures performed are recorded on the Procedure CRF. On the CM CRF, the CDASHIG variable CMAENO or on the PR CRF the CDASHIG variable PRAENO can be collected to identify the adverse event associated with this treatment by recording the appropriate AESPID. RELREC can be used to identify this relationship. e. AEACNDEV describes action taken with respect to a device in a study, which may or may not be the device under study. This field is usually collected as a free-text field. If possible/desired, the sponsor can create sponsor-defined controlled terminology. FDA Medical Device Reporting (MDR) guidelines (available at https://www.fda.gov/medicaldevices/) provide controlled terminology for the reporting of device problems.
7. Serious Adverse Events
a. If the details regarding a Serious AE are collected in the clinical database, then it is recommended that a separate "Yes/No" variable be defined for each Serious AE type (AESCAN, AESCONG, AESDISAB, AESDTH, AESHOSP, AESLIFE, AESOD, AESMIE). b. The sponsor should check if the regulatory agencies to which the data will be submitted require the collection of this data (see FDA Study Data Technical Conformance Guide, https://www.fda.gov/ForIndustry/DataStandards/StudyDataStandards/default.htm). The serious categories “Involves cancer” (AESCAN) and “Occurred with overdose” (AESOD) are not part of the ICH definition of a serious adverse event, but these categories are available for use in studies conducted under guidelines that existed prior to the FDA’s adoption of the ICH definition.
8. CDASHIG Variables AESEV, AETOXGR
a. In studies using a standard toxicity scale such as the National Cancer Institute's Common Terminology Criteria for Adverse Events v3.0 (CTCAE; available at https://ctep.cancer.gov/protocoldevelopment/), AETOXGR should be used instead of AESEV. In most cases, either AESEV or AETOXGR is populated but not both. b. There may be cases in which a sponsor may need to populate both variables. Whether populating either AESEV or AETOXGR or both, the sponsor is expected to provide the dictionary name and version or standard toxicity scale name and version used to map the terms utilizing the define.xml external codelist attributes.
9. CDASHIG Variable DTHDAT
a. The CDASH Model allows the Date of Death to be collected on any CRF deemed appropriate by the sponsor. Death Date is mapped to other SDTMIG domains, as deemed appropriate by the sponsor (e.g., DM, DS). b. The death date should only be collected on one form.
Example CRF for the CDASHIG AE - Adverse Events Domain
Example 1
Title: Adverse Events
|
Example CRF Completion Instructions Record all AEs except [list of protocol-defined exceptions] on the AE CRF after informed consent is obtained. All Serious Adverse Events (AEs), regardless of relationship to study drug, must be reported via telephone or fax within 24 hours of discovery. Safety information (e.g., AE, SAE) identified for all subjects must be recorded on source documents from the time informed consent is obtained. |
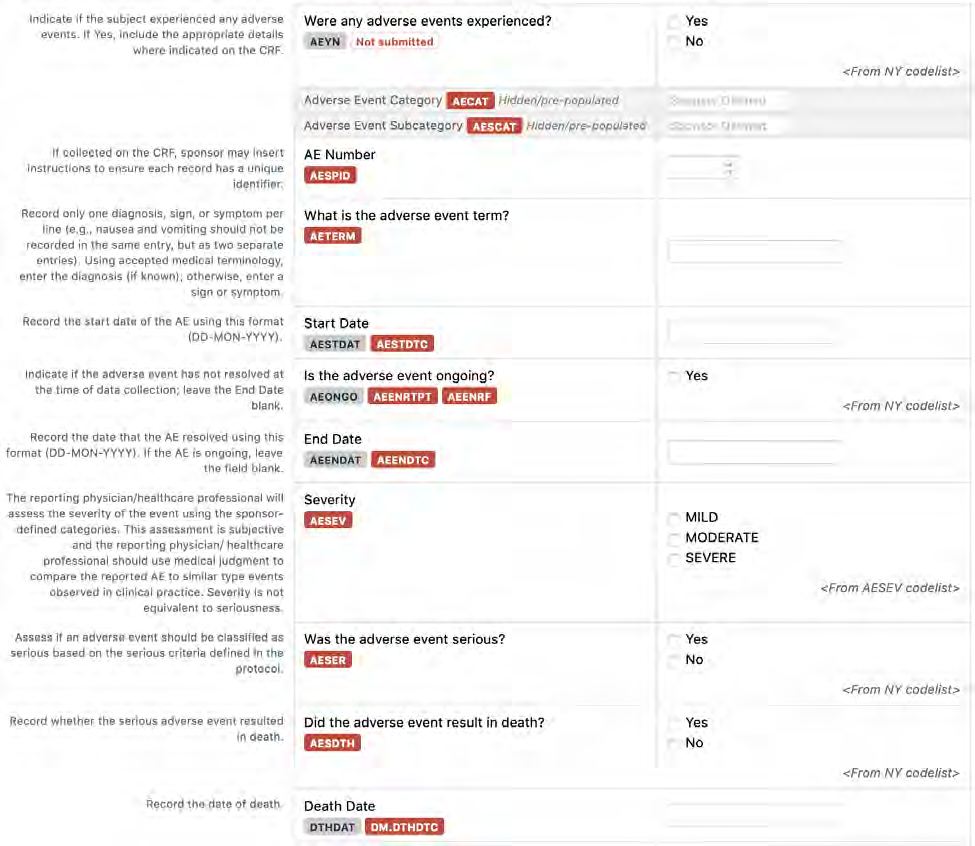

CRF Metadata
| Order | Question Text | Prompt | CRF Completion Instructions | Type | CDASH Variable | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology CodeList Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | Were any adverse events experienced? | Any Adverse Events | Indicate if the subject experienced any adverse events. If Yes, include the appropriate details where indicated on the CRF. | Text | AEYN | N/A | (NY) | Yes; No | |||||
2 | What is the category of the adverse event? | Adverse Event Category | Record the adverse event category, if not preprinted on the CRF. | Text | AECAT | AECAT | Sponsor Defined | Yes | |||||
3 | What is the subcategory of the adverse event? | Adverse Event Subcategory | Record the adverse event subcategory, if not preprinted on the CRF. | Text | AESCAT | AESCAT | Sponsor Defined | Yes | |||||
4 | What is the adverse event identifier? | AE Number | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | Integer | AESPID | AESPID | prompt | ||||||
5 | What is the adverse event term? | Adverse Event | Record only one diagnosis, sign, or symptom per line (e.g., nausea and vomiting should not be recorded in the same entry, but as two separate entries). Using accepted medical terminology, enter the diagnosis (if known); otherwise, enter a sign or symptom. | Text | AETERM | AETERM | |||||||
6 | What is the adverse event start date? | Start Date | Record the start date of the AE using this format (DD-MON- YYYY). | Date | AESTDAT | AESTDTC | prompt | ||||||
7 | Is the adverse event ongoing? | Ongoing | Indicate if the adverse event has not resolved at the time of data collection; leave the End Date blank. | Text | AEONGO | AEENRTPT; AEENRF | AEENRTPT; AEENRF | (NY) | Yes | checkbox | |||
8 | What was the adverse event end date? | End Date | Record the date that the AE resolved using this format (DD- MON-YYYY). If the AE is ongoing, leave the field blank. | date | AEENDAT | AEENDTC | prompt | ||||||
9 | What is the severity of the adverse event? | Severity | The reporting physician/healthcare professional will assess the severity of the event using the sponsor-defined categories. This assessment is subjective and the reporting physician/ healthcare professional should use medical judgment to compare the reported AE to similar type events observed in clinical practice. Severity is not equivalent to seriousness. | Text | AESEV | AESEV | (AESEV) | MILD; MODERATE; SEVERE | prompt | ||||
10 | Was the adverse event serious? | Serious | Assess if an adverse event should be classified as serious based on the serious criteria defined in the protocol. | Text | AESER | AESER | (NY) | Yes; No | |||||
11 | Did the adverse event result in death? | Death | Record whether the serious adverse event resulted in death. | Text | AESDTH | AESDTH | (NY) | Yes; No | |||||
12 | What [is/was] the subject’s date of death? | Death Date | Record the date of death. | date | DTHDAT | DM.DTHDTC | prompt | ||||||
13 | Was the adverse event life threatening? | Life Threatening | Record whether the serious adverse event is life threatening. | Text | AESLIFE | AESLIFE | (NY) | Yes; No | prompt | ||||
14 | Did the adverse event result in initial or prolonged hospitalization for the subject? | Hospitalization (initial or prolonged) | Record whether the serious adverse event resulted in an initial or prolonged hospitalization. | Text | AESHOSP | AESHOSP | (NY) | Yes; No | prompt | ||||
15 | Did the adverse event result in disability or permanent damage? | Disability or Permanent Damage | Record whether the serious adverse event resulted in a persistent or significant disability or incapacity. | Text | AESDISAB | AESDISAB | (NY) | Yes; No | prompt | ||||
16 | Was the adverse event associated with a congenital anomaly or birth defect? | Congenital Anomaly or Birth Defect | Record whether the serious adverse event was associated with congenital anomaly or birth defect. | Text | AESCONG | AESCONG | (NY) | Yes; No | prompt | ||||
17 | Did the adverse event require intervention to prevent permanent impairment or damage resulting from the use of a medical product? | Needs Intervention to Prevent Impairment | Record whether the serious adverse event required intervention to prevent permanent impairment or damage due to the use of a medical product. | Text | AESINTV | SUPPAE.QVAL | (NY) | Yes; No | prompt | ||||
18 | Was the adverse event a medically important event not covered by other serious criteria? | Other Serious (Important Medical Events) | Record whether the serious adverse event is an important medical event, which may be defined in the protocol or in the Investigator Brochure. | Text | AESMIE | AESMIE | (NY) | Yes; No | prompt | ||||
19 | Was this adverse event related to study treatment? | Relationship to Study Treatment | Indicate if the cause of the adverse event is related to the study treatment and cannot be reasonably explained by other factors (e.g., subject's clinical state, concomitant therapy, other interventions). | Text | AEREL | AEREL | NOT RELATED; UNLIKELY RELATED; POSSIBLY RELATED; RELATED | prompt | |||||
20 | What action was taken with study treatment? | Action Taken with Study Treatment | Record changes made to the study treatment resulting from the adverse event. | Text | AEACN | AEACN | (ACN) | DRUG WITHDRAWN; DOSE REDUCED; DOSE INCREASED; DOSE NOT CHANGED; UNKNOWN; NOT APPLICABLE | prompt | ||||
21 | What is the outcome of this adverse event? | Outcome | Record the appropriate outcome of the event in relation to the subject's status. | Text | AEOUT | AEOUT | (OUT) | RECOVERING / RESOLVING; NOT RECOVERED / NOT RESOLVED; RECOVERED / RESOLVED; RECOVERED / RESOLVED WITH SEQUELAE; FATAL | prompt |
8.2.3 CE - Clinical Events
Description/Overview for the CDASHIG CE - Clinical Events Domain
The CE domain includes clinical events of interest that would not be classified as Adverse Events. The clinical events included in the CE dataset should be consistent with the protocol requirements. Clinical events may be captured either as free text or via a prespecified list of terms. The structure of the CE domain is 1 record per clinical event per subject. It is the sponsor's responsibility to define a clinical event, and the event is usually described in the protocol.
As with all the data collection variables recommended in CDASH, it is assumed that sponsors will add other data variables as needed to meet protocol-specific and other data collection requirements (e.g., therapeutic area-specific data elements and others as required per protocol, business practice, or operating procedures). Sponsors should define the appropriate collection period for clinical events.
Specification for the CDASHIG CE - Clinical Events Domain
Clinical Events Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Events | CE | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Although this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Events | CE | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Events | CE | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Events | CE | N/A | N/A | 4 | CECAT | Category for Clinical Event | A grouping of topic-variable values based on user-defined characteristics. | What is the category of the clinical event? | [Clinical Event Category]; NULL | Char | O | Record the clinical event category, if not preprinted on the CRF. | CECAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Events | CE | N/A | N/A | 5 | CESCAT | Subcategory for Clinical Event | A sub-division of the CECAT values based on user-defined characteristics. | What is the subcategory of the clinical event? | [Clinical Event Subcategory]; NULL | Char | O | Record the clinical event subcategory, if not preprinted on the CRF. | CESCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. CESCAT can only be used if there is an CECAT and it must be a subcategorization of CECAT. |
Events | CE | N/A | N/A | 6 | CEYN | Any Clinical Event | An indication whether or not any clinical events were experienced during the study. | Were any clinical events experienced? | Any Clinical Events | Char | O | Indicate if the subject experienced any clinical events. If yes, include the appropriate details where indicated on the CRF. | N/A | Does not map to an SDTMIG variable. The SDTM aCRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification that all other fields on the CRF were deliberately left blank. |
Events | CE | N/A | N/A | 7 | CESPID | CE Sponsor- Defined Identifier | A sponsor-defined identifier. In CDASH, This is typically used for preprinted or auto-generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor defined question] | Sponsor defined | Char | O | If collected on the CRF, the sponsor may insert instructions to ensure each record has a unique identifier. | CESPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Because SPID is a sponsor-defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile concomitant medications, procedures and/or medical history records with CEs. If CMCENO or PRCENO is used, this is the identifier to which CMCENO or PRCENO refers. May be used to record preprinted number (e.g., line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Events | CE | N/A | N/A | 8 | CETERM | Reported Term for the Clinical Event | The reported or prespecified name of the clinical event. | What is the clinical event term? | [Clinical Event]; Specify (Other/Details) | Char | HR | Record the clinical event or [insert text corresponding to the specific clinical event]. | CETERM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Can be represented either as an open-entry field to capture verbatim terms reported by subjects or could be preprinted in the situation where solicited CEs of interest are captured. May be used as Specify Other field to collect more details on a prespecified CEDECOD value, or when CEDECOD=Other. In most cases, the verbatim term (i.e., investigator- reported term) will be coded to a standard medical dictionary such as MedDRA or WHO ART, after the data have been collected on the CRF. |
Events | CE | N/A | N/A | 9 | CEOCCUR | Clinical Event Occurrence | An indication whether a prespecified clinical event or a group of clinical events occurred when information about the occurrence of a specific event is solicited. | Did the subject have [prespecified clinical event/group of clinical events ]? | [Specified Clinical Event] | Char | O | Indicate if [specific clinical event] has occurred/is occurring by checking Yes or No. | CEOCCUR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | The CDASH variable CEOCCUR is used to report the occurrence of a prespecified clinical event not considered to be an adverse event by the sponsor. CEOCCUR should not be used for spontaneously reported events. The site should be able to indicate that the response was not asked or answered. |
Events | CE | N/A | N/A | 10 | CEPRESP | Clinical Event prespecified | An indication that a specific event, or group of events, are prespecified on a CRF. | N/A | N/A | Char | O | N/A | CEPRESP | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | A hidden field on a CRF defaulted to "Y", or added during the SDTM-based dataset creation when the clinical event is prespecified. Null for spontaneously reported events. If a study collects both prespecified clinical events as well as free-text events, the value of CEPRESP should be "Y" for all prespecified events and null for events reported as free-text. CEPRESP is a permissible field in SDTM and may be omitted from the SDTM-based dataset if all events were collected as free text. |
Events | CE | N/A | N/A | 11 | CESTDAT | Clinical Event Start Date | The start date of the clinical event, represented in an unambiguous date format (e.g., DD-MON-YYYY). | What was the [clinical event] start date? | Start Date | Char | O | Record the start date of the [clinical event ] using this format (DD- MON-YYYY). | CESTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable CESTDTC in ISO 8601 format. | N/A | N/A | N/A |
Events | CE | N/A | N/A | 12 | CESTTIM | Clinical Event Start Time | The start time of the clinical event represented in an unambiguous date format (e.g., hh:mm:ss). | What was the [clinical event] start time? | Start Time | Char | O | If appropriate, record the start time (as complete as possible) of the [clinical event]. | CESTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable CESTDTC in ISO 8601 format. | N/A | N/A | Collecting the time a CE started is only appropriate if it can be realistically determined and if there is a scientific reason for needing to know this level of detail. An example is where the subject is under the direct care of the site at the time the event started and the study design is such that it is important to know the CE start time with respect to dosing. |
Events | CE | N/A | N/A | 13 | CELOC | Clinical Event Location | A description of the anatomical location relevant for the clinical event. | What was the anatomical location of the [clinical event]? | Anatomical Location | Char | O | Indicate the anatomical location of the clinical event. | CELOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. LAT, DIR, PORTOT are used to further describe the anatomical location. |
Events | CE | N/A | N/A | 14 | CELAT | Clinical Event Laterality | Qualifier for anatomical location further detailing the side of the body relevant for the event. | What was the side of the anatomical location of the [clinical event]? | Side | Char | O | Record the side of the anatomical location of the clinical event. | CELAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Events | CE | N/A | N/A | 15 | CEDIR | Clinical Event Directionality | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What was the directionality of the anatomical location of the [clinical event]? | Directionality | Char | O | Record the directionality of the anatomical location of the clinical event. | CEDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Events | CE | N/A | N/A | 16 | CEPORTOT | CE Location Portion or Totality | Qualifier for anatomical location further detailing the distribution (i.e., arrangement of, apportioning of). | What was the portion or totality of the anatomical location of the clinical event? | Portion or Totality | Char | O | Indicate the portion or totality anatomical location of the clinical event. | CEPORTOT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (PORTOT) | N/A | Collected when the sponsor needs to identify the specific portionality for the anatomical locations. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Events | CE | N/A | N/A | 17 | CEONGO | Ongoing Clinical Event | Indication the clinical event is ongoing when no end date is provided. | Was the [clinical event] ongoing (as of [the study- specific timepoint or period]? | Ongoing (as of the [study-specific timepoint or period]) | Char | O | Indicate if the clinical event has not resolved at the time of data collection. If ongoing leave the End Date blank. | CEENRTPT; CEENRF | This does not map directly to an SDTMIG variable. May be used to populate a value into an SDTMIG relative timing variable such as CEENRF or CEENRTPT. When populating CEENRF, if the value of CEONGO is "Y", the value of "DURING", "AFTER" or "DURING/AFTER" may be used. When populating CEENRTPT, if the value of CEONGO is "Y", the value of "ONGOING" may be used. When CEONGO refers to the Study Reference Period (defined in DM.RFSTDTC to DM.RFENDTC) the SDTMIG variable CEENRF should be populated. When CEONGO is compared to another time point, the SDTMIG variables CEENRTPT and CEENTPT should be used. Note: CEENRTPT must refer to a time point anchor described in CEENTPT. | (NY) | N/A | Completed to indicate that the CE has not resolved at the time of data collection, when no End Date is collected. In some cases the ongoing status may be determined from CE Outcome. The purpose of collecting this field is to help with data cleaning and monitoring; this field provides further confirmation that the End Date was deliberately left blank. Often used as a tick/checkbox. |
Events | CE | N/A | N/A | 18 | CEENDAT | Clinical Event End Date | The date when the clinical event resolved/ ended, represented in an unambiguous date format (e.g., DD-MON-YYYY). | What was the [clinical event] end date? | End Date | Char | O | Record the date that the [clinical event] resolved using this format (DD- MON-YYYY). If the [clinical event] is ongoing, leave the field blank. | CEENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable CEENDTC in ISO 8601 format. | N/A | N/A | The definition of resolved is sponsor-specific. The preferred method is to collect a complete end date. Partial dates (e.g., providing year only, month and year only) may be acceptable. |
Events | CE | N/A | N/A | 19 | CEENTIM | Clinical Event End Time | The time when the clinical event ended/resolved, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the [clinical event] end time? | End Time | Char | O | Record the time (as complete as possible) that the [clinical event] resolved. | CEENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable CEENDTC in ISO 8601 format. | N/A | N/A | Collecting the time an CE resolved is only appropriate if it can be realistically determined and if there is a scientific reason for needing to know this level of detail. An example is where the subject is under the direct care of the site at the time the event resolved and the study design is such that it is important to know the CE end time with respect to dosing. |
Events | CE | N/A | N/A | 20 | CESEV | CE Severity/Intensity | The severity or intensity of the event. | What was the severity of the [clinical event]? | Severity | Char | O | The reporting physician/healthcare professional will assess the severity of the event using the sponsor- defined categories. This assessment is subjective and the reporting physician/ healthcare professional should use medical judgment to compare the reported clinical event to similar type events observed in clinical practice. | CESEV | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the toxicity scale name and version used to map the terms utilizing the Define-XML external code list attributes. | N/A | N/A | Some studies may collect Severity and/or Toxicity Grade. |
Events | CE | N/A | N/A | 21 | CETOX | Clinical Event Toxicity Description | A description of toxicity quantified by CETOXGR (e.g., NCI CTCAE Short Name). | What was the description of the toxicity? | [NCI CTCAE] Toxicity | Char | O | Record the description of the toxicity. | CETOX | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the toxicity scale name and version used to map the terms utilizing the Define-XML external code list attributes. | N/A | N/A | This would typically be the text description quantified by CETOXGR (e.g., HYPERCALCEMIA, HYPOCALCEMIA) |
Events | CE | N/A | N/A | 22 | CETOXGR | Clinical Event Toxicity Grade | The toxicity grade using a standard toxicity scale (e.g., NCI CTCAE). | What was the toxicity grade? | [NCI CTCAE Toxicity] Grade | Char | O | Record the severity of the clinical event using the CTCAE Toxicity Grades. | CETOXGR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the toxicity scale name and version used to map the terms utilizing the Define-XML external code list attributes. | N/A | N/A | Some studies may collect Severity and/or Toxicity Grade. CDISC Controlled Terminology (TOXGRV3); (TOXGRV4) may be used. |
Events | CE | N/A | N/A | 23 | CEMODIFY | Clinical Event Modified Term | If the value for CETERM is modified to facilitate coding, then CEMODIFY will contain the modified text. | N/A | N/A | Char | O | N/A | CEMODIFY | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This is not a data collection field that would appear on the CRF. Sponsors will populate this through the coding process. |
Events | CE | N/A | N/A | 24 | CEDECOD | CE Dictionary- Derived Term | The dictionary- or sponsor-defined standardized text description of CETERM or the modified topic variable (CEMODIFY), if applicable. | N/A | N/A | Char | O | N/A | CEDECOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This is typically not a data collection field that would appear on the CRF. Sponsors will populate this through the coding process. Equivalent to the Preferred Term (PT in MedDRA). |
Events | CE | N/A | N/A | 25 | CELLT | Clinical Event Lowest Level Term | MedDRA text description of the Lowest Level Term. | N/A | N/A | Char | O | N/A | CELLT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | CE | N/A | N/A | 26 | CELLTCD | Clinical Event Lowest Level Term Code | MedDRA Lowest Level Term code. | N/A | N/A | Num | O | N/A | CELLTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | CE | N/A | N/A | 27 | CEPTCD | Clinical Event Preferred Term Code | MedDRA code for the Preferred Term. | N/A | N/A | Num | O | N/A | CEPTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | CE | N/A | N/A | 28 | CEHLT | Clinical Event High Level Term | MedDRA text description of the High Level Term from the primary path. | N/A | N/A | Char | O | N/A | CEHLT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | CE | N/A | N/A | 29 | CEHLTCD | Clinical Event High Level Term Code | MedDRA High Level Term code from the primary path. | N/A | N/A | Num | O | N/A | CEHLTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | CE | N/A | N/A | 30 | CEHLGT | Clinical Event High Level Group Term | MedDRA text description of the High Level Group Term from the primary path. | N/A | N/A | Char | O | N/A | CEHLGT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | CE | N/A | N/A | 31 | CEHLGTCD | CE High Level Group Term Code | MedDRA High Level Group Term code from the primary path. | N/A | N/A | Num | O | N/A | CEHLGTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | CE | N/A | N/A | 32 | CESOC | CE Primary System Organ Class | MedDRA Primary System Organ Class description associated with the event. | N/A | N/A | Char | O | N/A | CESOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | CE | N/A | N/A | 33 | CESOCCD | CE Primary System Organ Class Code | MedDRA code for the primary System Organ Class. | N/A | N/A | Num | O | N/A | CESOCCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Assumptions for the CDASHIG CE - Clinical Events Domain
1. The determination of events to be considered clinical events versus adverse events should be done carefully and with reference to regulatory guidelines or consultation with a regulatory review division. Please note that all reportable adverse events that would contribute to AE incidence tables in a clinical study report must be included in the AE domain. AE data should not be commingled with CE data. The collection of write-in events on a CE CRF should be considered with caution.
2. Events considered to be clinical events may include episodes of symptoms of the disease under study (often known as signs and symptoms), or about events that do not constitute adverse events in themselves, although they might lead to the identification of an adverse event. For example, in a study of an investigational treatment for migraine headaches, migraine headaches may not be considered to be AEs per protocol. The occurrence of migraines or associated signs and symptoms might be reported in CE.
3. Some clinical events may escalate (e.g., increase in duration, severity) and require additional collection in AE. This should be clearly defined in the protocol.
4. The CDASHIG variable CEYN, with the question text “Were any clinical events experienced?”, is intended to assist in the cleaning of data and in confirming that no data are unintentionally missing. This CDASHIG variable is not included as part of the SDTM Event domain for submission. This field is annotated as NOT SUBMITTED on the CRF.
5. Categories CECAT and CESCAT should not be redundant with the domain code or dictionary classification provided by CEDECOD and CESOC (i.e., they should provide a different means of defining or classifying CE records).
6. Presence or Absence of Events: The CDASHIG variable CEOCCUR is used to record whether a subject had a prespecified clinical event. Example: "Does the subject have a fever?"
7. Coding
a. CEDECOD is the preferred term derived by the sponsor from the coding dictionary. It is permissible to code CETERM using a standard dictionary such as MedDRA. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the define.xml external codelist attributes.
b. CEMODIFY is a permissible variable and should be included if the sponsor’s coding procedure permits modification of a verbatim term. The modified term is listed in CEMODIFY. The variable should be populated as per the sponsor’s coding procedure. The CDASHIG elements CELLT, CELLTCD, CEPTCD, CEHLT, CEHLTCD, CEHLGT, CEHLGTCD, CEBDSYCD, CESOC, and CESOCCD are only applicable to events coded in MedDRA. These items are not expected for non- MedDRA coding.
8. Relative Timing Variables
a. CEONGO field does not map directly to an SDTMIG variable. It may be used to derive a value into an SDTMIG relative timing variable such as CEENRF or CEENRTPT when no end date is recorded. When populating CEENRF, if the value of CEONGO is "Y", a value (from the values "DURING", "AFTER" or "DURING/AFTER", as is appropriate to the study) may be derived.
b. When populating CEENRTPT, if the value of CEONGO is "Y", the value of "ONGOING" may be derived. CEENRTPT must refer to a "time point anchor" described in CEENTPT.
9. CEONGO is a special use case of Yes/No where the question is usually presented as a single possible response of Yes in conjunction with another question, as in this case, an end date. CEONGO is completed to indicate that the CE has not resolved at the time of data collection, and thus no end date is collected. The purpose of collecting this field is to help with data cleaning and monitoring; this field provides further confirmation that the end date was deliberately left blank
Example CRF for the CDASHIG CE - Clinical Events Domain
Example 1
Title: Hypoglycemic Events
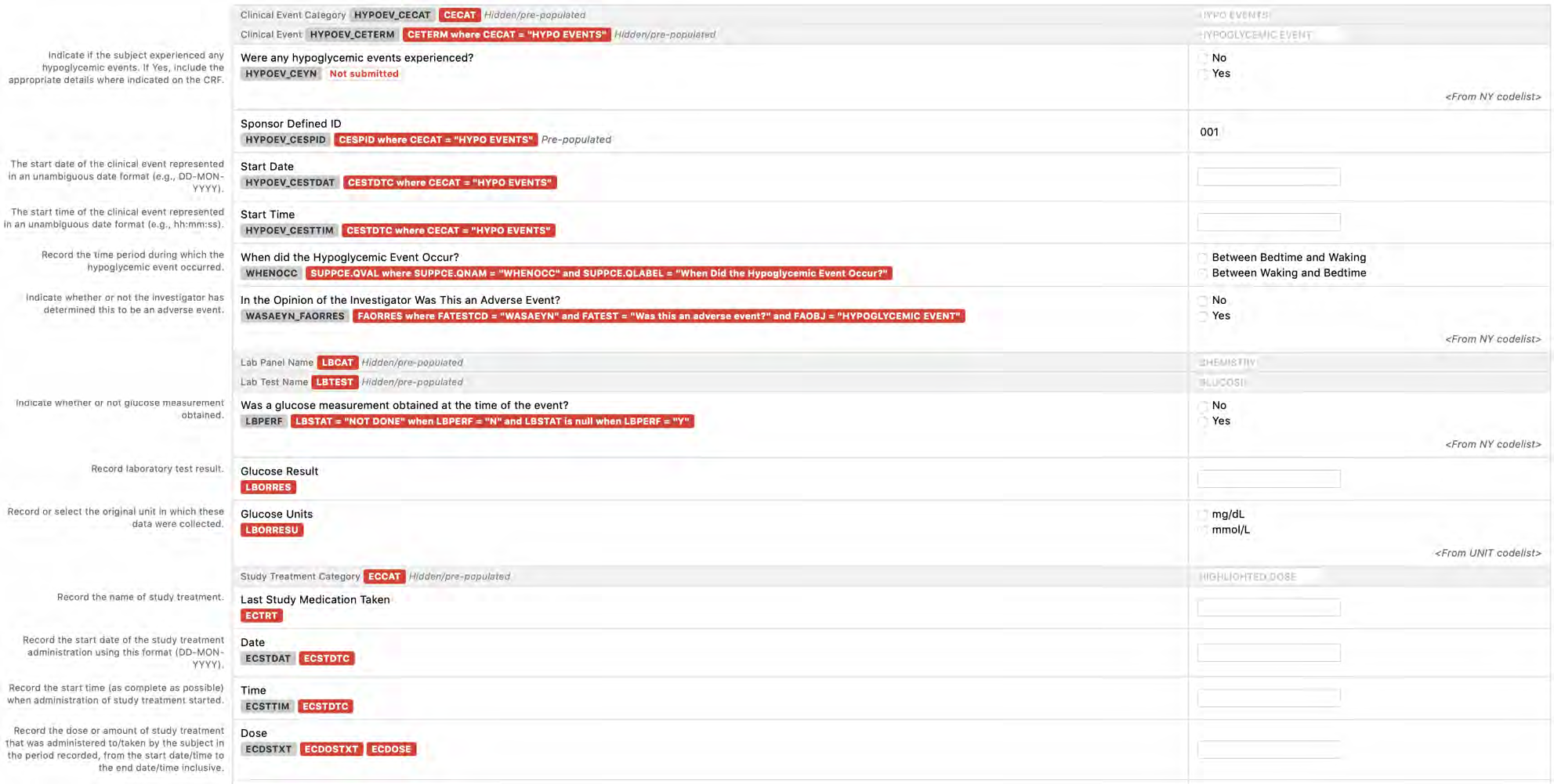
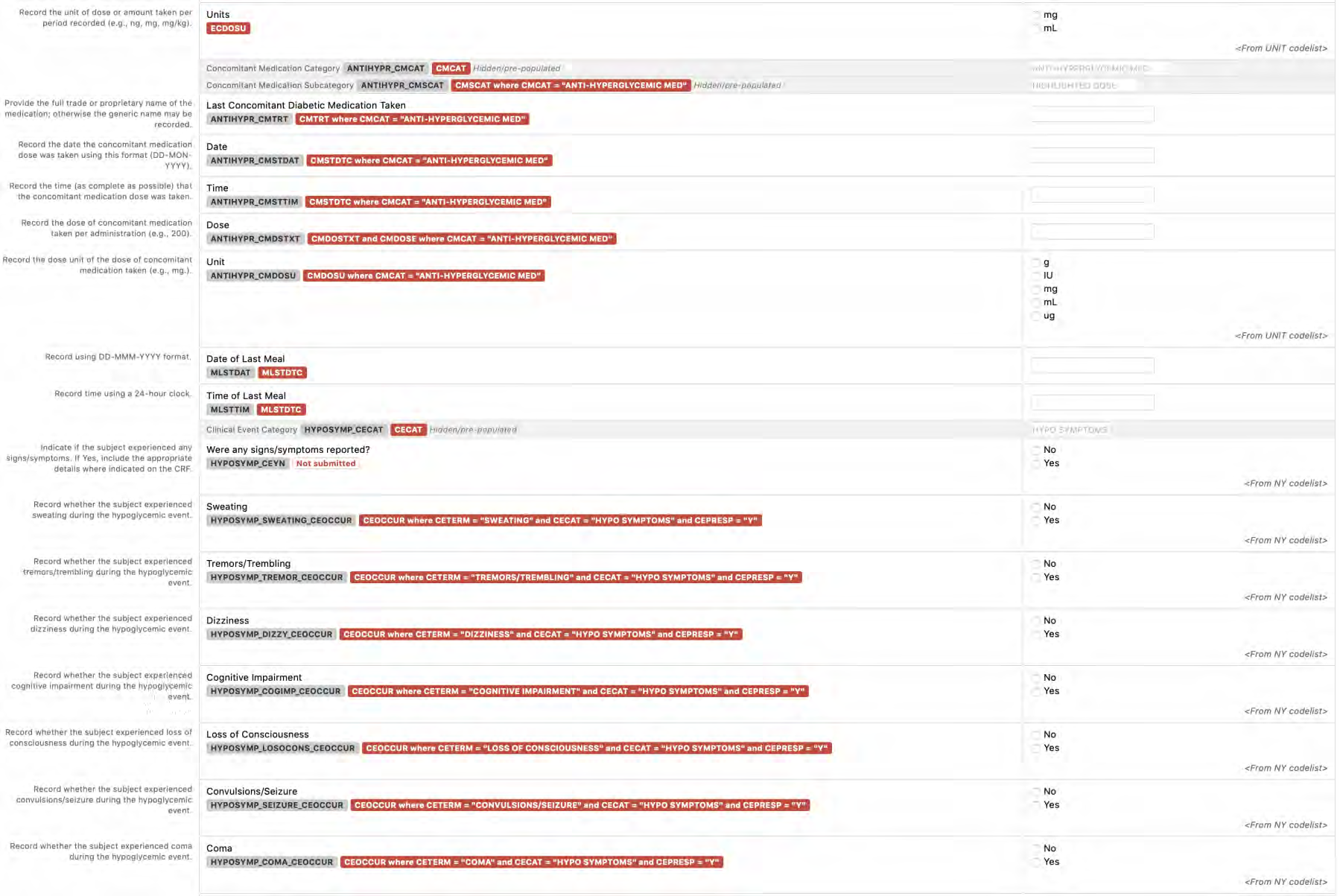
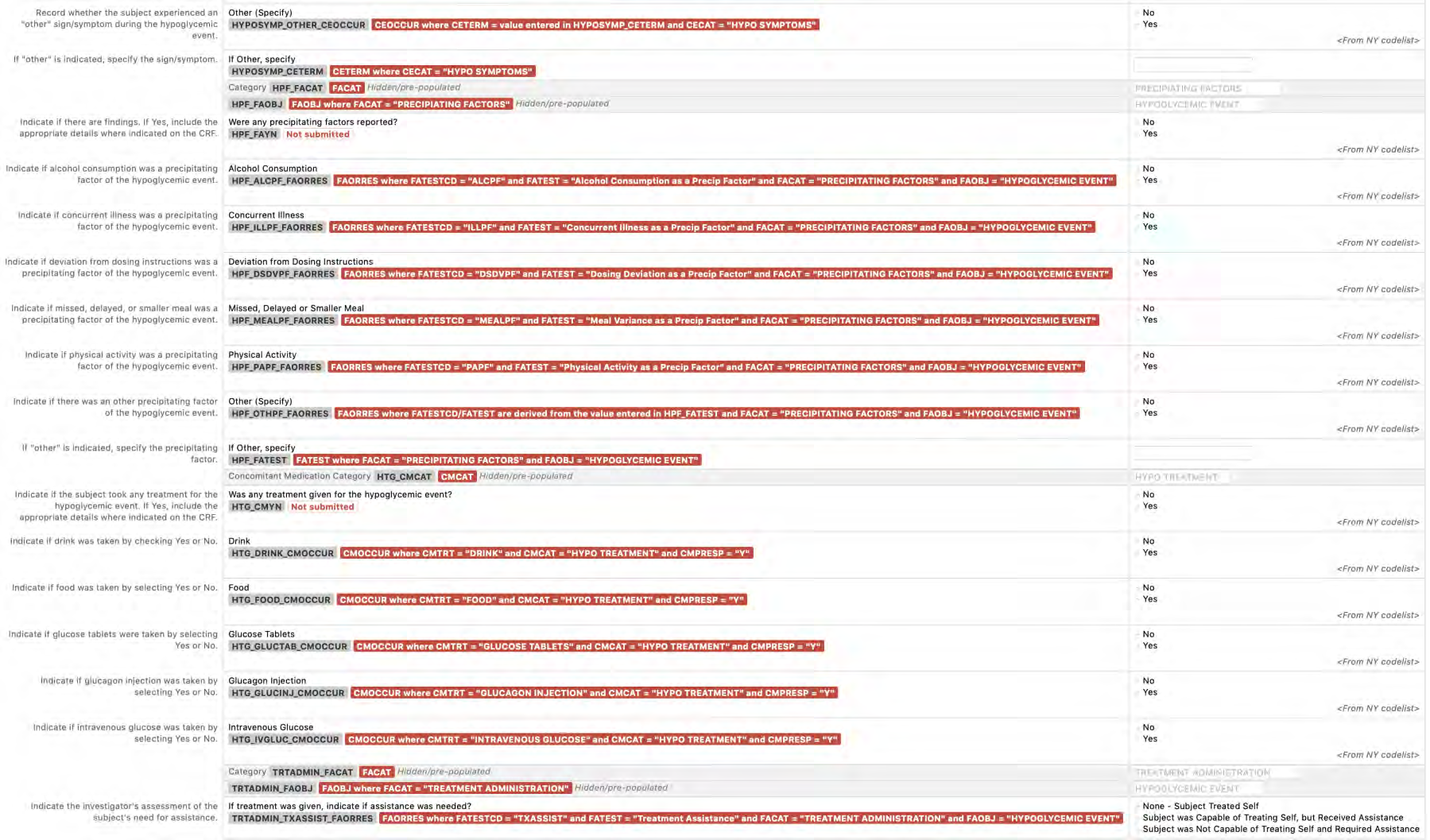
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre-Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
HYPOEV_CECAT | 1 | What is the category of the clinical event? | Clinical Event Category | Record the clinical event category, if not pre- printed on the CRF. | Text | CECAT | HYPO EVENTS | Prompt | Yes | ||||
HYPOEV_CETERM | 2 | What is the clinical event term? | Clinical Event | Record the clinical event or [insert text corresponding to the specific clinical event]. | Text | CETERM | CETERM where CECAT = "HYPO EVENTS" | HYPOGLYCEMIC EVENT | Prompt | Yes | |||
HYPOEV_CEYN | 3 | Were any hypoglycemic events experienced? | Any Hypoglycemic Events | Indicate if the subject experienced any hypoglycemic events. If Yes, include the appropriate details where indicated on the CRF. | Text | N/A | (NY) | No; Yes | radio | ||||
HYPOEV_CESPID | 4 | What is the clinical event identifier? | Sponsor Defined ID | If collected on the CRF, sponsors may insert instructions to ensure each record has a unique identifier. | Text | CESPID | CESPID where CECAT = "HYPO EVENTS" | 001 | Prompt | ||||
HYPOEV_CESTDAT | 5 | What was the clinical event start date? | Start Date | The start date of the clinical event represented in an unambiguous date format (e.g., DD-MON- YYYY). | Date | CESTDTC | CESTDTC where CECAT = "HYPO EVENTS" | Prompt | |||||
HYPOEV_CESTTIM | 6 | What was the clinical event start time? | Start Time | The start time of the clinical event represented in an unambiguous date format (e.g., hh:mm:ss). | Time | CESTDTC | CESTDTC where CECAT = "HYPO EVENTS" | Prompt | |||||
WHENOCC | 7 | When did the Hypoglycemic Event Occur? | Time Period | Record the time period during which the hypoglycemic event occurred. | Text | SUPPCE.QVAL | SUPPCE.QVAL where SUPPCE.QNAM = "WHENOCC" and SUPPCE.QLABEL = "When Did the Hypoglycemic Event Occur?" |
Between Bedtime and Waking; Between Waking and Bedtime | radio | ||||
WASAEYN_FAORRES | 8 | In the Opinion of the Investigator Was This an Adverse Event? | Adverse Event | Indicate whether or not the investigator has determined this to be an adverse event. | Text | FAORRES | FAORRES where FATESTCD = "WASAEYN" and FATEST = "Was this an adverse event?" and FAOBJ = "HYPOGLYCEMIC EVENT" | (NY) | No; Yes | radio | |||
LBCAT | 9 | What was the name of the lab panel? | Lab Panel Name | Record the lab test category, if not pre- printed on the CRF. | Text | LBCAT | CHEMISTRY | Prompt | Yes | ||||
LBTEST | 10 | What was the lab test name? | Lab Test Name | Record the name of the Lab measurement or finding, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | Text | LBTEST | GLUCOSE | Prompt | Yes | ||||
LBPERF | 11 | Was a glucose measurement obtained at the time of the event? | Lab Performed | Indicate whether or not glucose measurement obtained. | Text | LBSTAT | LBSTAT = "NOT DONE" when LBPERF = "N" and LBSTAT is null when LBPERF = "Y" | (NY) | No; Yes | radio | |||
LBORRES | 12 | What was the result of the glucose lab test? | Glucose Result | Record laboratory test result. | Text | LBORRES | Prompt | ||||||
LBORRESU | 13 | What was the unit of the glucose lab result? | Glucose Units | Record or select the original unit in which these data were collected. | Text | LBORRESU | (UNIT) | mg/dL; mmol/L | Prompt | radio | |||
ECCAT | 14 | What is the category of the study treatment? | Study Treatment Category | Record the study treatment category, if not pre-printed on the CRF. | Text | ECCAT | HIGHLIGHTED DOSE | Prompt | Yes | ||||
ECTRT | 15 | What was the last study medication taken? | Last Study Medication Taken | Record the name of study treatment. | Text | ECTRT | Prompt | ||||||
ECSTDAT | 16 | What was the dose date? | Date | Record the start date of the study treatment administration using this format (DD-MON- YYYY). | Date | ECSTDTC | Prompt | ||||||
ECSTTIM | 17 | What was the dose time? | Time | Record the start time (as complete as possible) when administration of study treatment started. | Date | ECSTDTC | Prompt | ||||||
ECDSTXT | 18 | What was the dose per administration of study treatment? | Dose | Record the dose or amount of study treatment that was administered to/taken by the subject in the period recorded, from the start date/time to the end date/time inclusive. | Text | ECDOSTXT; ECDOSE | Prompt | ||||||
ECDOSU | 19 | What were the units for the dose? | Units | Record the unit of dose or amount taken per period recorded (e.g., ng, mg, mg/kg). | Text | ECDOSU | (UNIT) | mg; mL | Prompt | radio | |||
ANTIHYPR_CMCAT | 20 | What is the category for the concomitant medication? | Concomitant Medication Category | Record the concomitant medication category, if not pre-printed on the CRF. | Text | CMCAT | ANTI- HYPERGLYCEMIC MED | Prompt | Yes | ||||
ANTIHYPR_CMSCAT | 21 | What is the subcategory for the concomitant medication? | Concomitant Medication Subcategory | Record the concomitant medication subcategory, if not pre-printed on the CRF. | Text | CMSCAT | CMSCAT where CMCAT = "ANTI-HYPERGLYCEMIC MED" | HIGHLIGHTED DOSE | Prompt | Yes | |||
ANTIHYPR_CMTRT | 22 | What was the last concomitant diabetic medication taken? | Last Concomitant Diabetic Medication Taken | Provide the full trade or proprietary name of the medication; otherwise the generic name may be recorded. | Text | CMTRT | CMTRT where CMCAT = "ANTI-HYPERGLYCEMIC MED" | Prompt | |||||
ANTIHYPR_CMSTDAT | 23 | What was the concomitant medication dose date? | Date | Record the date the concomitant medication dose was taken using this format (DD-MON- YYYY). | Date | CMSTDTC | CMSTDTC where CMCAT = "ANTI-HYPERGLYCEMIC MED" | Prompt | |||||
ANTIHYPR_CMSTTIM | 24 | What was the concomitant medication dose time? | Time | Record the time (as complete as possible) that the concomitant medication dose was taken. | Date | CMSTDTC | CMSTDTC where CMCAT = "ANTI-HYPERGLYCEMIC MED" | Prompt | |||||
ANTIHYPR_CMDSTXT | 25 | What was the individual dose of the concomitant mediation? | Dose | Record the dose of concomitant medication taken per administration (e.g., 200). | Text | CMDOSTXT; CMDOSE | CMDOSTXT and CMDOSE where CMCAT = "ANTI- HYPERGLYCEMIC MED" | Prompt | |||||
ANTIHYPR_CMDOSU | 26 | What is the unit for the dose of concomitant medication? | Unit | Record the dose unit of the dose of concomitant medication taken (e.g., mg.). | Text | CMDOSU | CMDOSU where CMCAT = "ANTI-HYPERGLYCEMIC MED" | (UNIT) | g; IU; mg; mL; ug | Prompt | radio | ||
MLSTDAT | 27 | What was the date of last meal? | Date of Last Meal | Record using DD-MMM- YYYY format. | Date | MLSTDTC | Prompt | ||||||
MLSTTIM | 28 | What was the time of last meal? | Time of Last Meal | Record time using a 24- hour clock. | Time | MLSTDTC | Prompt | ||||||
HYPOSYMP_CECAT | 29 | What is the category of the clinical event? | Clinical Event Category | Record the clinical event category, if not preprinted on the CRF. | Text | CECAT | HYPO SYMPTOMS | Prompt | Yes | ||||
HYPOSYMP_CEYN | 30 | Were any signs/symptoms reported? | Any Signs/Symptoms | Indicate if the subject experienced any signs/symptoms. If Yes, include the appropriate details where indicated on the CRF. | Text | N/A | (NY) | No; Yes | radio | ||||
HYPOSYMP_SWEATING_CEOCCUR | 31 | Did the subject experience sweating? | Sweating | Record whether the subject experienced sweating during the hypoglycemic event. | Text | CEOCCUR | CEOCCUR where CETERM = "SWEATING" and CECAT = "HYPO SYMPTOMS" and CEPRESP = "Y" | (NY) | No; Yes | Prompt | radio | ||
HYPOSYMP_TREMOR_CEOCCUR | 32 | Did the subject experience tremors/trembling? | Tremors/Trembling | Record whether the subject experienced tremors/trembling during the hypoglycemic event. | Text | CEOCCUR | CEOCCUR where CETERM = "TREMORS/TREMBLING" and CECAT = "HYPO SYMPTOMS" and CEPRESP = "Y" | (NY) | No; Yes | Prompt | radio | ||
HYPOSYMP_DIZZY_CEOCCUR | 33 | Did the subject experience dizziness? | Dizziness | Record whether the subject experienced dizziness during the hypoglycemic event. | Text | CEOCCUR | CEOCCUR where CETERM = "DIZZINESS" and CECAT = "HYPO SYMPTOMS" and CEPRESP = "Y" | (NY) | No; Yes | Prompt | radio | ||
HYPOSYMP_COGIMP_CEOCCUR | 34 | Did the subject experience cognitive impairment? | Cognitive Impairment | Record whether the subject experienced cognitive impairment during the hypoglycemic event. | Text | CEOCCUR | CEOCCUR where CETERM = "COGNITIVE IMPAIRMENT" and CECAT = "HYPO SYMPTOMS" and CEPRESP = "Y" | (NY) | No; Yes | Prompt | radio | ||
HYPOSYMP_LOSOCONS_CEOCCUR | 35 | Did the subject experience loss of consciousness? | Loss of Consciousness | Record whether the subject experienced loss of consciousness during the hypoglycemic event. | Text | CEOCCUR | CEOCCUR where CETERM = "LOSS OF CONSCIOUSNESS" and CECAT = "HYPO SYMPTOMS" and CEPRESP = "Y" | (NY) | No; Yes | Prompt | radio | ||
HYPOSYMP_SEIZURE_CEOCCUR | 36 | Did the subject experience convulsions/seizure? | Convulsions/Seizure | Record whether the subject experienced convulsions/seizure during the hypoglycemic event. | Text | CEOCCUR | CEOCCUR where CETERM = "CONVULSIONS/SEIZURE" and CECAT = "HYPO SYMPTOMS" and CEPRESP = "Y" | (NY) | No; Yes | Prompt | radio | ||
HYPOSYMP_COMA_CEOCCUR | 37 | Did the subject experience coma? | Coma | Record whether the subject experienced coma during the hypoglycemic event. | Text | CEOCCUR | CEOCCUR where CETERM = "COMA" and CECAT = "HYPO SYMPTOMS" and CEPRESP = "Y" | (NY) | No; Yes | Prompt | radio | ||
HYPOSYMP_OTHER_CEOCCUR | 38 | Did the subject experience any other sign/symptom? | Other (Specify) | Record whether the subject experienced an "other" sign/symptom during the hypoglycemic event. | Text | CEOCCUR | CEOCCUR where CETERM = value entered in HYPOSYMP_CETERM and CECAT = "HYPO SYMPTOMS" | (NY) | No; Yes | Prompt | radio | ||
HYPOSYMP_CETERM | 39 | If Other, specify | Specify | If "other" is indicated, specify the sign/symptom. | Text | CETERM | CETERM where CECAT = "HYPO SYMPTOMS" | ||||||
HPF_FACAT | 40 | What is the category? | Category | Record the FA category, if not pre-printed on the CRF. | Text | FACAT | PRECIPIATING FACTORS | Prompt | Yes | ||||
HPF_FAOBJ | 41 | N/A | N/A | N/A | Text | FAOBJ | FAOBJ where FACAT = "PRECIPIATING FACTORS" | HYPOGLYCEMIC EVENT | Prompt | Yes | |||
HPF_FAYN | 42 | Were any precipitating factors reported? | Any Precipitating Factors | Indicate if there are findings. If Yes, include the appropriate details where indicated on the CRF. | Text | N/A | (NY) | No; Yes | radio | ||||
HPF_ALCPF_FAORRES | 43 | Was alcohol consumption a precipitating factor? | Alcohol Consumption | Indicate if alcohol consumption was a precipitating factor of the hypoglycemic event. | Text | FAORRES | FAORRES where FATESTCD = "ALCPF" and FATEST = "Alcohol Consumption as a Precip Factor" and FACAT = "PRECIPITATING FACTORS" and FAOBJ = "HYPOGLYCEMIC EVENT" | (NY) | No; Yes | Prompt | radio | ||
HPF_ILLPF_FAORRES | 44 | Was concurrent illness a precipitating factor? | Concurrent Illness | Indicate if concurrent illness was a precipitating factor of the hypoglycemic event. | Text | FAORRES | FAORRES where FATESTCD = "ILLPF" and FATEST = "Concurrent Illness as a Precip Factor" and FACAT = "PRECIPITATING FACTORS" and FAOBJ = "HYPOGLYCEMIC EVENT" | (NY) | No; Yes | Prompt | radio | ||
HPF_DSDVPF_FAORRES | 45 | Was deviation from dosing instructions a precipitating factor? | Deviation from Dosing Instructions | Indicate if deviation from dosing instructions was a precipitating factor of the hypoglycemic event. | Text | FAORRES | FAORRES where FATESTCD = "DSDVPF" and FATEST = "Dosing Deviation as a Precip Factor" and FACAT = "PRECIPITATING FACTORS" and FAOBJ = "HYPOGLYCEMIC EVENT" | (NY) | No; Yes | Prompt | radio | ||
HPF_MEALPF_FAORRES | 46 | Was missed, delayed or smaller meal a precipitating factor? | Missed, Delayed or Smaller Meal | Indicate if missed, delayed, or smaller meal was a precipitating factor of the hypoglycemic event. | Text | FAORRES | FAORRES where FATESTCD = "MEALPF" and FATEST = "Meal Variance as a Precip Factor" and FACAT = "PRECIPITATING FACTORS" and FAOBJ = "HYPOGLYCEMIC EVENT" | (NY) | No; Yes | Prompt | radio | ||
HPF_PAPF_FAORRES | 47 | Was physical activity a precipitating factor? | Physical Activity | Indicate if physical activity was a precipitating factor of the hypoglycemic event. | Text | FAORRES | FAORRES where FATESTCD = "PAPF" and FATEST = "Physical Activity as a Precip Factor" and FACAT = "PRECIPITATING FACTORS" and FAOBJ = "HYPOGLYCEMIC EVENT" | (NY) | No; Yes | Prompt | radio | ||
HPF_OTHPF_FAORRES | 48 | Was there any other precipitating factor? | Other (Specify) | Indicate if there was an other precipitating factor of the hypoglycemic event. | Text | FAORRES | FAORRES where FATESTCD/FATEST are derived from the value entered in HPF_FATEST and FACAT = "PRECIPITATING FACTORS" and FAOBJ = "HYPOGLYCEMIC EVENT" | (NY) | No; Yes | Prompt | radio | ||
HPF_FATEST | 49 | If Other, specify | Specify | If "other" is indicated, specify the precipitating factor. | Text | FATEST | FATEST where FACAT = "PRECIPITATING FACTORS" and FAOBJ = "HYPOGLYCEMIC EVENT" |
||||||
HTG_CMCAT | 50 | What is the category for the concomitant medication? | Concomitant Medication Category | Record the concomitant medication category, if not pre-printed on the CRF. | Text | CMCAT | HYPO TREATMENT | Prompt | Yes | ||||
HTG_CMYN | 51 | Was any treatment given for the hypoglycemic event? | Any Treatment | Indicate if the subject took any treatment for the hypoglycemic event. If Yes, include the appropriate details where indicated on the CRF. | Text | N/A | (NY) | No; Yes | radio | ||||
HTG_DRINK_CMOCCUR | 52 | Did the subject take drink? | Drink | Indicate if drink was taken by checking Yes or No. | Text | CMOCCUR | CMOCCUR where CMTRT = "DRINK" and CMCAT = "HYPO TREATMENT" and CMPRESP = "Y" | (NY) | No; Yes | Prompt | radio | ||
HTG_FOOD_CMOCCUR | 53 | Did the subject take food? | Food | Indicate if food was taken by selecting Yes or No. | Text | CMOCCUR | CMOCCUR where CMTRT = "FOOD" and CMCAT = "HYPO TREATMENT" and CMPRESP = "Y" | (NY) | No; Yes | Prompt | radio | ||
HTG_GLUCTAB_CMOCCUR | 54 | Did the subject take glucose tablets? | Glucose Tablets | Indicate if glucose tablets were taken by selecting Yes or No. | Text | CMOCCUR | CMOCCUR where CMTRT = "GLUCOSE TABLETS" and CMCAT = "HYPO TREATMENT" and CMPRESP = "Y" | (NY) | No; Yes | Prompt | radio | ||
HTG_GLUCINJ_CMOCCUR | 55 | Did the subject take a glucagon injection? | Glucagon Injection | Indicate if glucagon injection was taken by selecting Yes or No. | Text | CMOCCUR | CMOCCUR where CMTRT = "GLUCAGON INJECTION" and CMCAT = "HYPO TREATMENT" and CMPRESP = "Y" | (NY) | No; Yes | Prompt | radio | ||
HTG_IVGLUC_CMOCCUR | 56 | Did the subject take intravenous glucose? | Intravenous Glucose | Indicate if intravenous glucose was taken by selecting Yes or No. | Text | CMOCCUR | CMOCCUR where CMTRT = "INTRAVENOUS GLUCOSE" and CMCAT = "HYPO TREATMENT" and CMPRESP = "Y" | (NY) | No; Yes | Prompt | radio | ||
TRTADMIN_FACAT | 57 | What is the category? | Category | Record the FA category, if not pre-printed on the CRF. | Text | FACAT | TREATMENT ADMINISTRATION | Prompt | Yes | ||||
TRTADMIN_FAOBJ | 58 | N/A | N/A | N/A | Text | FAOBJ | FAOBJ where FACAT = "TREATMENT ADMINISTRATION" | HYPOGLYCEMIC EVENT | Prompt | Yes | |||
TRTADMIN_TXASSIST_FAORRES | 59 | If treatment was given, indicate if assistance was needed? | Need for Assistance | Indicate the investigator's assessment of the subject's need for assistance. | Text | FAORRES | FAORRES where FATESTCD = "TXASSIST" and FATEST = "Treatment Assistance" and FACAT = "TREATMENT ADMINISTRATION" and FAOBJ = "HYPOGLYCEMIC EVENT" | None - Subject Treated Self; Subject was Capable of Treating Self, but Received Assistance; Subject was Not Capable of Treating Self and Required Assistance | radio |
For another way to represent data collected using the CE domain, see the CDISC Therapeutic Area Data Standards User Guide for Diabetes v1.0 Section 3.3.2, Example 5 (available at https://www.cdisc.org/standards/therapeutic-areas/diabetes).
8.2.4 DS - Disposition
Description/Overview for the CDASHIG DS - Disposition Domain
The DS domain includes disposition events and protocol milestones (e.g., informed consent obtained, randomized).
Specification for the CDASHIG DS - Disposition Domain
Disposition Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Events | DS | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Events | DS | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Events | DS | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Events | DS | N/A | N/A | 4 | DSCAT | Category for Disposition Event | A categorization of the disposition events which is used to distinguish between disposition events, protocol milestones, and other events. | What was the category of the disposition? | [Disposition Category] | Char | HR | If collected on the CRF, the sponsor provides instructions to ensure the data is entered as intended. | DSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DSCAT) | N/A | This would most commonly be a heading on the CRF or screen, not a question to which the site would provide an answer. This is used to distinguish a DISPOSITION EVENT, a PROTOCOL MILESTONE, or an OTHER EVENT. |
Events | DS | N/A | N/A | 5 | DSSCAT | Subcategory for Disposition Event | A sub-division of the DSCAT values based on user- defined characteristics. | What was the subcategory of the disposition? | [Disposition Subcategory]; NULL | Char | O | If collected on the CRF, the sponsor provides instructions to ensure the data is entered as intended. | DSSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. DSSCAT can only be used if there is an DSCAT with a and it must be a subcategorization of the Disposition event. |
Events | DS | N/A | N/A | 6 | EPOCH | Epoch | Trial epoch (e.g., DOUBLE BLIND, SINGLE BLIND, TREATMENT, SCREENING, RUN IN) for which subject disposition is being collected. | What is the trial period for this disposition event? | Trial Period | Char | R/C | Check the [epoch, or insert more appropriate wording] for which disposition is being recorded. | EPOCH | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EPOCH) | N/A | If disposition events are collected more than once in the study, EPOCH may be needed to differentiate them. Typically, the trial epoch will be preprinted on the CRF as the title of the page; however, some companies have a standard CRF module that includes a pick-list of epochs. See SDTMIG for further information regarding EPOCH. EPOCH would only be populated for disposition event and not for protocol milestones. |
Events | DS | N/A | N/A | 7 | DSDECOD | Standardized Disposition Term | The standardized terminology of the disposition term that describes whether a subject completed the study or a portion of a study (epoch) or the reason they did not complete. | What was the subject's status (at the EPOCH/study specific time frame)? | Status (at the EPOCH/study specific time frame) | Char | R/C | Document the subject's status at [insert text corresponding to the selected trial epoch/study specific time frame]. If the subject discontinued prematurely, record the primary reason for discontinuation. | DSDECOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. Both DSDECOD and DSTERM must be populated in the SDTM-based datasets. If DSTERM was collected as an "Other, Specify" free text, populate DSTERM with the free text and populate DSDECOD with the sponsor-defined standardized text. If DSDECOD was collected with no free text, populate DSTERM and DSDECOD in the SDTM-based dataset with the DSDECOD value that was collected. | (NCOMPLT) | N/A | DSDECOD can be used as the standardized coded list with DSTERM used to capture any "Specify, Other" information, or DSDECOD can be used on its own. For Sponsor- and/or study-specific reasons for discontinuation, it is recommended that these reasons be preprinted on the CRF, as subcategories of the appropriate standardized DSDECOD item. However, it is recommended to limit the use of sponsor and study- specific reasons in order to promote consistent use of terminology and hence permit the combination of data across multiple sponsors. Either DSTERM or DSDECOD must be on the CRF. Both may be used if DSTERM is used as a "Specify, Other" field. |
Events | DS | N/A | N/A | 8 | DSTERM | Reported Term for the Disposition Event | The verbatim or prespecified name of the event. The reported or prespecified name for how a subject completed the study or a portion of a study (epoch) or the reason they did not complete. | What was the subject's status?; If [DSDECOD], specify | [Status]; [Specify] | Char | R/C | Document the subject's status at [insert text corresponding to the selected trial epoch]. If the subject discontinued prematurely, record the primary reason for discontinuation. (Or, if used with a DECOD list), if Other is selected from the Status list, provide the verbatim reason. | DSTERM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variables DSDECOD and DSTERM must be populated in SDTM. If DSTERM was collected as an "Other, Specify" free text, populate the SDTMIG variable DSTERM with the free text and populate DSDECOD with the sponsor-defined standardized text. If DSDECOD was collected with no free text, populate DSTERM and DSDECOD in the SDTM-based dataset with the DSDECOD value that was collected. | N/A | N/A | If used with a DECOD list, free text description of the subject's "Other" status. DSTERM is the verbatim term for subject status when Other is selected from the DSDECOD code list. This field would only be used with the Prompt and Completion Instructions provided as an Specify, Other field in conjunction with DSDECOD. DSTERM may be used by itself when no code list is provided for DSDECOD on the CRF. Either DSTERM or DSDECOD must be on the CRF. DSTERM is required in the SDTM-based dataset. |
Events | DS | N/A | N/A | 9 | DSSTDAT | Disposition Event Start Date | The date of the specified protocol milestone (e.g., informed consent, randomization) or disposition event (e.g., study completion or discontinuation), represented in an unambiguous date format (e.g., DD- MON-YYYY). | What was the [protocol milestone/disposition event/other event name] date? | [Protocol Milestone/Disposition Event/Other Event Name] Date | Char | R/C | Record the date of [protocol milestone/disposition event] as defined in the protocol and/or CRF Completion Instructions using this format (DD- MON-YYYY). | DSSTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable DSSTDTC in ISO 8601 format. | N/A | N/A | Define in the protocol and/or CRF completion instructions the criteria for completion of each protocol milestone or disposition event for which a disposition CRF will be provided. Only collect the date of each protocol milestone or disposition event once. For example, if the date of the last dose is defined to mark the end of the Treatment Phase epoch, and is collected on the Exposure form, then this field would not be collected on the Disposition CRF module. If not collected elsewhere, this field should be collected on the Disposition CRF module. |
Events | DS | N/A | N/A | 10 | DSSTTIM | Disposition Event Start Time | The time of the specified protocol milestone (e.g., informed consent, randomization) or disposition event (e.g., study completion or discontinuation), represented in an unambiguous time format (e.g., hh:mm:ss). | What was the [protocol milestone/disposition event/other event name] time? | [Protocol Milestone/Disposition Event/Other Event Name] Time | Char | O | Record the time (as complete as possible) that the subject completed the study or portion of the study as defined in the protocol and/or CRF Completion Instructions. If the subject did not complete the study or portion of the study, record the time (as complete as possible) as defined in the protocol and/or CRF Completion Instructions. | DSSTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable DSSTDTC in ISO 8601 format. | N/A | N/A | Define in the protocol and/or CRF completion instructions the criteria for completion of each epoch for which a disposition CRF will be provided. Define also the date of completion or discontinuation. Collecting the time of completion or discontinuation is only appropriate if it can be realistically determined and if there is a scientific reason for needing to know this level of detail. Typically, it is not recommended that a time be collected unless the subject is under the direct care of the site at the time of the event. Only collect the time of completion or discontinuation on the disposition CRF module if the same information is not being collected on another CRF module. For example, if the time of the last dose is defined to mark the end of the Treatment Phase epoch, and is collected on the Drug Exposure form, then this field would not be collected on the Disposition CRF module. |
Events | DS | N/A | N/A | 11 | DTHDAT | Death Date | Date of death for any subject who died. | What [is/was] the subject's date of death? | Death Date | Char | O | Record the date of death | DM.DTHDTC | This field does not map directly to an SDTMIG variable. For the SDTM dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTM variable DTHDTC in ISO 8601 format. | N/A | N/A | The CDASH model defines Death Date as a timing variable; this is not included as a timing variable in the SDTMIG. It may be collected on any CRF deemed appropriate by the sponsor, but should only be collected once. The SDTMIG variable DTHDTC is mapped to the DM domain during the SDTM submission dataset creation process. The SDTMIG variable DM.DTHFLG is not a CDASH variable, but it is typically populated during the SDTM submission dataset creation process. Death Date may be mapped to other SDTM domains (e.g., DS) as deemed appropriate by the sponsor. |
Events | DS | N/A | N/A | 12 | DSUNBLND | Unblinded | An indication the subject's treatment information was revealed to any unauthorized site personnel during the trial. | Was (study) treatment unblinded by the site? | Unblinded | Char | O | Record "Yes" if the subject's treatment assignment was unblinded/unmasked intentionally due to an AE, or unintentionally for other reasons (e.g., an administrative action) during the subject's blinded/masked treatment. Record the primary reason for discontinuation if the unblinding resulted in the subject discontinuing the trial prematurely. | DSTERM; DSDECOD | This does not map directly to an SDTM variable. If the CDASH field DSUNBLIND="Y", then the SDTMIG variables DSDECOD and DSTERM="TREATMENT UNBLINDED" and DSCAT ="OTHER EVENT". If DSUNBLIND="N", then the CRF should be annotated to indicate that this value is NOT SUBMITTED. | (NY) | N/A | There may be multiple rows in the SDTM DS dataset to represent this information, each with the appropriate DSCAT values. One row could indicate the treatment was unblinded using DSCAT="OTHER EVENT" and another row could indicate the status of the subject at the end of participation in the trial using DSCAT= "DISPOSITION". If DSUNBLND is "Yes" and information was collected about the reason for the unblinding, populate DSCAT with "OTHER EVENT" and the SDTMIG variables DSTERM with the free text and DSDECOD with the sponsor-defined standardized text (e.g., TREATMENT UNBLINDED). If DSUNBLND is "Yes", and the unblinding also resulted in the subject discontinuing the trial prematurely, populate DSCAT with "DISPOSTION" and use the SDTM IG variables DSTERM and DSDECOD to capture the applicable discontinuation details. If the unblinding occurred due to an Adverse Event, DSTERM contains the text of the AE, and in the AE domain the SDTMIG variable AEACNOTH "Were any other actions taken in response to this adverse event?" may include text of "Treatment Unblinded". DSUNBLND may also be used to document intentional unblinding at a protocol defined point in the trial. |
Events | DS | N/A | N/A | 13 | DSCONT | Subject Continue | The plan for subject continuation to the next phase of the study or another study at the time of completion of the CRF. | Will the subject continue? | Subject Continue | Char | O | Record if the subject will be continuing to [the next phase of this study/related study] (sponsor to specify as appropriate). | SUPPDS.QVAL | This information could be submitted in a SUPPDS dataset as the value of SUPPDS.QVAL when SUPPDS.QNAM= "DSCONT" and SUPPDS.QLABEL="Subject Continue". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | Sponsors should specify the next phase of the study or the related study on the CRF. Typically this is a prompt question to aid in monitoring and data cleaning, and usually not submitted in a SUPPDS dataset. |
Events | DS | N/A | N/A | 14 | DSNEXT | Next EPOCH | Identifies the study epoch or new study in which the subject will participate. | What is the next [epoch/period/study/trial] the subject will [continue to/enter/enroll]? | Next [Epoch/Period/Study/Trial] | Char | O | Record the planned subsequent [study epoch/study] in which the subject intends to participate. | N/A | Sponsor-defined SDTMIG mapping. | N/A | N/A | Sponsors should specify the next phase of the study or the related study on the CRF. The data are to be used to aid in monitoring and data cleaning. No specific SDTM-based dataset mapping rules are provided since the mapping depends on the situation (next epoch or next trial). Per sponsor decision, plans to enter the next epoch within a study may be included in the SDTM submission datasets (e.g., SE). Actual subject entry into the next study is submitted as part of Trial Design datasets of that study. |
Assumptions for the CDASHIG DS - Disposition Domain
1. Sponsors may choose which disposition events and milestones to collect for a study. A milestone is a protocol-specified “point in time” that is not assigned to an epoch. A disposition event describes whether a subject completed the study or portion of a study (epoch), or the reason they did not complete. A sponsor may collect 1 disposition event for the trial as a whole, or they may collect disposition for each epoch of the trial.
2. Disposition data may be collected on a form dedicated to disposition data, or it may be collected across several forms that also contain data that are not DS. When disposition data are collected on forms also containing data that are not DS, the disposition should be mapped to the appropriate DS SDTMIG variable (e.g., DSCAT, DSSTDTC, DSTERM, DSDECOD). The same disposition data should not be collected on both domain-specific forms and on a disposition form.
a. The CDASHIG DS domain does not specify where to collect protocol milestones within the CRF. Protocol milestones may be included in convenient places in the CRF. For example, Informed Consent Date or Randomization Date may be collected on the same CRF page as demographics data and mapped for submission to the SDTMIG DS domain.
b. The CDASH Model allows for collection of the Date of Death to be collected on any CRF deemed appropriate by the sponsor and mapped for submission to the SDTMIG DS domain or the Date of Death may be collected as part of a Disposition form. Consideration should be given to design the CRF to collect the Date of Death only once in a study.
3. Controlled Terminology (NCOMPLT) is focused on disposition events, and is used when DSCAT is "DISPOSITION EVENT". Because the complete list of controlled terminology may not be appropriate, sponsors may choose to include only subsets of CT on the CRF. The choices that appear for a DS Event are dependent upon the event itself, and the contents of the list can vary if data are collected for multiple epochs in a study. For example, "Non-Compliance with Study Drug" and "Lack of Efficacy” are not applicable choices prior to the start of treatment; "Failure to Meet Randomization Criteria" is an applicable choice only for the epoch that immediately follows the date of subject's randomization.
4. If DSUNBLND is "Yes", and the unblinding/unmasking resulted in the subject discontinuing the trial prematurely, DSTERM and DSDECOD capture the applicable details. If the unblinding/unmasking occurred due to an adverse event, DSTERM contains the text of the AE, and the AE “Were any other actions taken in response to this adverse event?” (AEACNOTH [optional]) free text may include text of “Treatment Unblinded”. DSUNBLND may also be used to document intentional unblinding at a protocol defined point in the trial.
5. DSCONT and DSNEXT data are used to aid in monitoring and data cleaning. Because the responses are about future plans, the validity of the responses cannot be ascertained until the subject enters the subsequent epoch or new study.
Example CRFs for the CDASHIG DS - Disposition Domain
Example 1
Title: Informed Consent
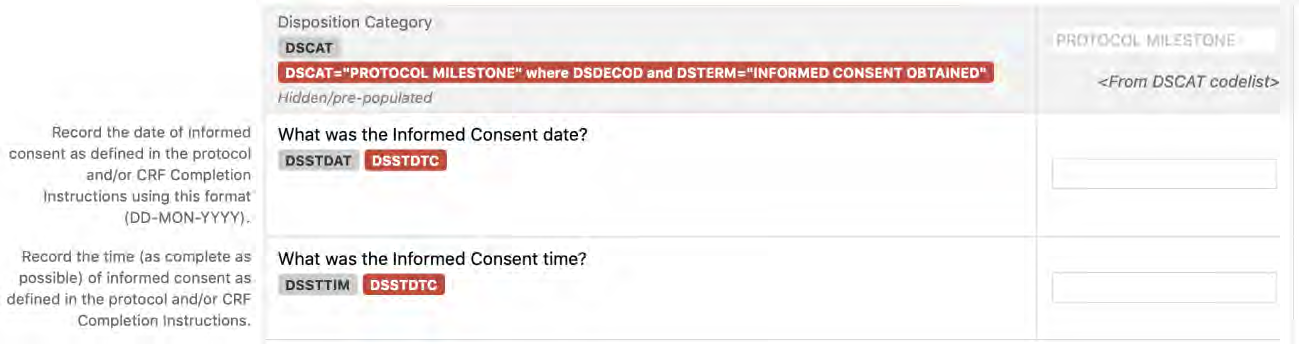
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Value | SDTMIG Target Mapping | Controlled Terminology Code List Name | CRF Implementation Notes | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DSCAT | 1 | What was the category of the disposition? | Disposition Category | If collected on the CRF, the sponsor provides instructions to ensure the data is entered as intended. | Text | DSCAT | DSCAT="PROTOCOL MILESTONE" where DSDECOD and DSTERM="INFORMED CONSENT OBTAINED" | (DSCAT) | Refer to the SDTMIG for guidelines for assigning DSCAT, DSTERM, and DSDECOD for events considered Protocol Milestones/ | PROTOCOL MILESTONE | prompt | Yes | ||
DSSTDAT | 2 | What was the Informed Consent date? | Informed Consent Date | Record the date of informed consent as defined in the protocol and/or CRF Completion Instructions using this format (DD- MON-YYYY). | Date | DSSTDTC | qtext | |||||||
DSSTTIM | 3 | What was the Informed Consent time? | Informed Consent Time | Record the time (as complete as possible) of informed consent as defined in the protocol and/or CRF Completion Instructions. | Time | DSSTDTC | qtext |
Example 2
In this example, the Disposition CRF simply collects whether or not the subject completed the study, so there is only 1 record per subject.
Title: Study Disposition
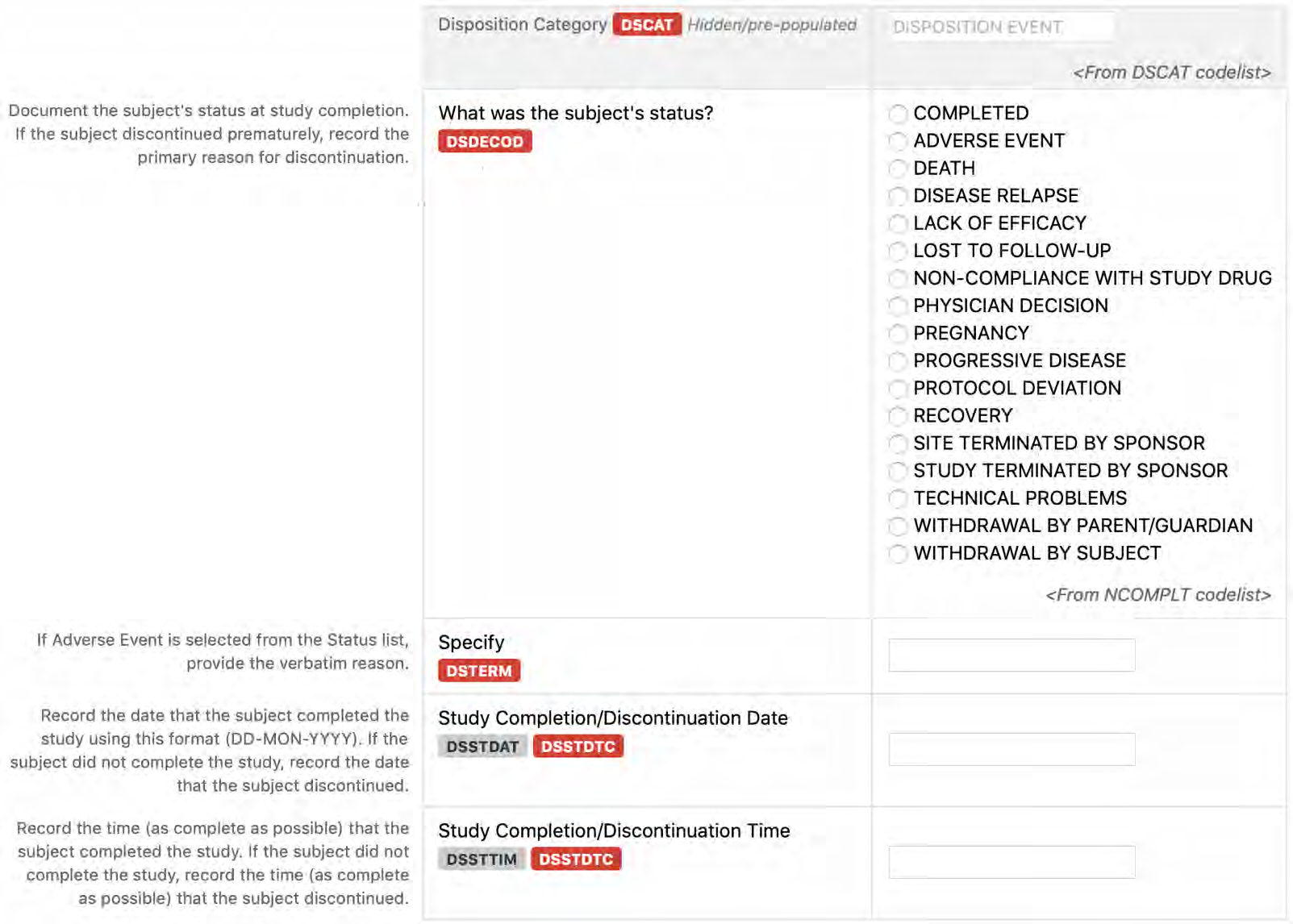
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Value | SDTMIG Target Mapping | Controlled Terminology Code List Name | CRF Implementation Notes | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DSCAT | 1 | What was the category of the disposition? | Disposition Category | If collected on the CRF, the sponsor provides instructions to ensure the data is entered as intended. | Text | DSCAT | (DSCAT) | DISPOSITION EVENT | prompt | Yes | ||||
DSDECOD | 2 | What was the subject's status? | Status | Document the subject's status at study completion. If the subject discontinued prematurely, record the primary reason for discontinuation. | Text | DSDECOD | (NCOMPLT) | The Controlled Terminology (NCOMPLT) is focused on disposition events, and is used when DSCAT is "DISPOSITION EVENT". | COMPLETED; ADVERSE EVENT; DEATH; DISEASE RELAPSE; LACK OF EFFICACY; LOST TO FOLLOW-UP; NON-COMPLIANCE WITH STUDY DRUG; PHYSICIAN DECISION; PREGNANCY; PROGRESSIVE DISEASE; PROTOCOL DEVIATION; RECOVERY; SITE TERMINATED BY SPONSOR; STUDY TERMINATED BY SPONSOR; TECHNICAL PROBLEMS; WITHDRAWAL BY PARENT/GUARDIAN; WITHDRAWAL BY SUBJECT | radio | ||||
DSTERM | 3 | If adverse event, specify | Specify | If Adverse Event is selected from the Status list, provide the verbatim reason. | Text | DSTERM | If DSTERM was collected as an adverse event, Specify", populate the SDTMIG variable DSTERM with the free text and populate DSDECOD with the standardized text from (NCOMPLT). | prompt | ||||||
DSSTDAT | 4 | What was the study completion/discontinuation date? | Study Completion/Discontinuation Date | Record the date that the subject completed the study using this format (DD-MON-YYYY). If the subject did not complete the study, record the date that the subject discontinued. | Date | DSSTDTC | ||||||||
DSSTTIM | 5 | What was the study completion/discontinuation time? | Study Completion/Discontinuation Time | Record the time (as complete as possible) that the subject completed the study. If the subject did not complete the study, record the time (as complete as possible) that the subject discontinued. | Time | DSSTDTC |
Example 3
In this example, the Disposition CRF collects multiple disposition events at different time points in the study as indicated by the trial period (epoch).
Title: Trial Period Disposition
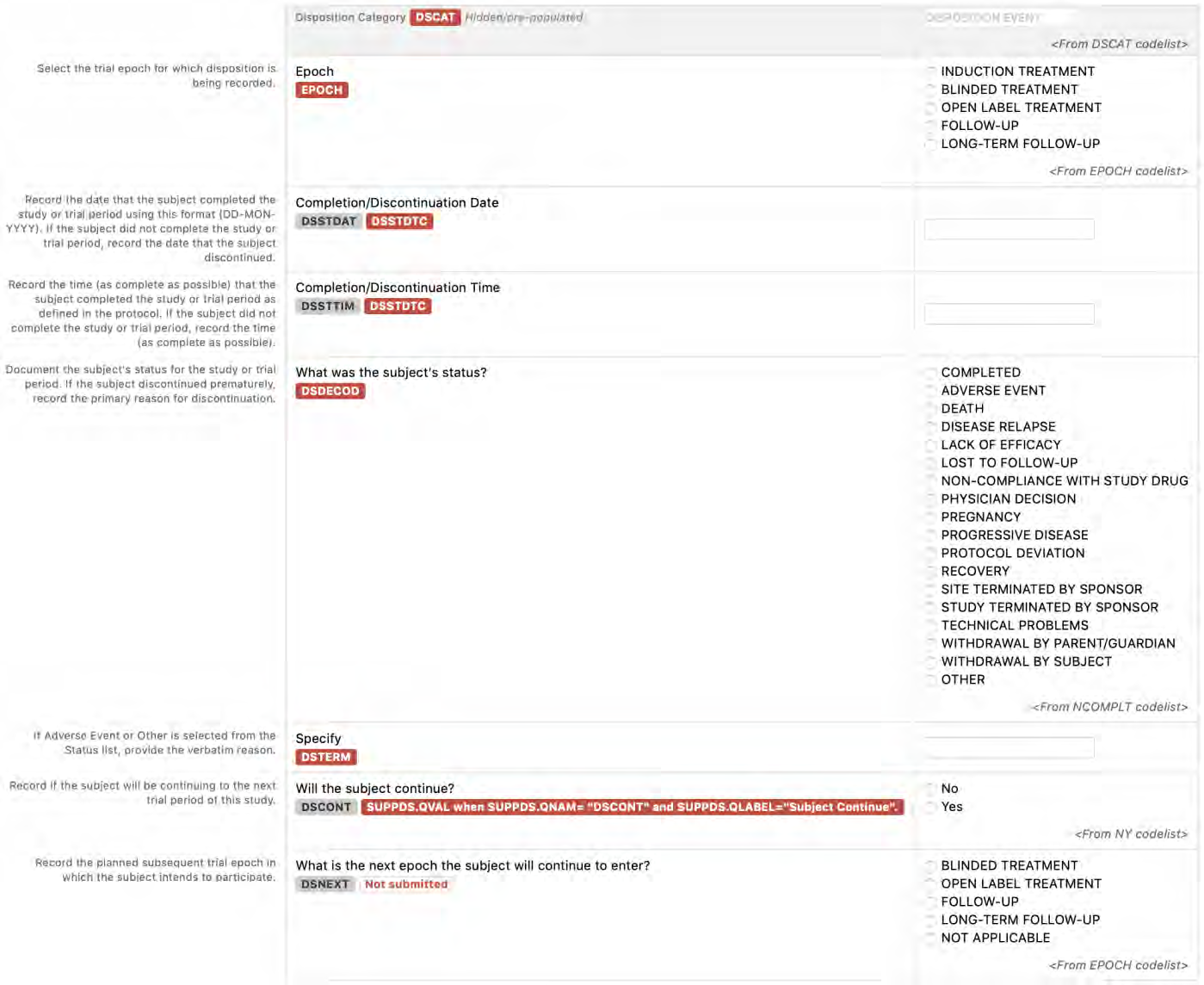
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Value | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DSCAT | 1 | What was the category of the disposition? | Disposition Category | If collected on the CRF, the sponsor provides instructions to ensure the data is entered as intended. | Text | DSCAT | (DSCAT) | DISPOSITION EVENT | prompt | Yes | |||
EPOCH | 2 | What is the trial epoch for this disposition event? | Epoch | Select the trial epoch for which disposition is being recorded. | Text | EPOCH | (EPOCH) | INDUCTION TREATMENT; BLINDED TREATMENT; OPEN LABEL TREATMENT; FOLLOW-UP; LONG-TERM FOLLOW-UP | prompt | radio | |||
DSSTDAT | 3 | What was the completion/discontinuation date? | Completion/Discontinuation Date | Record the date that the subject completed the study or trial period using this format (DD-MON- YYYY). If the subject did not complete the study or trial period, record the date that the subject discontinued. | Date | DSSTDTC | |||||||
DSSTTIM | 4 | What was the completion/discontinuation time? | Completion/Discontinuation Time | Record the time (as complete as possible) that the subject completed the study or trial period as defined in the protocol. If the subject did not complete the study or trial period, record the time (as complete as possible). | Time | DSSTDTC | |||||||
DSDECOD | 5 | What was the subject's status? | Status | Document the subject's status for the study or trial period. If the subject discontinued prematurely, record the primary reason for discontinuation. | Text | DSDECOD | (NCOMPLT) | COMPLETED; ADVERSE EVENT; DEATH; DISEASE RELAPSE; LACK OF EFFICACY; LOST TO FOLLOW-UP; NON-COMPLIANCE WITH STUDY DRUG; PHYSICIAN DECISION; PREGNANCY; PROGRESSIVE DISEASE; PROTOCOL DEVIATION; RECOVERY; SITE TERMINATED BY SPONSOR; STUDY TERMINATED BY SPONSOR; TECHNICAL PROBLEMS; WITHDRAWAL BY PARENT/GUARDIAN; WITHDRAWAL BY SUBJECT; OTHER | radio | ||||
DSTERM | 6 | If other, specify | Specify | If Adverse Event or Other is selected from the Status list, provide the verbatim reason. | Text | DSTERM | prompt | ||||||
DSCONT | 7 | Will the subject continue? | Continue | Record if the subject will be continuing to the next trial period of this study. | Text | SUPPDS.QVAL | SUPPDS.QVAL when SUPPDS.QNAM= "DSCONT" and SUPPDS.QLABEL="Subject Continue". | (NY) | No; Yes | radio | |||
DSNEXT | 8 | What is the next epoch the subject will continue to enter? | Next epoch | Record the planned subsequent trial epoch in which the subject intends to participate. | Text | N/A | (EPOCH) | BLINDED TREATMENT; OPEN LABEL TREATMENT; FOLLOW-UP; LONG-TERM FOLLOW-UP; NOT APPLICABLE |
8.2.5 DV - Protocol Deviations
Description/Overview for the CDASHIG DV - Protocol Deviations Domain
The DV domain is intended to collect protocol deviations or violations that occur after enrollment. It is not intended to collect information about inclusion/exclusion criteria; that data should be collected in IE.
Considerations Regarding Usage of a Protocol Deviations CRF
Sponsors must employ a robust and systematic method for recording protocol deviations; this may include the use of a dedicated CRF for this purpose, or the intentional inclusion of data collection fields throughout the entire set of CRFs that will detect protocol deviations.
The DV domain metadata and example CRF were developed as a guide that sponsors could use for designing a Protocol Deviations CRF and associated database, should they choose to do so. See Appendix B, Glossary and Abbreviations, for definitions of Protocol Deviation and Protocol Violation are available.
Specification for the CDASHIG DV - Protocol Deviations Domain
Protocol Deviations Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Events | DV | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Events | DV | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted for the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Events | DV | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Events | DV | N/A | N/A | 4 | DVCAT | Category for Protocol Deviation | A grouping of topic- variable values based on user- defined characteristics. | What is the category of the protocol deviation? | [Protocol Deviation Category]; NULL | Char | O | Record the deviation category, if not preprinted on the CRF. | DVCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Events | DV | N/A | N/A | 5 | DVSCAT | Subcategory for Protocol Deviation | A sub-division of the DVCAT values based on user- defined characteristics. | What is the subcategory of the protocol deviation? | [Protocol Deviation Subcategory]; NULL | Char | O | Record the deviation event subcategory, if not preprinted on the CRF. | DVSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. DVSCAT can only be used if there is an DVCAT and it must be a subcategorization of DVCAT. |
Events | DV | N/A | N/A | 6 | DVYN | Any Protocol Deviation | An indication whether or not there were any protocol deviations. | Were there any protocol deviations? | Any Deviations | Char | O | Enter Yes if a protocol deviation occurred and No if none occurred. Ensure that any adverse event which triggers a protocol deviation (e.g., concomitant medication use, newly discovered medical history) is noted in the respective CRF. | N/A | Does not map to an SDTMIG variable. The SDTM aCRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification that all other fields on the CRF were deliberately left blank. |
Events | DV | N/A | N/A | 7 | DVDECOD | Protocol Deviation Coded Term | The sponsor-defined standardized text for the name of the protocol deviation. | What was the (standardized) protocol deviation (term/(code)? | (Standardized) Protocol Deviation (Term) | Char | R/C | Record protocol deviations identified and/or select the appropriate code from the list of protocol deviation terms. | DVDECOD | Maps directly to the SDTMIG variable listed in the column with the heading "SDTMIG Targe". | N/A | N/A | DVTERM and DVDECOD may have the same value. If the CRF is collecting protocol deviations using a code list of responses, then DVDECOD should be used to store the code list response. Sponsors must use either DVDECOD or DVTERM on the CRF and in some cases, both may be used. For example, if the CRF collects "Specify, Other" or similar additional free text descriptions of code list items, then DVTERM should be used to store the detailed descriptive text |
Events | DV | N/A | N/A | 8 | DVTERM | Protocol Deviation Term | The reported or prespecified name of the protocol deviation. | What was the protocol deviation term? | (Specify) Protocol Deviation | Char | R/C | Record the appropriate code from the list of protocol deviation terms. | DVTERM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | DVTERM and DVDECOD may have the same value. If the CRF is collecting protocol deviations using a free text field, then DVTERM should be used to store the free text response. Sponsors may use either DVDECOD or DVTERM on the CRF, but a value in DVTERM is required in the SDTM-based datasets. |
Events | DV | N/A | N/A | 9 | DVSTDAT | Deviation Start Date | The start date of deviation, represented in an unambiguous date format (e.g., DD- MON-YYYY). | What was the protocol deviation start date? | Start Date | Char | O | Record the start date that the protocol deviation using this format (DD-MON- YYYY). This should be the start or occurrence of the protocol deviation and not the date it was discovered or reported. | DVSTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable DVSTDTC in ISO 8601 format. | N/A | N/A | This may be derived if not collected on a CRF. |
Events | DV | N/A | N/A | 10 | DVSTTIM | Deviation Start Time | The start time of the deviation, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the protocol deviation start time? | Start Time | Char | O | If appropriate, record the start time (as complete as possible) of the protocol deviation in an unambiguous time format (e.g.,., hh:mm:ss). This should be the start or occurrence of the protocol deviation and not the time it was discovered or reported. | DVSTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable DVSTDTC in ISO 8601 format. | N/A | N/A | N/A |
Events | DV | N/A | N/A | 11 | DVENDAT | Deviation End Date | The end date of the deviation ,represented in an unambiguous date format (e.g., DD- MON-YYYY). | What was the protocol deviation end date? | End Date | Char | O | Record the end date of the protocol deviation using this format (DD-MON- YYYY). This should be the date the protocol deviation stopped and not the date it was discovered or reported. | DVENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable DVENDTC in ISO 8601 format. N/A | N/A | N/A | |
Events | DV | N/A | N/A | 12 | DVENTIM | Deviation End Time | The end time of the deviation, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the protocol deviation end time? | End Time | Char | O | If appropriate, record the end time (as complete as possible) of the protocol deviation in an unambiguous time format (e.g., hh:mm:ss). This should be the time the protocol deviation stopped and not the time it was discovered or reported. | DVENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable DVENDTC in ISO 8601 format. | N/A | N/A | N/A |
Events | DV | N/A | N/A | 13 | DVSPID | DV Sponsor- Defined Identifier | A sponsor-defined identifier. In CDASH, This is typically used for preprinted or auto-generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor-defined question] | [Sponsor defined] | Char | O | If collected on the CRF, the sponsor may insert instructions to ensure each record has a unique identifier. | DVSPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Because SPID is a sponsor- defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile data. May be used to record preprinted number (e.g. line number, record |
Assumptions for the CDASHIG DV - Protocol Deviations Domain
If a sponsor decides to use a DV CRF, the sponsor should not rely on this CRF as the only source of protocol deviation information for a study. Rather, they should also utilize monitoring, data review, and programming tools to assess whether there were protocol deviations in the study that may affect the usefulness of the datasets for analysis of efficacy and safety.
Example CRF for the CDASHIG DV - Protocol Deviations Domain
Example 1
Title: Protocol Deviations
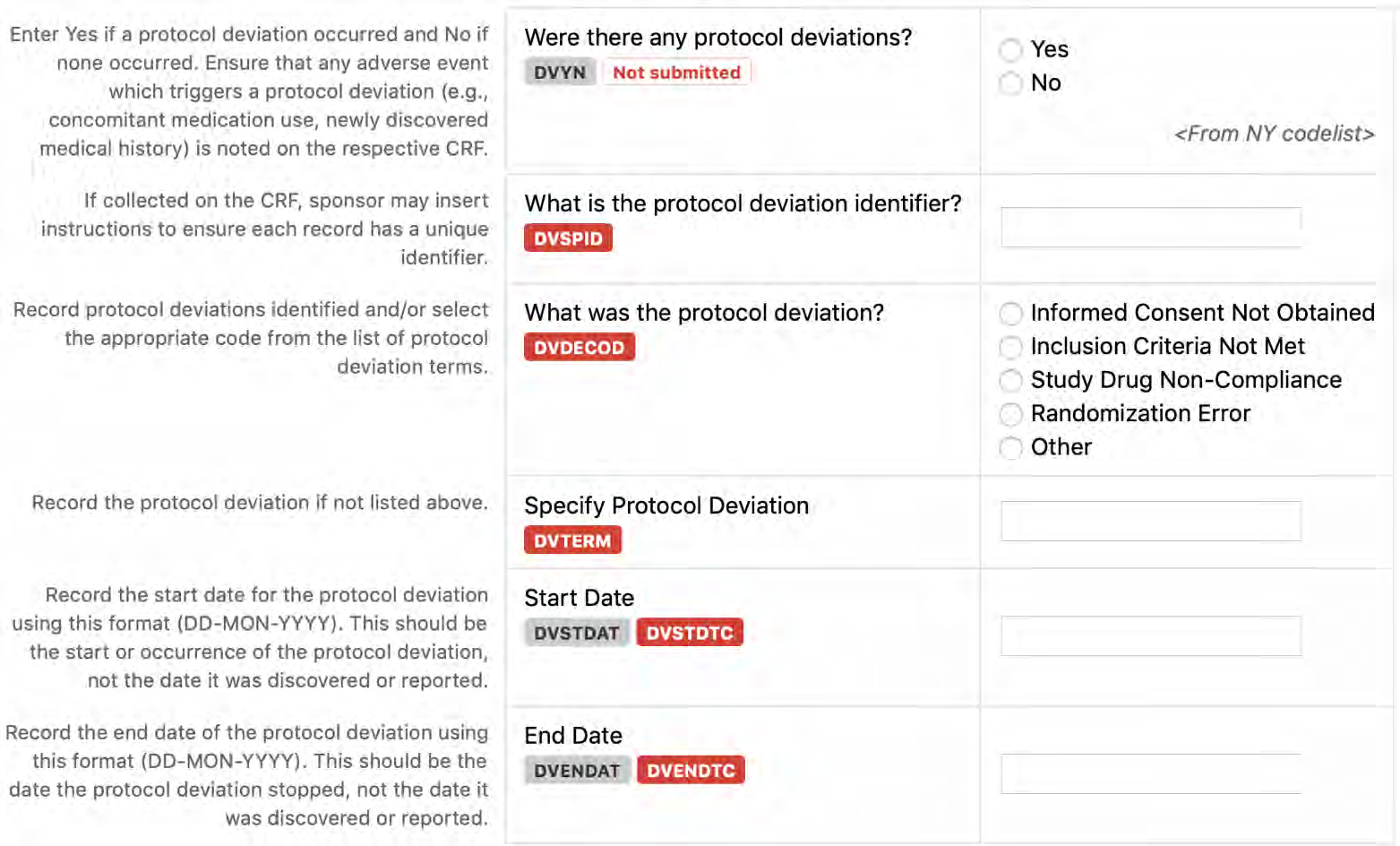
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Value | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DVYN | 1 | Were there any protocol deviations? | Any Deviations | Enter Yes if a protocol deviation occurred and No if none occurred. Ensure that any adverse event which triggers a protocol deviation (e.g., concomitant medication use, newly discovered medical history) is noted on the respective CRF. | Text | N/A | (NY) | Yes; No | |||||
DVSPID | 2 | What is the protocol deviation identifier? | DV Number | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | Text | DVSPID | Yes | ||||||
DVDECOD | 3 | What was the protocol deviation? | Protocol Deviation | Record protocol deviations identified and/or select the appropriate code from the list of protocol deviation terms. | Text | DVDECOD | Informed Consent Not Obtained; Inclusion Criteria Not Met; Study Drug Non- Compliance; Randomization Error; Other | ||||||
DVTERM | 4 | What was the protocol deviation term? | Specify Protocol Deviation | Record the protocol deviation if not listed above. | Text | DVTERM | Prompt | ||||||
DVSTDAT | 5 | What was the protocol deviation start date? | Start Date | Record the start date for the protocol deviation using this format (DD-MON-YYYY). This should be the start or occurrence of the protocol deviation, not the date it was discovered or reported. | Date | DVSTDTC | |||||||
DVENDAT | 6 | What was the protocol deviation end date? | End Date | Record the end date of the protocol deviation using this format (DD-MON-YYYY). This should be the date the protocol deviation stopped, not the date it was discovered or reported. | Date | DVENDTC |
8.2.6 HO - Healthcare Encounters
Description/Overview for the CDASHIG HO - Healthcare Encounters Domain
The HO dataset includes inpatient and outpatient healthcare events (e.g., hospitalizations, nursing home stay, rehabilitation facility stays, medical practitioner office visits).
This domain is used to record information about the event (e.g., type of encounter, timing). Other data about any interventions administered or any findings measured, observed, or tested during the healthcare encounter should be collected for submission in those respective domains. For example:
• Information about imaging—for example, MRI or endoscopic retrograde cholangiopancreatography (ERCP), which is a combination of an upper GI endoscopy and x-ray—would be collected in the Procedures (PR) domain.
• Laboratory results would be reported in Lab Results (LB).
It may not be necessary to collect data for the HO domain if the planned reporting or analysis is about the interventions and/or findings data rather than to the number/types of encounters, the duration of encounters, and so on.
Assumptions for the CDASHIG HO - Healthcare Encounters Domain
Please refer to SDTMIG for Healthcare Encounters Domain Assumptions.
Specification for the CDASHIG HO - Healthcare Encounters Domain
Healthcare Encounters Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Events | HO | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Events | HO | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Events | HO | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Events | HO | N/A | N/A | 4 | HOYN | Any Healthcare Encounters | An indication whether or not there were any healthcare encounters. | Were there any healthcare encounters? | Any Healthcare Encounters | Char | O | Indicate if the subject experienced any healthcare encounters. If yes, include the appropriate details where indicated on the CRF. | N/A | Does not map to an SDTMIG variable. The SDTM Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification if all other fields on the CRF were deliberately left blank. |
Events | HO | N/A | N/A | 5 | HOCAT | Category for Healthcare Encounter | A grouping of topic-variable values based on user-defined characteristics. | What was the category of the healthcare encounter? | [Healthcare Encounter Category]; NULL | Char | O | If collected on the CRF, the sponsor provides instructions to ensure the data is entered as intended. | HOCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. Examples include HOSPITALIZATION and OUTPATIENT. |
Events | HO | N/A | N/A | 6 | HOSCAT | Subcategory for Healthcare Encounter | A sub-division of the HOCAT values based on user-defined characteristics. | What was the subcategory of the healthcare encounter? | [Healthcare Encounter Subcategory]; NULL | Char | O | If collected on the CRF, the sponsor provides instructions to ensure the data is entered as intended. | HOSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. HOSCAT can only be used if there is an HOCAT and it must be a subcategorization of HOCAT. |
Events | HO | N/A | N/A | 7 | HOOCCUR | Healthcare Encounter Occurrence | An indication whether a health encounter occurred when information about the occurrence of a specific event is solicited. | Did the subject have [prespecified healthcare encounter term]? | [prespecified Healthcare Encounter Term] | Char | O | Indicate if [specific healthcare encounter/event topic] has occurred/is occurring by checking Yes or No. | HOOCCUR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | The CDASH variable HOOCCUR is used is used to report the occurrence of a prespecified healthcare encounter. HOOCCUR is not used if the healthcare encounters are collected on the CRF in a manner that requires a free-flow text response. The site should be able to indicate in a separate item (or system variable) that the question was not asked or answered. |
Events | HO | N/A | N/A | 8 | HOPRESP | prespecified Healthcare Encounter | An indication that a specific event, or group of events, are prespecified on a CRF. | N/A | N/A | Char | O | N/A | HOPRESP | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | A hidden field on a CRF defaulted to "Y", or added during the SDTM- based dataset creation, when the healthcare encounter is prespecified. Null for spontaneously reported events. If a study collects both prespecified healthcare encounters as well as free-text events, the value of HOPRESP should be "Y" for all prespecified events and null for events reported as free-text. HOPRESP is a permissible field in SDTM and may be omitted from the SDTM-based dataset if all events were collected as free text. |
Events | HO | N/A | N/A | 9 | HOREASND | Reason Healthcare Encounter Not Done | An explanation of why the data are not available. | What was the reason the data was not collected? | Reason Not Collected | Char | O | Provide the reason why the subject's Healthcare Encounters experience was not assessed. | HOREASND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | The reason the data are not available may be chosen from a sponsor defined list (e.g., subject not asked) or entered as free text. When HOREASND is used, the SDTMIG variable HOSTAT should also be populated in the SDTM-based dataset. |
Events | HO | N/A | N/A | 10 | HOSPID | HO Sponsor- Defined Identifier | A sponsor- defined identifier. In CDASH, This is typically used for preprinted or auto-generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor defined question] | [Sponsor defined] | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | HOSPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Because SPID is a sponsor-defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile data. May be used to record preprinted number (e.g. line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Events | HO | N/A | N/A | 11 | HOTERM | Reported Term for Healthcare Encounter | The reported or prespecified name of the healthcare encounter. | What was the healthcare encounter?; If [HODECOD], specify | [Healthcare Encounter]; [Specify] | Char | HR | Record the healthcare encounter or if used with a HODECOD list, "Other" specify. | HOTERM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | If collected on the CRF, sponsors must use either HODECOD or HOTERM and in some cases, both may be used. If the CRF is collecting healthcare encounters using a standardized sponsor-defined code list (e.g., INPATIENT ER, ICU, SURGERY, GENERAL WARD, PROVIDER OFFICE - PRIVATE, PROVIDER OFFICE - HOSPITAL, GASTROENTEROLOGIST), then HODECOD may be used to store the code list response. If the CRF collects "Specify, Other" or similar additional free text descriptions of code list items, then HOTERM may be used to store the detailed descriptive text. If HOTERM is entered only as free text, HODECOD would be populated through the sponsor's coding process. |
Events | HO | N/A | N/A | 12 | HODECOD | HO Standardized Term | The sponsor- defined standardized text description of HOTERM or the modified topic variable (HOMODIFY), if applicable. | What was the (standardized) healthcare encounter (term/code)? | (Standardized) Healthcare Encounter (Term) | Char | R/C | Select the healthcare encounter. | HODECOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | If collected on the CRF, sponsors must use either HODECOD or HOTERM and in some cases, both may be used. If the CRF is collecting healthcare encounters using a standardized sponsor defined code list (e.g., INPATIENT ER, ICU, SURGERY, GENERAL WARD, PROVIDER OFFICE-PRIVATE, PROVIDER OFFICE-HOSPITAL, GASTROENTEROLOGIST), then HODECOD may be used to store the code list response. If the CRF collects "Specify, Other" or similar additional free text descriptions of code list items, then HOTERM may be used to store the detailed descriptive text. If HOTERM is entered only as free text, HODECOD would be populated through the sponsor's coding process. |
Events | HO | N/A | N/A | 13 | HOSTDAT | Healthcare Encounter Start Date | The start date the healthcare encounter, represented in an unambiguous date format (e.g., DD-MON-YYYY). | What was the [healthcare encounter/HOTERM] [start/admission] date? | [Start/Admission] Date | Char | O | Record the start date of the healthcare encounter (e.g., date of admission) using this format (DD- MON-YYYY). | HOSTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable HOSTDTC in ISO 8601 format. | N/A | N/A | The preferred method is to collect a complete Start Date. Partial dates (e.g., providing year only, month and year only) may be acceptable. |
Events | HO | N/A | N/A | 14 | HOSTTIM | Healthcare Encounter Start Time | The start time of the healthcare encounter, (e.g., time of admission) represented in an unambiguous time format (e.g., hh:mm:ss). | What was the [healthcare encounter/HOTERM] [start/admission] time? | [Start/Admission] Time | Char | O | Record the start time (as complete as possible ) of the healthcare encounter in an unambiguous time format (e.g., hh:mm:ss). | HOSTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable HOSTDTC in ISO 8601 format. | N/A | N/A | N/A |
Events | HO | N/A | N/A | 15 | HOENDAT | Healthcare Encounter End Date | The end date of the healthcare encounter, (date of discharge) represented in an unambiguous date format (e.g., DD-MON-YYYY). | What was the [healthcare encounter/HOTERM] [end/discharge] date? | [End/Discharge] Date | Char | O | Record the end date of the healthcare encounter using this format (DD- MON-YYYY). | HOENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable HOENDTC in ISO 8601 format. | N/A | N/A | The preferred method is to collect a complete end date. Partial dates (e.g., providing year only, month and year only) may be acceptable. |
Events | HO | N/A | N/A | 16 | HOENTIM | Healthcare Encounter End Time | The end time of the healthcare encounter, (date of discharge) represented in an unambiguous time format (e.g., hh:mm:ss). | What was the [healthcare encounter/HOTERM] [end/discharge] time? | [End/Discharge]Time | Char | O | Record the end time (as complete as possible) of the healthcare encounter in an unambiguous time format (e.g., hh:mm:ss). | HOENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable HOENDTC in ISO 8601 format. | N/A | N/A | N/A |
Events | HO | N/A | N/A | 17 | HOCDUR | Healthcare Encounter Collected Duration | Collected duration of the healthcare encounter. | What was the duration of the [healthcare encounter/HOTERM]? | Duration | Char | O | Provide the duration of the healthcare encounter. | HODUR | This does not map directly to an SDTMIG variable. For the SDTM-based dataset, concatenate the CDASH collected duration and collected duration unit and populate the SDTMIG variable HODUR in ISO 8601 Period format. Example: P1DT2H (for 1 day, 2 hours). | N/A | N/A | Collected duration of the healthcare encounter. Used only if collected on the CRF and not derived from the start and end date/times. |
Events | HO | N/A | N/A | 18 | HOCDURU | HO Collected Duration Unit | Unit of the collected duration of the healthcare encounter. Used only if duration was collected on the CRF. | What was the duration unit of the [healthcare encounter/HOTERM]? | (Duration) Unit | Char | O | Select the appropriate duration unit of the healthcare encounter. | HODUR | This does not map directly to an SDTMIG variable. For the SDTM-based dataset, concatenate the CDASH collected duration and collected duration unit and populate the SDTMIG variable HODUR in ISO 8601 Period format. Example: P1DT2H (for 1 day, 2 hours). | (UNIT) | N/A | To ensure data quality, it is recommended that the appropriate duration unit(s) be preprinted on the (e)CRF. If only one unit is appropriate, no data entry would be required. |
Events | HO | N/A | N/A | 19 | HOONGO | Ongoing Healthcare Encounter | Indication that the encounter is ongoing when no end date is provided. | Was the [healthcare encounter/HOTERM] ongoing(as of the[study-specific timepoint or period? | Ongoing as of the[study-specific timepoint or period] | Char | O | Record the healthcare encounter as ongoing ('Y') if it has not stopped at[at the timepoint defined by the study]. | HOENRF; HOENRTPT | This does not map directly to an SDTM variable. May be used to populate a value into an SDTMIG relative timing variable such as HOENRF or HOENRTPT. When populating HOENRF, if the value of HOONGO is "Y", the value of "DURING", "AFTER" or "DURING/AFTER" may be used. When populating HOENRTPT, if the value of HOONGO is "Y", the value of "ONGOING" may be used. When HOONGO refers to the Study Reference Period (defined in DM.RFSTDTC to DM.RFENDTC) the SDTMIG variable HOENRF should be populated. When HOONGO is compared to any other time point, the SDTMIG variables HOENRTPT and HOENTPT should be used. Note: HOENRTPT must refer to a time point anchor described in HOENTPT. | (NY) | N/A | Completed to indicate that the healthcare encounter has not ended at the time of data collection. It is expected that every reported encounter has either an End Date or the Ongoing field is populated, but not both. The purpose of collecting this field is to help with data cleaning and monitoring; this field provides further confirmation that the End Date was deliberately left blank. Often used as a tick/checkbox. See Section 3.7, Mapping Relative Times from Collection to Submissions, and SDTMIG v3.2 Section 4.1.4.7 for more information. |
Events | HO | N/A | N/A | 20 | HOREAS | Reason for the Healthcare Encounter | Denotes the reason for the healthcare encounter. | What was the reason for the [healthcare encounter/HOTERM]? | Reason for the Healthcare Encounter | Char | O | Provide the reason for the subject's Healthcare Encounters. | SUPPHO.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPHO dataset as the value of SUPPHO_QVAL where SUPPHO.QNAM ="HOREAS" and SUPPHO.QLABEL="Healthcare Encounter Reason". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | N/A | N/A | To ensure data quality, it is recommended that the sponsor develop controlled terminology (e.g., LACK OF EFFICACY, ADVERSE EVENT, CHEMOTHERAPY, PHYSICAL THERAPY, INDICATION UNDER STUDY, NOT INDICATION UNDER STUDY). The sponsor may, however choose to collect this as free text or as a combination of preprinted options and free text in the form of "Specify, Other". |
Events | HO | N/A | N/A | 21 | HOAENO | Related Adverse Event ID | Identifier for the adverse event that precipitated this encounter. | What was the identifier for the adverse event(s), which precipitated the [healthcare encounter/HOTERM]? | Adverse Event Identifier | Char | O | Record the identifier for the adverse event that precipitated this encounter. | N/A | This does not map directly to an SDTMIG variable. For the SDTM submission datasets, may be used to create RELREC to link this record with a record in the Adverse Events domain. | N/A | N/A | Intent is to establish a link between the healthcare encounter record and the AE that was the primary cause of the encounter. HOAENO can be used in RELREC to identify a relationship between records in HO dataset and records in the AE |
Example CRF for the CDASHIG HO - Healthcare Encounters Domain
Example 1
This example shows the collection of admission date/time and discharge date/time for admission to hospitals and to rehabilitation facilities.
Title: Healthcare Encounters
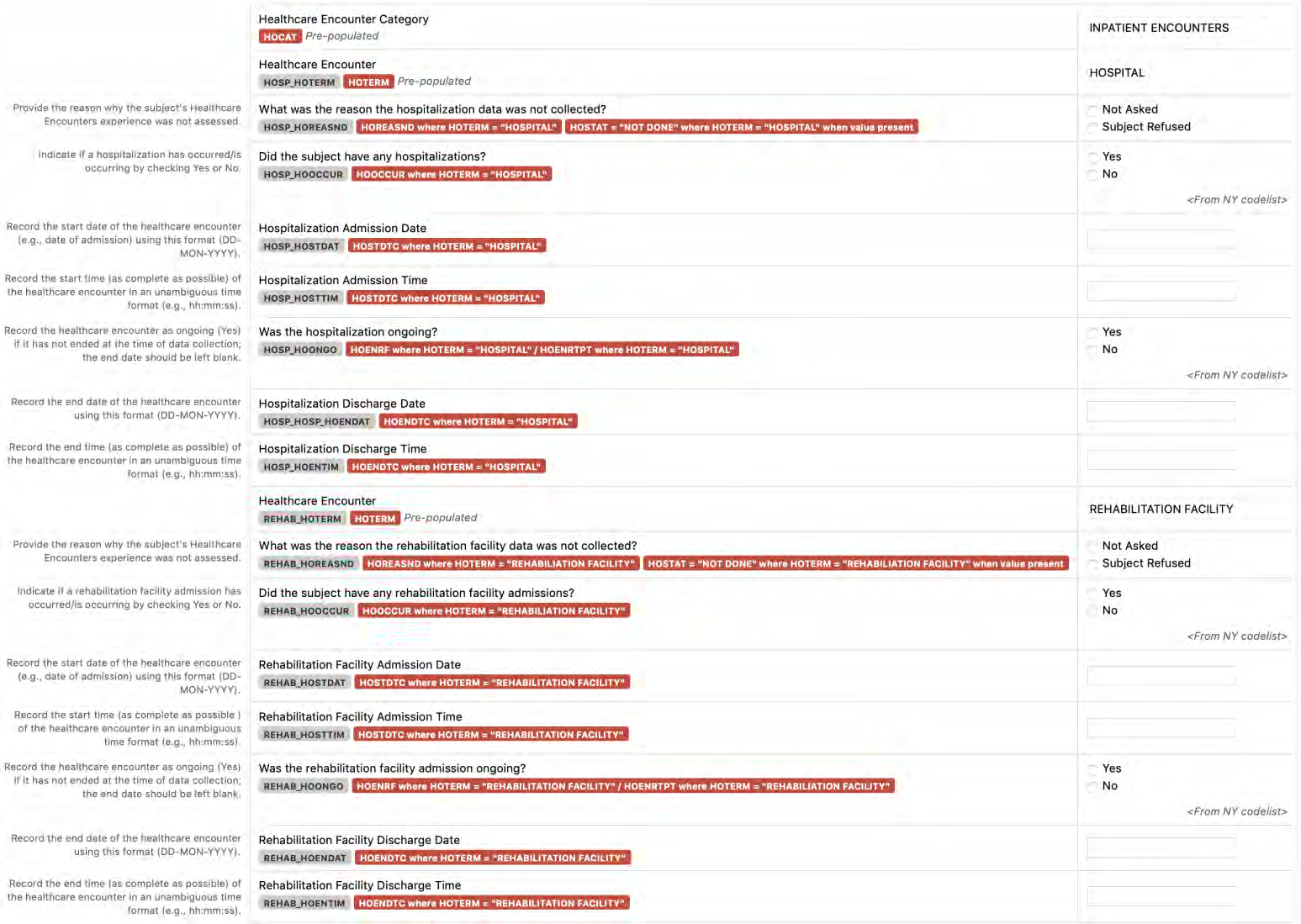
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre-Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
HOCAT | 1 | What was the category of the healthcare encounter? | Healthcare Encounter Category | If collected on the CRF, the sponsor provides instructions to ensure the data is entered as intended. | Text | HOCAT | INPATIENT ENCOUNTERS | Prompt | |||||
HOSP_HOTERM | 2 | What was the healthcare encounter? | Healthcare Encounter | Record the healthcare encounter name. | Text | HOTERM |
| HOSPITAL | Prompt | ||||
HOSP_HOREASND | 3 | What was the reason the hospitalization data was not collected? | Reason Not Collected | Provide the reason why the subject's Healthcare Encounters experience was not assessed. | Text | HOREASND | HOREASND where HOTERM = "HOSPITAL"; HOSTAT = "NOT DONE" where HOTERM = "HOSPITAL" when value present | Not Asked; Subject Refused | |||||
HOSP_HOOCCUR | 4 | Did the subject have any hospitalizations? | Hospitalizations | Indicate if a hospitalization has occurred/is occurring by checking Yes or No. | Text | HOOCCUR | HOOCCUR where HOTERM = "HOSPITAL" | (NY) | Yes; No | Radio | |||
HOSP_HOSTDAT | 5 | What was the hospitalization admission date? | Hospitalization Admission Date | Record the start date of the healthcare encounter (e.g., date of admission) using this format (DD-MON-YYYY). | Date | HOSTDTC | HOSTDTC where HOTERM = "HOSPITAL" | Prompt | |||||
HOSP_HOSTTIM | 6 | What was the hospitalization admission time? | Hospitalization Admission Time | Record the start time (as complete as possible) of the healthcare encounter in an unambiguous time format (e.g., hh:mm:ss). | Time | HOSTDTC | HOSTDTC where HOTERM = "HOSPITAL" | Prompt | |||||
HOSP_HOONGO | 7 | Was the hospitalization ongoing? | Hospitalization Ongoing | Record the healthcare encounter as ongoing (Yes) if it has not ended at the time of data collection; the end date should be left blank. | Text | HOENRF; HOENRTPT | HOENRF where HOTERM = "HOSPITAL" / HOENRTPT where HOTERM = "HOSPITAL" | (NY) | Yes; No | Radio | |||
HOSP_HOSP_HOENDAT | 8 | What was the hospitalization discharge date? | Hospitalization Discharge Date | Record the end date of the healthcare encounter using this format (DD-MON-YYYY). | Date | HOENDTC | HOENDTC where HOTERM = "HOSPITAL" | Prompt | |||||
HOSP_HOENTIM | 9 | What was the hospitalization discharge time? | Hospitalization Discharge Time | Record the end time (as complete as possible) of the healthcare encounter in an unambiguous time format (e.g., hh:mm:ss). | Time | HOENDTC | HOENDTC where HOTERM = "HOSPITAL" | Prompt | |||||
REHAB_HOTERM | 10 | What was the healthcare encounter? | Healthcare Encounter | Record the healthcare encounter name. | Text | HOTERM | REHABILITATION FACILITY | Prompt | |||||
REHAB_HOREASND | 11 | What was the reason the rehabilitation facility data was not collected? | Reason Rehabilitation Facility Not Collected | Provide the reason why the subject's Healthcare Encounters experience was not assessed. | Text | HOREASND | HOREASND where HOTERM = "REHABILIATION FACILITY"; HOSTAT = "NOT DONE" where HOTERM = "REHABILIATION FACILITY" when value present | Not Asked; Subject Refused | |||||
REHAB_HOOCCUR | 12 | Did the subject have any rehabilitation facility admissions? | Rehabilitation Facility | Indicate if a rehabilitation facility admission has occurred/is occurring by checking Yes or No. | Text | HOOCCUR | HOOCCUR where HOTERM = "REHABILIATION FACILITY" | (NY) | Yes; No | Radio | |||
REHAB_HOSTDAT | 13 | What was the rehabilitation facility admission date? | Rehabilitation Facility Admission Date | Record the start date of the healthcare encounter (e.g., date of admission) using this format (DD-MON-YYYY). | Date | HOSTDTC | HOSTDTC where HOTERM = "REHABILITATION FACILITY" | Prompt | |||||
REHAB_HOSTTIM | 14 | What was the rehabilitation facility admission time? | Rehabilitation Facility Admission Time | Record the start time (as complete as possible ) of the healthcare encounter in an unambiguous time format (e.g., hh:mm:ss). | Time | HOSTDTC | HOSTDTC where HOTERM = "REHABILITATION FACILITY" | Prompt | |||||
REHAB_HOONGO | 15 | Was the rehabilitation facility admission ongoing? | Rehabilitation Facility Ongoing | Record the healthcare encounter as ongoing (Yes) if it has not ended at the time of data collection; the end date should be left blank. | Text | HOENRF; HOENRTPT | HOENRF where HOTERM = "REHABILITATION FACILITY" / HOENRTPT where HOTERM = "REHABILIATION FACILITY" | (NY) | Yes; No | Radio | |||
REHAB_HOENDAT | 16 | What was the rehabilitation facility discharge date? | Rehabilitation Facility Discharge Date | Record the end date of the healthcare encounter using this format (DD-MON-YYYY). | Date | HOENDTC | HOENDTC where HOTERM = "REHABILITATION FACILITY" | Prompt | |||||
REHAB_HOENTIM | 17 | What was the rehabilitation facility discharge time? | Rehabilitation Facility Discharge Time | Record the end time (as complete as possible) of the healthcare encounter in an unambiguous time format (e.g., hh:mm:ss). | Time | HOENDTC | HOENDTC where HOTERM = "REHABILITATION FACILITY" | Prompt |
8.2.7 MH - Medical History
Description/Overview for the CDASHIG MH - Medical History Domain
The MH dataset includes the subject's medical history at the start of the trial. The CDASH metadata tables contain the most common general medical history data collection fields. In cases where more indication- specific medical history is required by the protocol, sponsors should add fields as needed from the CDASH Events model.
Specification for the CDASHIG MH - Medical History Domain
Medical History Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Events | MH | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM- based dataset creation before submission. |
Events | MH | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Events | MH | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Events | MH | N/A | N/A | 4 | MHYN | Any Medical History Event | An indication whether or not there was any medical history to report. | Were any medical conditions or events reported?; Has the subject had any medical conditions or events? | Any Medical History | Char | O | Indicate if the subject experienced any medical conditions or events. If yes, include the appropriate details where indicated on the CRF. | N/A | Does not map to an SDTMIG variable. The SDTM Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification that all other fields on the CRF were deliberately left blank. |
Events | MH | N/A | N/A | 5 | MHCAT | Category for Medical History | A grouping of topic- variable values based on user- defined characteristics. | What was the category of the medical history? | [Medical History Category]; NULL | Char | R/C | If collected on the CRF, the sponsor provides instructions to ensure the data is entered as intended. | MHCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology (e.g., CARDIAC, GENERAL). This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. This would be used when specific medical history (e.g., disease under study details) is captured, in addition to the general medical history. |
Events | MH | N/A | N/A | 6 | MHSCAT | Subcategory for Medical History | A sub-division of the MHCAT values based on user- defined characteristics. | What was the subcategory of the medical history? | [Medical History Subcategory]; NULL | Char | O | If collected on the CRF, the sponsor provides instructions to ensure the data is entered as intended. | MHSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. Typically would be used when specific medical history (e.g., disease diagnosis) is captured, in addition to the general medical history. MHSCAT can only be used if there is an MHCAT and it must be a subcategorization of MHCAT. |
Events | MH | N/A | N/A | 7 | MHDAT | Medical History Collection Date | The date on which the medical history was collected, represented in an unambiguous date format (e.g., DD- MON-YYYY). | What was the date the medical history was collected? | Collection Date | Char | O | Record the date on which the Medical History was collected using this format (DD-MON- YYYY). | MHDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable MHDTC in ISO 8601 format. | N/A | N/A | This should be a complete date. The date of collection may be determined from a collected visit date. |
Events | MH | N/A | N/A | 8 | MHSPID | MH Sponsor- Defined Identifier | A sponsor-defined identifier. In CDASH, This is typically used for preprinted or auto-generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor-defined question] | [Sponsor defined] | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | MHSPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Because SPID is a sponsor- defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile concomitant medications and/or procedure records with MH. May be used to record preprinted number (e.g. line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. If CMMHNO or PRMHNO is used, this is the identifier to which CMMHNO or PRMHNO refers. |
Events | MH | N/A | N/A | 9 | MHEVDTYP | Medical History Event Date Type | Specifies the aspect of the medical condition or event by which MHSTDTC and/or the MHENDTC is defined. | What was the medical history event date type? | Medical History Event Date Type | Char | O | The instructions depend upon the format of the CRF. Sponsors may pre- print these values on the CRF or use them as defaulted or hidden text. | SUPPMH.QVAL | This field does not map directly to an SDTM variable. This information could be submitted in a SUPPMH dataset as the value of SUPPMH.QVAL when SUPPMH.QNAM = "MHEVDTYP" and SUPPMH.QLABEL= "Medical History Event Date Type". Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. | N/A | N/A | The type of start/ and or end date (e.g., DIAGNOSIS, EPISODE, EXACERBATION, SYMPTOM ONSET). It is not related to the trial's condition. This date type cannot be a PRIMARY DIAGNOSIS, SECONDARY DIAGNOSIS since these terms do not define the date type. |
Events | MH | N/A | N/A | 10 | MHTERM | Reported Term for the Medical History | The reported or prespecified name of the medical condition or event. | What is the medical condition or event term? | Medical History Term | Char | HR | Record all relevant medical conditions or events, as defined in the protocol. Record only 1 medical condition or event per line. Ensure that the medical conditions or events listed on the Medical History page do not meet any of the exclusion criteria. | MHTERM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsors should collect all relevant medical conditions or events, as defined in the protocol. It is a best practice for sponsors to collect all relevant history of surgeries or procedures using the associated diagnosis in the MH domain, while reporting relevant surgeries and procedures in the SDTM PR domain. Sponsors should provide instructions on how surgeries and procedures will be handled based on the protocol requirements. Information on prespecified surgeries, or procedures should be collected in the PR domain. |
Events | MH | N/A | N/A | 11 | MHOCCUR | Medical History Occurrence | An indication whether a prespecified medical condition/event or a group of medical conditions/events occurred when information about the occurrence of a specific event is solicited. | Did the subject have [prespecified medical condition/event/group of medical conditions]; Is the [prespecified medical occurring]? | [Medical condition/Event] | Char | O | Indicate if [specific medical condition/event] has occurred/is occurring by checking Yes or No. | MHOCCUR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | MHOCCUR is used to report the occurrence of a prespecified medical conditions or events. MHOCCUR is not used if the medical conditions or events are collected on the CRF in a manner that requires spontaneously free- text response. The site should be able to indicate that the response was not asked or answered. |
Events | MH | N/A | N/A | 12 | MHPRESP | Medical History Event prespecified | An indication that a specific event, or group of events, are prespecified on a CRF. | N/A | N/A | Char | O | N/A | MHPRESP | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | A hidden field on a CRF defaulted to "Y", or added during the SDTM- based dataset creation when the medical condition or event is prespecified. Null for spontaneously reported events. If a study collects both prespecified medical history as well as free- text events, the value of MHPRESP should be "Y" for all prespecified events and null for medical conditions or events reported as free-text. MHPRESP is a permissible field in SDTM and may be omitted from the SDTM- based dataset if all events were collected as free text. |
Events | MH | N/A | N/A | 13 | MHPRIOR | Prior Medical History Event | An indication whether the event occurred prior to study start. | Did the medical condition or event start prior to [MHSTTPT]?; Did the medical condition or event start prior to study start? | Prior to [MHSTTPT]; Prior to Study | Char | O | Check if the medical condition or event started [before the study]. | MHSTRTPT; MHSTRF | This does not map directly to an SDTM variable. May be used to populate a value into an SDTMIG relative timing variable such as MHSTRF or MHSTRTPT. When populating MHSTRF, or MHSTRTPT, if the value of the CDASH field MHPRIOR is "Y" a value from the CDISC CT (STENRF) may be used. When MHPRIOR refers to the Study Reference Period (defined in DM.RFSTDTC to DM.RFENDTC) the SDTMIG variable MHSTRF should be populated. When MHPRIOR is compared to another time point, the SDTMIG variables MHSTRTPT and MHSTTPT should be used. Note: MHSTRTPT must refer to the time point anchor described in MHSTTPT. | (NY) | N/A | Sponsors may collect this information rather than start dates. See Section 3.7, Mapping Relative Times from Collection to Submissions, and SDTMIG v3.2 Section 4.1.4.7 for more information. |
Events | MH | N/A | N/A | 14 | MHONGO | Ongoing Medical History Event | Indication the medical condition or event is ongoing when no end date is provided. | Is the medical condition or event ongoing (as of the [study-specific timepoint or period])? | Ongoing (as of the [study-specific timepoint or period]) | Char | O | Record the medical condition or event as ongoing ("Y") if it has not ended at the time of data collection. If the medical condition or event, is ongoing, the end date should be left blank. | MHENRF; MHENRTPT | This does not map directly to an SDTM variable. May be used to populate a value into an SDTMIG relative timing variable such as MHENRF or MHENRTPT. When populating MHENRF, if the value of MHONGO is "Y", the value of "DURING", "AFTER" or "DURING/AFTER" may be used. When populating MHENRTPT, if the value of MHONGO is "Y", the value of "ONGOING" may be used. When MHONGO refers to the Study Reference Period (defined in DM.RFSTDTC to DM.RFENDTC) the SDTMIG variable MHENRF should be populated. When MHONGO is compared to another time point, the SDTMIG variables MHENRTPT and MHENTPT should be used. Note: MHENRTPT must refer to a time point anchor described in MHENTPT. | (NY) | N/A | Completed to indicate that the condition has not resolved at the time of data collection. It is expected that every reported condition has either an End Date or the Ongoing field is populated, but not both. See Section 3.7, Mapping Relative Times from Collection to Submissions, and SDTMIG v3.2 Section 4.1.4.7 for more information. |
Events | MH | N/A | N/A | 15 | MHCTRL | MH Disease or Symptom Under Control | Indication whether the medical condition or event is under control at the time of data collection. | Is the medical condition or event under control? | Medical Condition Under Control | Char | O | Select the most appropriate response. | SUPPMH.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPMH dataset as the value of SUPPMH.QVAL where SUPPMH.QNAM ="MHCTRL" and SUPPMH.LABEL="Medical Condition Under Control". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | MHCTRL is not defined in the SDTMIG MH domain. If collected, it should be submitted in the SUPPMH dataset. If MHCTRL is collected, the sponsor must provide information on the relative timeframe. Generally, MHDAT is collected or determined using the visit date of the collection to indicate this is the subject's status at the time of data collection. |
Events | MH | N/A | N/A | 16 | MHSTDAT | Medical History Event Start Date | The start date of medical history event or condition represented in an unambiguous date format (e.g., DD- MON-YYYY). | What [is/was] the [medical event or condition/category of the event] start date? | Start Date | Char | O | Record the start date of the medical event or condition using this format (DD-MON-YYYY). | MHSTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH START DATE and TIME components and populate the SDTMIG variable MHSTDTC in ISO 8601 format. | N/A | N/A | The sponsor may choose to capture a complete date or any variation thereof (e.g., month and year, year). |
Events | MH | N/A | N/A | 17 | MHENDAT | Medical History Event End Date | The end date of medical history event or condition represented in an unambiguous date format (e.g., DD- MON-YYYY). | What[is/was] the[medical event or condition/category of the event] end date? | End Date | Char | O | Record the end date of the medical event or condition using this format (DD-MON-YYYY). | MHENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable MHENDTC in ISO 8601 format. | N/A | N/A | The sponsor may choose to capture a complete date or any variation thereof (e.g., month and year, year). |
Events | MH | N/A | N/A | 18 | MHLOC | Medical History Event Location | A description of the anatomical location relevant for the medical condition or event. | What was the anatomical location of the medical condition or event? | Anatomical Location | Char | O | Indicate the anatomical location of the medical event or condition. | MHLOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location (e.g., ARM for skin rash). Could be a defaulted or hidden field on the CRF for prespecified [MHTERM/Event Topic]. Sponsors may collect the data using a subset list of Controlled Terminology on the CRF. LAT, DIR, PORTOT are used to further describe the anatomical location. |
Events | MH | N/A | N/A | 19 | MHLAT | Medical History Event Laterality | Qualifier for anatomical location further detailing the side of the body relevant for the event. | What was the side of the anatomical location of the medical condition or event? | Side | Char | O | Record the side of the anatomical location of the medical event. | MHLAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Events | MH | N/A | N/A | 20 | MHDIR | Medical History Directionality | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What was the directionality of the anatomical location of the medical condition or event? | Directionality | Char | O | Record the directionality of the anatomical location of the medical event. | MHDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Events | MH | N/A | N/A | 21 | MHPORTOT | MH Event Location Portion or Totality | Qualifier for anatomical location further detailing the distribution, which means arrangement of, apportioning of. | What was the portion or totality of the anatomical location of the of the medical condition or event? | Portion or Totality | Char | O | Indicate the portion or totality anatomical location of the medical event. | MHPORTOT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (PORTOT) | N/A | Collected when the sponsor needs to identify the specific portionality for the anatomical locations. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Events | MH | N/A | N/A | 22 | MHMODIFY | MH Modified Reported Term | If the value for MHTERM is modified to facilitate coding, then MHMODIFY will contain the modified text. | N/A | N/A | Char | O | N/A | MHMODIFY | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This is not a data collection field that would appear on the CRF. Sponsors will populate this through the coding process. |
Events | MH | N/A | N/A | 23 | MHDECOD | MH Dictionary- Derived Term | The dictionary text description of MHTERM or the modified topic variable (MH MODIFY), if applicable. | N/A | N/A | Char | O | N/A | MHDECOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the Define- XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This is typically not a data collection fieldthat will appear on the CRF.Sponsors will populate this through the coding process. Equivalent to the Preferred Term (PT in MedDRA). |
Events | MH | N/A | N/A | 24 | MHLLT | Medical History Event Lowest Level Term | MedDRA Dictionary text description of the Lowest Level Term. | N/A | N/A | Char | O | N/A | MHLLT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | MH | N/A | N/A | 25 | MHLLTCD | MH Event Lowest Level Term Code | MedDRA Lowest Level Term code. | N/A | N/A | Num | O | N/A | MHLLTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | MH | N/A | N/A | 26 | MHPTCD | MH Event Preferred Term Code | MedDRA code for the Preferred Term. | N/A | N/A | Num | O | N/A | MHPTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | MH | N/A | N/A | 27 | MHHLT | Medical History Event High Level Term | MedDRA text description of the High Level Term from the primary path. | N/A | N/A | Char | O | N/A | MHHLT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | MH | N/A | N/A | 28 | MHHLTCD | MH Event High Level Term Code | MedDRA High Level Term code from the primary path. | N/A | N/A | Num | O | N/A | MHHLTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | MH | N/A | N/A | 29 | MHHLGT | MH Event High Level Group Term | MedDRA text description of the High Level Group Term from the primary path. | N/A | N/A | Char | O | N/A | MHHLGT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | MH | N/A | N/A | 30 | MHHLGTCD | MH Event High Level Group Term Code | MedDRA High Level Group Term code from the primary path. | N/A | N/A | Num | O | N/A | MHHLGTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | MH | N/A | N/A | 31 | MHSOC | MH Event Primary System Organ Class | MedDRA Primary System Organ Class description associated with the event. | N/A | N/A | Char | O | N/A | MHSOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Events | MH | N/A | N/A | 32 | MHSOCCD | MH Event Primary System Organ Class Code | MedDRA code for the primary System Organ Class. | N/A | N/A | Num | O | N/A | MHSOCCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the dictionary name and version used to code utilizing the Define-XML external codelist attributes. Sponsors should include an Origin in the define metadata document to indicate that the data was "ASSIGNED". | N/A | N/A | This field does not typically appear on the CRF. Sponsors will populate this through the coding process. This is applicable to items using MedDRA coding. |
Assumptions for the CDASHIG MH - Medical History Domain
1. Medical History Collection Period
a. Sponsors should define the appropriate collection period for medical history in the protocol. The evaluation interval may be provided in the SDTMIG variable MHEVLINT or MHEVINTX. These intervals are not recorded by the investigator, but are populated by the sponsor in the SDTMIG MH dataset. These intervals may be preprinted on the CRF as instruction text.
2. Medical History Coding
a. Sponsors who code medical history should use appropriate dictionary variables for the coding. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the define.xml External Codelist attributes.
b. Coding using MedDRA, though not required by the FDA, is recommended by CDASH. Coding of medical history is recommended because it provides methodology to compare medical history to adverse events and facilitate the identification of unexpected safety concerns or potential relationships to past treatments. It is recommended that coding be done during the execution phase of a study rather than after it is completed, as this facilitates efficient resolution of any coding queries.
c. For uncoded medical history, a sponsor-defined categorization of medical history events is recommended. One approach is to use the MHCAT variable.
d. Coding variables are not a data collection field that will appear on the CRF itself; sponsors will populate this through the coding process. When MedDRA is used as the coding dictionary, the MedDRA coding variables are included in the SDTM dataset.
3. Date of Collection (DAT)
a. This is the date that the data was recorded, not the date that the condition started or the event occurred. The date of collection can be "derived" from the date of the visit.
4. Relative Timing Variables
a. The date of data collection in conjunction with a collected time point anchor date and the MHONGO CDASHIG fields would determine how the SDTMIG relative timing variables would be populated.
b. The MHONGO field does not map directly to an SDTMIG variable but it may be used to derive a value into an SDTMIG relative timing variable such as MHENRF or MHENRTPT. When populating MHENRF, if the MHONGO box is checked, a value of "DURING", "AFTER" or "DURING/AFTER" may be derived. When populating MHENRTPT, if the MHONGO box is checked, the value of "ONGOING" may be derived. Note: MHENRTPT must refer to a "time point anchor" described in MHENTPT. See Section 3.7, Mapping Relative Times from Collection to Submissions, and SDTMIG v3.2 Section 4.1.4.7 for more information.
c. MHONGO is a special-use case of "Yes/No", where the question is usually presented as a single possible response of "Yes" when there is no applicable end date at time of collection. In this case, if the box is checked and the end date is blank, the desired SDTMIG relative timing variable can be derived according to note 4b. If the box is not checked (MHONGO is NULL) and an end date is present, no SDTMIG relative timing variable would be derived. MHONGO is only used to derive an appropriate SDTMIG relative timing variable and should not be submitted on its own in the MH or SUPPMH dataset. The purpose of collecting this field is to help with data cleaning and monitoring; this field provides further confirmation that the end date was deliberately left blank.
d. MHPRIOR can be added to this domain from the CDASH Model and used when the sponsor elects not to collect start dates (even partial dates) on the MH CRF. The sponsor would derive a value into an SDTMIG relative timing variable such as MHSTRF or MHSTRTPT. When populating MHSTRF, if the value of MHPRIOR is “Y”, the value of “BEFORE” may be derived. When populating MHSTRTPT, if the value of MHPRIOR is “Y”, the value of “BEFORE” may be derived. Note: MHSTRTPT must refer to a “time point anchor” as described in MHSTTPT.
5. Start and End Dates
a. Partial dates are commonly collected in MH when the subject does not remember the complete date of when a medical history condition started or ended. The sponsor may choose to capture a complete date or any variation thereof (e.g., month and year, year).
6. Non-standard Supplemental Qualifier Variables
a. Medical History Event Type (MHEVDTYP) is used to specify the aspect of the medical condition or event by which its start date is defined. This variable (MHEVDTYP) is only to be used in the MH domain. This variable is used when the CRF records "multiple" dates such as the date when the condition was diagnosed, when symptoms were first reported prior to diagnosis, when the subject had a relapse, or when the infection associated with the diagnosis was reported. Example values for MHEVDTYP include DIAGNOSIS, EPISODE, EXACERBATION and SYMPTOM ONSET..
b. Condition under control (MHCTRL) is used to indicate that the disease/symptoms are under control at the time of data collection. It is recommended that, because this non-standard variable (NSV) has an implied time frame, the sponsor should provide the MHDTC data in the SDTMIG MH domain when using this NSV. Refer to the CDASHIG Metadata Tables for detailed mapping instructions.
Example CRFs for the CDASHIG MH - Medical History Domain
Example 1
Title: Cirrhosis History
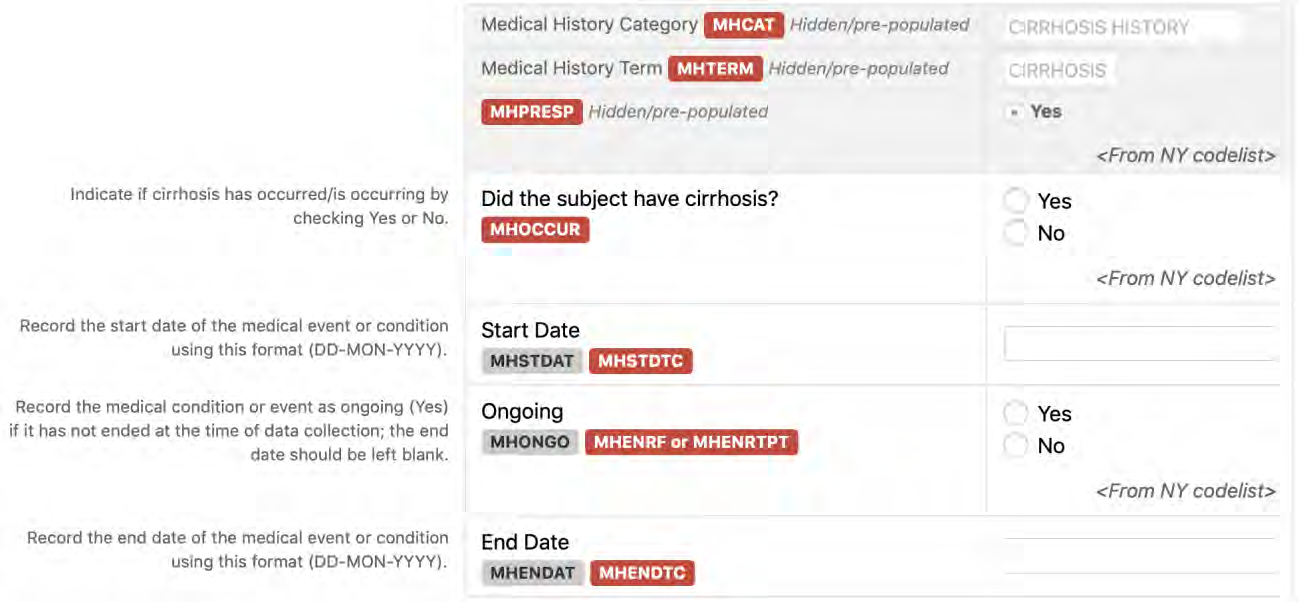
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
MHCAT | 1 | What was the category of the medical history? | Medical History Category | If collected on the CRF, the sponsor provides instructions to ensure the data is entered as intended. | Text | MHCAT | CIRRHOSIS HISTORY | Prompt | Yes | ||||
MHTERM | 2 | What is the medical condition or event term? | Medical History Term | Record all relevant medical conditions or events, as defined in the protocol. Record only one medical condition or event per line. Ensure that the medical conditions or events listed on the Medical History page do not meet any of the exclusion criteria. | Text | MHTERM | CIRRHOSIS | Prompt | Yes | ||||
MHPRESP | 3 | N/A | N/A | N/A | Text | MHPRESP | (NY) | Yes | Yes | radio | Yes | ||
MHOCCUR | 4 | Did the subject have cirrhosis? | Cirrhosis | Indicate if cirrhosis has occurred/is occurring by checking Yes or No. | Text | MHOCCUR | (NY) | Yes; No | radio | ||||
MHSTDAT | 5 | What was the medical condition or event start date? | Start Date | Record the start date of the medical event or condition using this format (DD-MON-YYYY). | Date | MHSTDTC | Prompt | ||||||
MHONGO | 6 | Is the medical condition or event ongoing? | Ongoing | Record the medical condition or event as ongoing (Yes) if it has not ended at the time of data collection; the end date should be left blank. | Text | MHENRF; MHENRTPT | MHENRF or MHENRTPT | (NY) | Yes; No | Prompt | radio | ||
MHENDAT | 7 | What was the medical condition or event end date? | End Date | Record the end date of the medical event or condition using this format (DD-MON-YYYY). | Date | MHENDTC | Prompt |
Example 2
Title: General Medical History
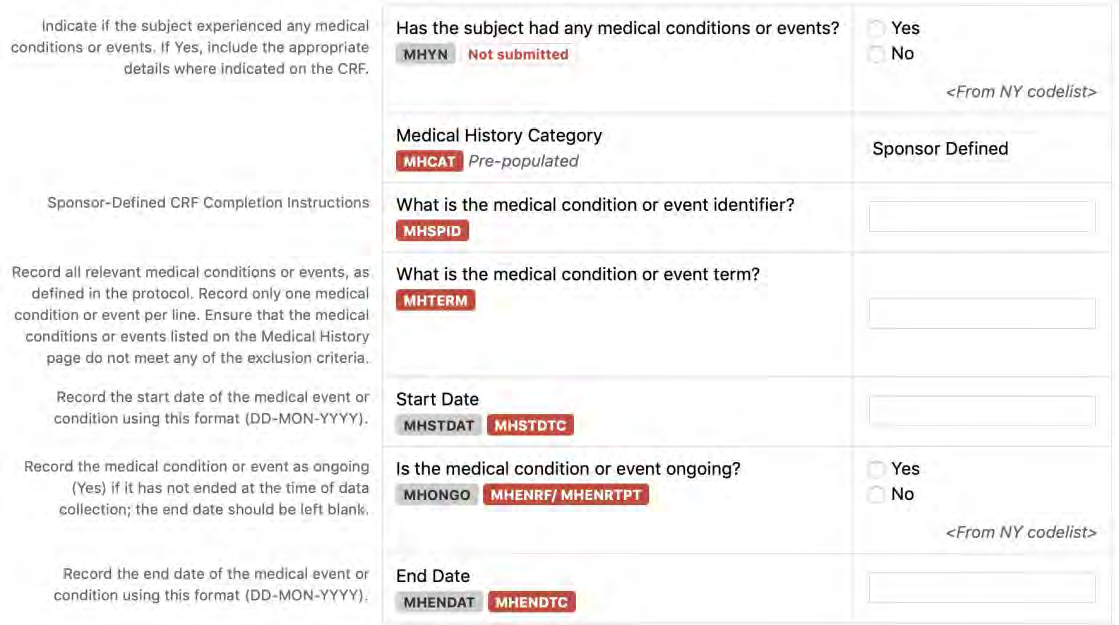
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
MHYN | 1 | Has the subject had any medical conditions or events? | Any Medical History | Indicate if the subject experienced any medical conditions or events. If Yes, include the appropriate details where indicated on the CRF. | Text | N/A | (NY) | Yes; No | radio | ||||
MHCAT | 2 | What was the category of the medical history? | Medical History Category | If collected on the CRF, the sponsor provides instructions to ensure the data is entered as intended. | Text | MHCAT | Sponsor Defined | prompt | |||||
MHSPID | 3 | What is the medical condition or event identifier? | MH Number | Sponsor-Defined CRF Completion Instructions | Text | MHSPID |
| ||||||
MHTERM | 4 | What is the medical condition or event term? | Medical History Term | Record all relevant medical conditions or events, as defined in the protocol. Record only one medical condition or event per line. Ensure that the medical conditions or events listed on the Medical History page do not meet any of the exclusion criteria. | Text | MHTERM | |||||||
MHSTDAT | 5 | What was the medical condition or event start date? | Start Date | Record the start date of the medical event or condition using this format (DD-MON-YYYY). | Date | MHSTDTC | prompt | ||||||
MHONGO | 6 | Is the medical condition or event ongoing? | Ongoing | Record the medical condition or event as ongoing (Yes) if it has not ended at the time of data collection; the end date should be left blank. | Text | MHENRF/ MHENRTPT | (NY) | Yes; No | radio | ||||
MHENDAT | 7 | What was the medical condition or event end date? | End Date | Record the end date of the medical event or condition using this format (DD-MON-YYYY). | Date | MHENDTC | prompt |
Example 3
Title: Psychiatric History: Symptom Start and Diagnosis Dates
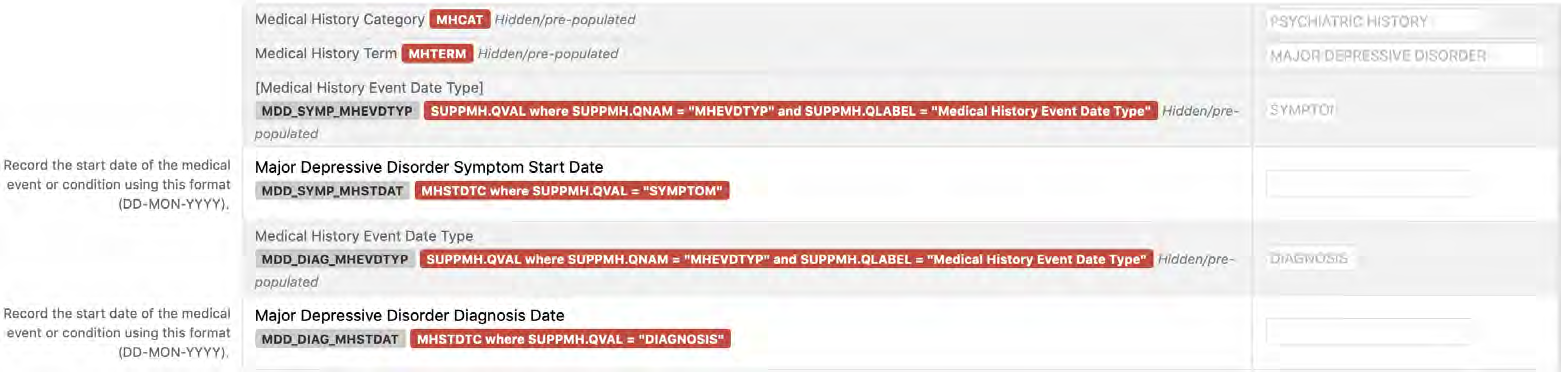
8.3 Findings Class Domains
The Findings class includes CDASH domains that define collection standards for results from evaluations such as physical examinations, laboratory tests, electrocardiogram (ECG) testing, and responses to questionnaires.
8.3.1 General CDASH Assumptions for Findings Domains
1. CDASH --CAT and/or --SCAT are generally not entered on the CRF by sites. Implementers may prepopulate and display these category values to help sites understand what data should be recorded on the CRF. Implementers may also prepopulate hidden variables with the values assigned within their operational database. Categories and subcategories that are not collected and are self-evident from the protocol design are populated during the creation of the SDTM submission dataset.
2. CDASH --PERF
a. --PERF variables having the Question Text "[Were any/Was the] [--TEST/ topic] [measurement(s)/test(s) /examinations (s)/specimen(s) /sample(s) ] [performed/collected]?" are intended to assist in the cleaning of data and in confirming that there are no missing values. (Refer to CDASH Model v1.1 for more information on creating and using --PERF variables.)
b. --PERF may be used at the page, panel, or question level. --PERF may be used during the creation of the STDM submission datasets to derive a value into the SDTM variable --STAT. The implementer can use a combination of --CAT, --SCAT, with the --TESTCD= "--ALL" and --TEST= "
3. The CDASH variable --REASND is used with SDTM variable --STAT only. The value NOT DONE in -- STAT indicates that the findings test was not performed.
4. The CDASH --SPID variable may be populated by the sponsor's data collection system. If collected, it can be beneficial to use an identifier in a data query to communicate clearly to the site the specific record in question. This field may be populated by the sponsor's data collection system.
5. Date and Time Variables
a. CDASH variables (--DAT, --TIM) are used in Findings domains to collect the date or date and time that the test was done or performed. The SDTM --DTC variable contains either a date or date and time when a specimen is collected or the start date or start date and time when a specimen is collected over time.
b. Collecting the time is only appropriate if it can be realistically determined and if there is a scientific reason for needing to know this level of detail. An example is where the subject is under the direct care of the site at the time the test was performed and the study design is such that it is important to know the time the test was performed with respect to dosing time.
c. Implementers must not use these elements to record a date that is the result of a test (e.g., date of last day on the job). The date of the last day on the job would be recorded in the CDASH variable -- ORRES. See SDTMIG v3.2 Section 4.1.4.9 for more information.
d. The date of collection of a test may be derived from the date of visit. If so, a separate date of observation field is not required to be present on the CRF.
6. Horizontal (Denormalized) and Vertical Data Structures (Normalized)
a. In the CDASHIG Metadata Table, many of the CDASH Findings Class Domains are presented in a normalized structure (1 record for each test) that is similar to the SDTM, even though many data management systems hold the data in a denormalized structure (1 variable for each test). When implementing CDASH in a denormalized structure, create variable names for the Findings --TEST and/or --TESTCD values. To do this, define the denormalized variable names using available CDISC Controlled Terminology for --TESTCD. Alternatively, CDASH variable names for data management systems allowing more than 8-character variable names can use CDASH variables using the following naming convention: <--TESTCD>_<-- SDTM variable name> where --TESTCD is the appropriate CT for the test code (e.g., DIABP_VSORRES, DIABP_VSLOC).
b. In the horizontal (denormalized) setting, SDTM variables such as --PERF, --LOC , --STAT may be collected once for the whole horizontal record and applying the value to all of the observations on that record, or they can be collected by test using the CDASH variable such as <--TESTCD>_--PERF. When SDTM submission datasets are created, any variables collected for the entire horizontal record must be mapped to each vertical record (as appropriate).
c. In the horizontal (denormalized) setting, an identifier (e.g., --GRPID) may be used to identify all -- TESTCD collected on the same record. This facilitates transformation from the CDASH horizontal setting to the SDTM vertical setting and creation of RELRECs.
7. Tests and Original Results
a. The value in --TEST cannot be longer than 40 characters. The corresponding codelist value for the short test name (at most 8 characters) must also be populated in the SDTM variable --TESTCD.
b. --TESTCD should be used to create a variable name and --TEST be used as the Prompt on the CRF. Both --TESTCD and --TEST are recommended for use in the operational database. See Section 5, Conformance to the CDASH Standard, for more details.
c. CDASH variable --ORRES is used to collect test results or findings in the original units in character format.
d. If the results are modified for coding, the --MODIFY variable contains any modified text.
e. If normal or reference ranges are collected for results, the CDASH variables --ORNRLO and -- ORNRHI and --NIND are used.
f. CDASH does not define the SDTM variable used to standardize the findings results (e.g., --STRESC, - -STRESN) or to standardize the normal/reference ranges (--STNRLO,--STNRHI, --STNRC). The standardization of the original findings results and normal/reference ranges is expected to be performed during the creation of the SDTM submission datasets. Extensive discussion on the standardization of findings results is provided in the SDTMIG.
8. Location Variables (--LOC, --LAT, --DIR, --PORTOT)
a. These variables are used to collect the location of the test. The SDTM acknowledges that the results themselves may not be at the same location as the test. This is a known issue with the SDTM.
b. Sponsors may collect the data using a subset list of Controlled Terminology on the CRF. --LOC could be a defaulted or hidden field on the CRF.
9. CDASH uses the variables –ORRES, --RES, --DESC, and --RESOTH to collect results for the observation class findings. The variable pairs --RES/--DESC, and --RES/--RESOTH are often used when a finding result is collected using 2 CRF questions but is combined into a single row in the SDTM dataset. The variable --ORRES is used when the finding result is based on a single question, and is mapped directly to a single row in SDTM. CDASH suggests:
a. --ORRES variable is used when the finding result is collected using a single question. The result should map directly to the SDTM variable --ORRES.
b. --RES and --DESC are used when a question is asked to collect the finding result, with a follow-up question for a description of the finding. Typically, the question would be “Is the
i. --ORRES result is normal/absent, or
ii. --ORRES is the actual abnormality/observed finding and the collected value abnormal/present are not represented.
c. --RES and --RESOTH are used when a question is asked that allows the selection of a prespecified finding, with a follow-up question to ask about the prespecified response "OTHER". Typically, the question would be "What is the result?" with a set of prespecified responses, including the choice “OTHER” with a follow-up question “Specify, Other”. --RES is used to collect the finding with prespecified responses, and --RESOTH is used to collect "Specify, Other". Typically, --RESOTH data is modeled in SDTM as --ORRES instead of the response "OTHER".
10. The Findings About Events and Intervention domains use the same root variables as the Findings domain with the addition of the --OBJ variable. The CDASHIG Metadata Table contains a generic FA domain.
It is assumed that implementers will add other data variables as needed to meet protocol-specific and other data collection requirements (e.g., therapeutic area-specific data elements and others as required per protocol, business practice, or operating procedures) to Findings and Findings About domains.
8.3.2 DA - Drug Accountability
Description/Overview for the CDASHIG DA - Drug Accountability Domain
The CDASHIG DA domain is used to collect information about dispensing and returning of study treatment materials used in a clinical trial.
The SDTMIG separates drug accountability from the Exposure (EX) domain, which contains the data about the subjects' actual exposure to study treatment.
SDTMIG definitions:
• "Drug Accountability is for data regarding the accountability of study drug, such as information on receipt, dispensing, return, and packaging." See the current SDTMIG for more information.
• "The Exposure domain model records the details of a subject’s exposure to protocol-specified study treatment. Study treatment may be any intervention that is prospectively defined as a test material within a study, and is typically but not always supplied to the subject." Examples include but are not limited to placebo, active comparators, and investigational products. Treatments that are not protocol-specified should be recorded in the Concomitant Medications (CM) domain. See the current SDTMIG for more information.
Care should be taken not to confuse drug accountability with study treatment compliance or study drug exposure. Comparing the amount dispensed to the subject and the amount returned by the subject does not necessarily mean the difference equates to the amount of treatment consumed by the subject or the subject's compliance with the treatment plan. For example, the subject could have dropped 2 tablets into the sink drain, which would not be reflected in the returned amount and could provide a false estimate of compliance.
Because the actual treatment name may not be known to the site at the time of dispensing or returning, the word treatment in the context of the CDASHIG DA domain refers to the identifier that references the treatment (e.g., Bottle A, Bottle B, Drug A, Drug B) rather than the actual (unblinded) treatment name.
The term dispensed refers to when the study treatment is provided to the subject, not when the subject uses or consumes the study treatment. The term returned refers to when the subject returns the unused study treatment to the investigational site.
In some cases sponsors may wish to link DA data to EX data. This may be accomplished by using the appropriate identifier variables and the relationship (RELREC) dataset as described in the SDTMIG.
This domain, which is in the Findings class, has been modeled in both normalized and denormalized structures to provide users with examples of how each structure could be implemented. Findings domains are typically represented in the vertical/normalized structure as this is usually the easiest and quickest way to collect, process, and clean the data. However, users may have system constraints that prevent them from collecting this data in the vertical/normalized manner. The horizontal/denormalized version provides the structure necessary to collect the variables in this manner.
Depending on the study design, the DA CRF/eCRF may not be required.
Specification for the CDASHIG DA - Drug Accountability Domain
Drug Accountability Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | DA | N/A | Horizontal- Generic | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | DA | N/A | Horizontal- Generic | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | DA | N/A | Horizontal- Generic | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | DA | N/A | Horizontal- Generic | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | DA | N/A | Horizontal- Generic | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD- MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable DADTC in ISO 8601 format. | N/A | N/A | The date the drug accountability assessments were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the observations at that visit, or the collection date can be included on the DA CRF using the date (DADAT) field. |
Findings | DA | N/A | Horizontal- Generic | 6 | DAGRPID | Drug Accountability Group ID | A sponsor- defined identifier used to tie a block of related records in a single domain. | What is the test group identifier? | Test Group Identifier | Char | O | Record unique group identifier. Sponsor may insert additional instructions to ensure each record has a unique group identifier. | DAGRPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | It can be beneficial to use an identifier in a data query to communicate clearly to the site the specific record in question. This group identifier ties together all the tests collected on this horizontal record. This field may be populated by the sponsor's data collection system. |
Findings | DA | N/A | Horizontal- Generic | 7 | [DATESTCD]_DAPERF | Drug Accountability Performed | An indication whether or not a planned drug accountability assessment was performed. | Was [DATEST] collected? | [DATEST] Collected | Char | O | Indicate whether or not drug accountability was performed. | DASTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTMIG variable DASTAT. If DAPERF="N", the value of DASTAT will be "NOT DONE". If DAPERF="Y", DASTAT should be null. A combination of SDTMIG variables ( e.g., DACAT and DASCAT, DATPT ) is used to indicate that multiple tests were not done. In this situation, the SDTM variable DATESTCD would be populated as DAALL and an appropriate test name (DATEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This general prompt question is used as a data management tool to verify that missing results are confirmed missing. This may be implemented for all tests collected on the same horizontal record or for each specific test. When mapped to SDTM, the value of DAPERF would apply to all tests on the same record. Use the CDASH variable [DATESTCD]_DAPERF when implemented on a specific test basis. This CDASH variable name is an example of what "variable name" can be used in a denormalized data structure. |
Findings | DA | N/A | Horizontal- Generic | 8 | [DATESTCD]_DACAT | DA Category of Assessment | A grouping of topic-variable values based on user-defined characteristics. | What was the type of treatment for which drug accountability was assessed? | Treatment Type | Char | O | Record the type of study treatment for which drug accountability is assessed (e.g., STUDY MEDICATION, RESCUE MEDICATION, COMPARATOR, PLACEBO). | DACAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. If the protocol allows dispensing different types of study treatment (e.g., study medication, rescue medication, run-in medication) the CRF can capture the type of treatment using DACAT. This may be preprinted on the CRF. If DACAT is not collected (e.g., it is self- evident from the protocol design), it could be populated during the SDTM-based dataset creation process. The value of DACAT would apply to all measurements on that record when mapped to SDTM. If needed, the CDASH variable [DATESTCD]_DACAT may be used to collect a category for each DATEST. See SDTMIG v3.2 Section 6.3.8.2 for examples on populating DACAT and DASCAT. This CDASH variable name is an example of what "variable name" can be used in a denormalized data structure |
Findings | DA | N/A | Horizontal- Generic | 9 | [DATESTCD]_DASCAT | DA Subcategory of Assessment | A sub-division of the DACAT values based on user-defined characteristics. | What was the name of the treatment for which drug accountability was assessed? | [DATEST] Treatment Name | Char | O | Record the name of the study treatment dispensed (e.g., DRUG A, DRUG B, BOTTLE 1). | DASCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. If it is known at the time of data collection, the treatment name may be collected in DASCAT (with appropriate, but different than, DACAT grouping values). The value of DASCAT would apply to all measurements on that record when mapped to SDTM. If needed, the CDASH variable [DATESTCD]_DASCAT may be used to collect a category for each DATEST. See SDTMIG Section 6.3 for examples on populating DACAT and DASCAT. DASCAT can only be used if there is an DACAT and it must be a subcategorization of DACAT. |
Findings | DA | N/A | Horizontal- Generic | 10 | [DATESTCD]_DAREFID | Drug Accountability Reference ID | An internal or external identifier such as treatment label identifier (e.g., kit number, bottle label, vial identifier). | What is the [DATEST] treatment label identifier? | [DSTEST] Treatment Label Identifier | Char | O | Record dispensed treatment label identifier. | DAREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in EX domain. | N/A | N/A | This packaging identifier (e.g., kit number, bottle label, vial identifier) may be collected in different ways (e.g., affixing label onto CRF, scanning a bar code). For some study dosing regimens, greater granularity for treatment identifiers may be needed. In this situation, sponsors may need to use additional variables. This CDASH variable name is an example of what "variable name" can be used in a denormalized data structure. |
Findings | DA | N/A | Horizontal- Generic | 11 | [DATESTCD]_DADAT | Drug Accountability Date of Assessment | The date the study treatment was dispensed or returned, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the date [DATEST] drug accountability was assessed? | [DATEST] Date | Char | R/C | Record the date drug accountability was performed, using this format ( DD-MON-YYYY). | DADTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable DADTC in ISO 8601 format. | N/A | N/A | The date study treatment dispensed/returned should be recorded for each dispensation for a study with multiple periods or multiple products dispensed. A single date may be collected for all the observations when they are performed on the same date. The date of each observation can also be collected using a CDASH variable [DATESTCD]_DADAT. The date of the observation may be determined from a collected date of visit and in such cases a separate measurement date field is not required. This CDASH variable name is an example of what "variable name" can be used in a denormalized data structure. |
Findings | DA | N/A | Horizontal- Generic | 12 | [DATESTCD]_DAORRES | DA Assessment Result in Original Units | Result of the drug accountability assessment (e.g., actual amount). | What is the amount of the [DATEST] drug accountability assessment? | [DATEST] Amount | Char | HR | Record the result of the drug accountability assessment. | DAORRES; DATEST; DATESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. In addition to the SDTMIG variable DAORRES, create DATESTCD from the CDASH variable name and derive the value of DATEST from DATESTCD. The CDASH prompt may also contain the DATEST. Use appropriate CDISC Controlled Terminology for the test and test code. | N/A | N/A | Each test may be collected using the CDASH variable [TESTCD] ( e.g., RETAMT) or [TESTCD]_DAORRES where TESTCD is the appropriate CT for the DA test code (e.g., RETAMT_DAORRES). For a study with multiple periods or multiple products dispensed, drug accountability amounts should be assessed for each dispensation and return. For the SDTM submission dataset, DAREFID should be used to link related records. This CDASH variable name is an example of what "variable name" can be used in a denormalized data structure. |
Findings | DA | N/A | Horizontal- Generic | 13 | [DATESTCD]_DAORRESU | DA Original Units | The unit of the result as originally received or collected. | What was the unit of the [DATEST] result? | [DATEST] Unit | Char | HR | Record or select the original units in which these data were collected, if not preprinted on CRF. | DAORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | (DAORRESU) | The unit should be preprinted on the CRF or a field provided on the CRF to capture it. This CDASH variable name is an example of what "variable name" can be used in a denormalized data structure. |
Findings | DA | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | DA | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | DA | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What is the subject identifier? | Subject | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | DA | N/A | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, see SDTM Table 2.2.4. |
Findings | DA | N/A | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD- MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable DADTC in ISO 8601 format. | N/A | N/A | The date the drug accountability assessments were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the observations at that visit, or the collection date can be included on the DA CRF using the date (DADAT) field. |
Findings | DA | N/A | N/A | 6 | DAPERF | Drug Accountability Performed | An indication whether or not a planned drug accountability assessment was performed. | Was drug accountability performed? | Drug Accountability Performed | Char | O | Indicate whether or not drug accountability was performed. | DASTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTMIG variable DASTAT. If DAPERF="N", the value of DASTAT will be "NOT DONE". If DAPERF="Y", DASTAT should be null. A combination of SDTMIG variables ( e.g., DACAT and DASCAT, DATPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable DATESTCD would be populated as DAALL and an appropriate test name (DATEST) provided. See SDTMIG Section 4.1.5.1.2. | (NY) | N/A | This may be implemented on a CRF page level on a visit-by-visit basis. This general prompt question is used as a data management tool to verify that missing results are confirmed missing. |
Findings | DA | N/A | N/A | 7 | DACAT | DA Category of Assessment | A grouping of topic-variable values based on user-defined characteristics. | What was the type of treatment for which drug accountability was assessed? | [Treatment Type]; NULL | Char | O | Record the type of treatment dispensed/returned. | DACAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. If the protocol allows dispensing different types of treatment (e.g., study medication, rescue medication, run-in medication) the CRF can capture the type of treatment using DACAT. This may be preprinted on the CRF. If DACAT is not collected (e.g., it is self- evident from the protocol design), it can be populated during the SDTM-based dataset creation process. See SDTMIG DA domain for examples on populating DACAT and DASCAT. |
Findings | DA | N/A | N/A | 8 | DASCAT | DA Subcategory of Assessment | A sub-division of the DACAT values based on user-defined characteristics. | What was the name of the treatment for which drug accountability was assessed? | [Treatment Name]; NULL | Char | O | Record the name of the study treatment dispensed/returned (e.g., Bottle A, Bottle B). | DASCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. If it is known at the time of data collection, the treatment name may be collected in DASCAT (with appropriate, but different, than DACAT grouping values). See SDTMIG DA domain for examples on populating DACAT and DASCAT. DASCAT can only be used if there is an DACAT and it must be a subcategorization of DACAT. |
Findings | DA | N/A | N/A | 9 | DADAT | Drug Accountability Date | The date the study treatment was dispensed or returned, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the date the drug accountability assessment was performed? | Date | Char | R/C | Record the exact date the study treatment was (dispensed or returned), using this format (DD-MON- YYYY). | DADTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable DADTC in ISO 8601 format. | N/A | N/A | The date study treatment dispensed/returned should be recorded for each dispensation for a study with multiple periods or multiple products dispensed. |
Findings | DA | N/A | N/A | 10 | DAREFID | Drug Accountability Reference ID | An internal or external identifier such as treatment label identifier (e.g., kit number, bottle label, vial identifier). | What was the treatment label identifier? | Treatment Label Identifier | Char | O | Record treatment label identifier. | DAREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | For the SDTM submission dataset, DAREFID should be used to tie together a block of related records and to link dispensed product to returned product. This packaging identifier (e.g., kit number, bottle label, vial identifier) may be collected in different ways (e.g., affixing label onto CRF, scanning a bar code). For some study dosing regimens, greater granularity for treatment identifiers may be needed. In this situation, sponsors may need to use additional identifier variables. |
Findings | DA | N/A | N/A | 11 | DATEST | Name of Accountability Assessment | Descriptive name of the measurement or finding (e.g., dispensed, returned). | What was the drug accountability being assessed? | [Drug Accountability Test Name] | Char | HR | Record the name of the drug accountability assessment if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | DATEST; DATESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable DATESTCD may be determined from the value collected in the CDASH field DATEST. The SDTMIG variables DATESTCD and DATEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | (DATEST) | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as Test can be included as the column header. For a study with multiple periods or multiple products dispensed, drug accountability amounts should be assessed for each dispensation. |
Findings | DA | N/A | N/A | 12 | DAORRES | DA Assessment Result in Original Units | Result of the drug accountability assessment as originally dispensed or returned (e.g., actual amount). | What is the result of the drug accountability assessment? | Amount | Char | HR | Record the actual amount of treatment dispensed or returned. | DAORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | For a study with multiple periods or multiple products dispensed, drug accountability amounts should be assessed for each dispensation. |
Findings | DA | N/A | N/A | 13 | DAORRESU | DA Original Units | The unit of the result as originally received or collected. | What was the unit? | Unit | Char | HR | Record or select the original units in which these data were collected, if not preprinted on CRF. | DAORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | (DAORRESU) | Should be preprinted on the CRF with the associated test when possible, rather than collected in a field that requires the site to enter text. |
Assumptions for the CDASHIG DA - Drug Accountability Domain
1. The DA domain is only needed if this information will be collected for the study.
2. There may need to be a clear understanding of how the drugs are identified (e.g., by subject, by masked ID) in order to set up data collection in a manner that makes sense. This is a cross-functional team issue that needs to be addressed early in planning, if applicable.
3. Drug accountability may be implemented for an entire study or on a visit-by-visit basis depending on the most logical approach for each protocol.
4. The DA panel can be used for studies that allow dispensing different types of study treatment (e.g., study medication, rescue medication, run-in medication) by using the DACAT variable to differentiate treatment types.
Example CRFs for the CDASHIG DA - Drug Accountability Domain
Example 1
This example shows normalized data collection for drug accountability information
Title: Study Drug Accountability
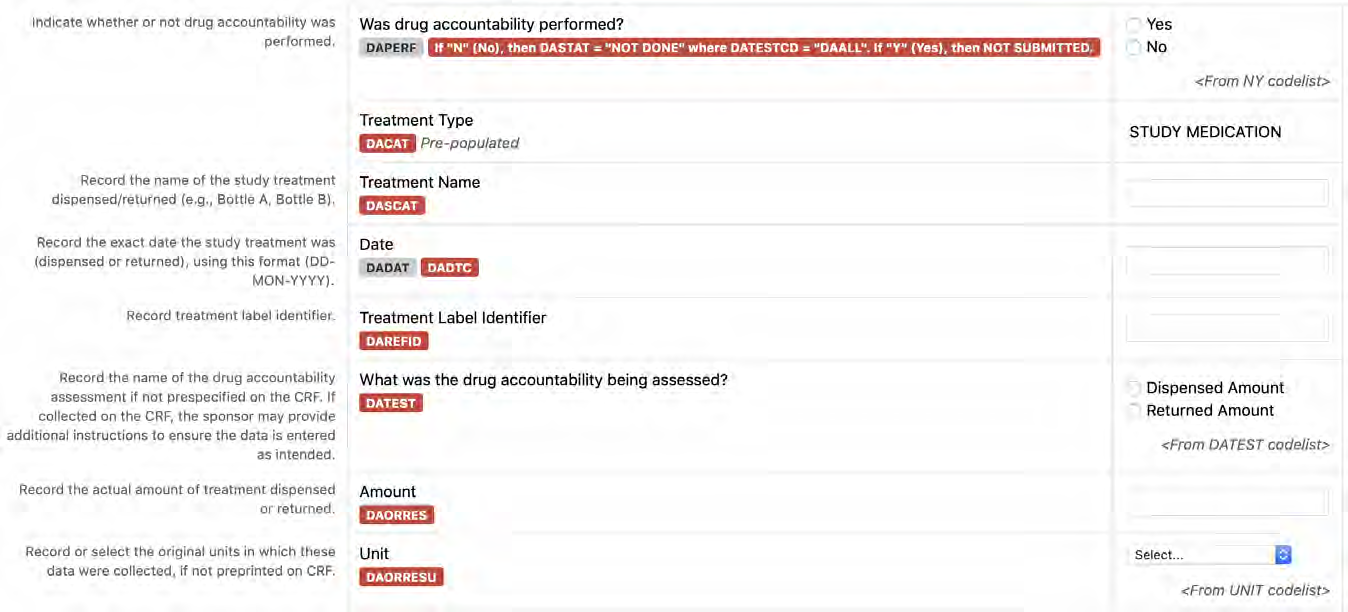
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DAPERF | 1 | Was drug accountability performed? | Drug Accountability Performed | Indicate whether or not drug accountability was performed. | Text | DASTAT | If "N" (No), then DASTAT = "NOT DONE" where DATESTCD = "DAALL". If "Y" (Yes), then NOT SUBMITTED. | (NY) | Yes; No | ||||
DACAT | 2 | What was the type of treatment for which drug accountability was assessed? | Treatment Type | Record the type of treatment dispensed/returned. | Text | DACAT | STUDY MEDICATION | Prompt | |||||
DASCAT | 3 | What was the name of the treatment for which drug accountability was assessed? | Treatment Name | Record the name of the study treatment dispensed/returned (e.g., Bottle A, Bottle B). | Text | DASCAT | Prompt | ||||||
DADAT | 4 | What was the date the drug accountability assessment was performed? | Date | Record the exact date the study treatment was (dispensed or returned), using this format (DD- MON-YYYY). | Date | DADTC | Prompt | ||||||
DAREFID | 5 | What was the treatment label identifier? | Treatment Label Identifier | Record treatment label identifier. | Text | DAREFID | Prompt | ||||||
DATEST | 6 | What was the drug accountability being assessed? | Drug Accountability Test Name | Record the name of the drug accountability assessment if not prespecified on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | Text | DATEST | (DATEST) | Dispensed Amount; Returned Amount | |||||
DAORRES | 7 | What is the result of the drug accountability assessment? | Amount | Record the actual amount of treatment dispensed or returned. | Text | DAORRES | Prompt | ||||||
DAORRESU | 8 | What was the unit? | Unit | Record or select the original units in which these data were collected, if not preprinted on CRF. | Text | DAORRESU | (UNIT) | Bag Dosing Unit; Bottle Dosing Unit; Box Dosing Unit; Capsule Dosing Unit; Container Dosing Unit; Disk Dosing Unit; Gram; Milligram; Milliliter; Package Dosing Unit; Packet Dosing Unit; Patch Dosing Unit; Syringe Dosing Unit; Tablet Dosing Unit; Tube Dosing Unit; Vial Dosing Unit | Prompt |
Example 2
This example shows denormalized data collection for drug accountability information.
Title: Study Treatment Accountability
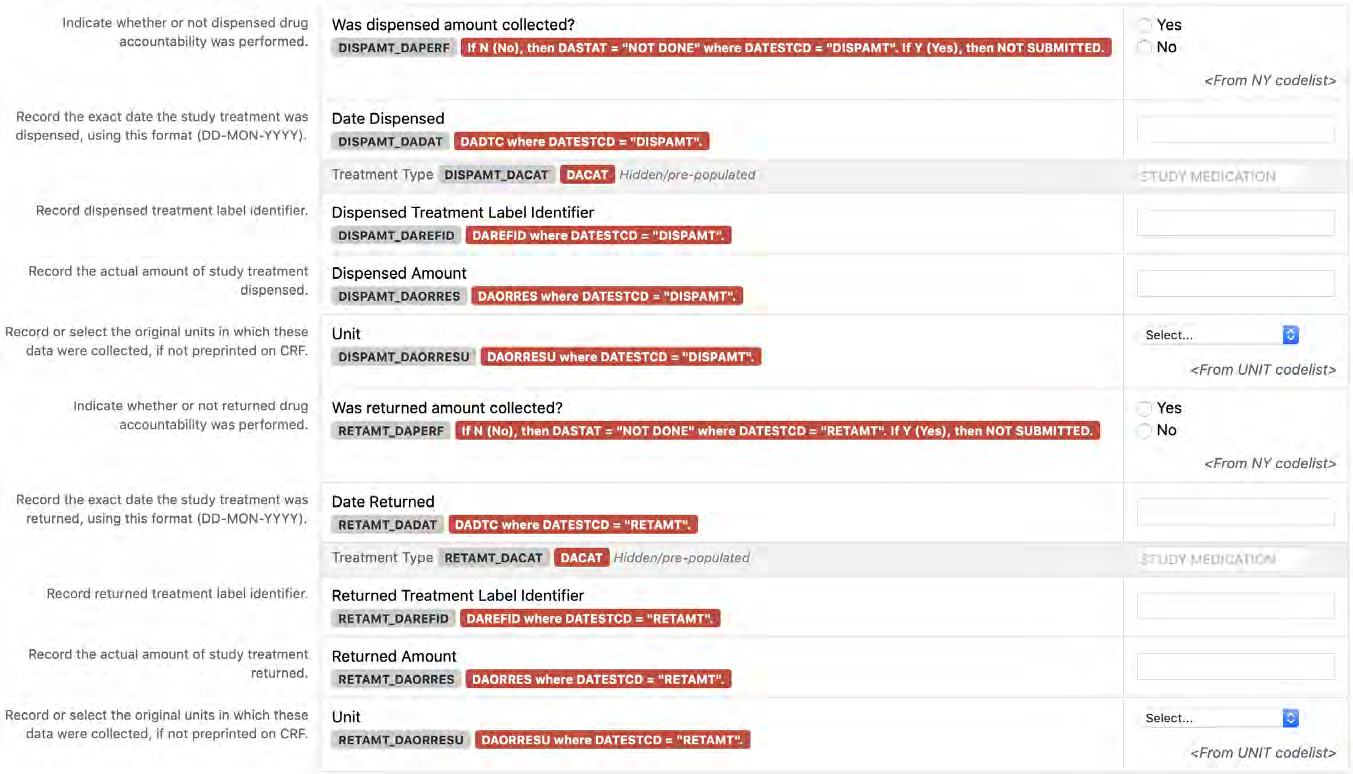
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DISPAMT_DAPERF | 1 | Was dispensed amount collected? | Dispensed Amount Collected | Indicate whether or not dispensed drug accountability was performed. | Text | DASTAT | If N (No), then DASTAT = "NOT DONE" where DATESTCD = "DISPAMT". If Y (Yes), then NOT SUBMITTED. | (NY) | Yes; No | ||||
DISPAMT_DADAT | 2 | What was the date the drug accountability assessment was performed? | Date Dispensed | Record the exact date the study treatment was dispensed, using this format (DD-MON- YYYY). | Date | DADTC | DADTC where DATESTCD = "DISPAMT". | ||||||
DISPAMT_DACAT | 3 | What was the type of treatment dispensed? | Treatment Type | Record the type of study treatment dispensed (e.g., STUDY MEDICATION, RESCUE MEDICATION, COMPARATOR, PLACEBO). | Text | DACAT | STUDY MEDICATION | Prompt | Yes | ||||
DISPAMT_DAREFID | 4 | What was the dispensed treatment label identifier? | Dispensed Treatment Label Identifier | Record dispensed treatment label identifier. | Text | DAREFID | DAREFID where DATESTCD = "DISPAMT". | Prompt | |||||
DISPAMT_DAORRES | 5 | What is the amount dispensed? | Dispensed Amount | Record the actual amount of study treatment dispensed. | Text | DAORRES; DATEST; DATESTCD | DAORRES where DATESTCD = "DISPAMT". | Prompt | |||||
DISPAMT_DAORRESU | 6 | What was the unit? | Unit | Record or select the original units in which these data were collected, if not preprinted on CRF. | Text | DAORRESU | DAORRESU where DATESTCD = "DISPAMT". | (UNIT) | Bag Dosing Unit; Bottle Dosing Unit; Box Dosing Unit; Capsule Dosing Unit; Container Dosing Unit; Disk Dosing Unit; Gram; Milligram; Milliliter; Package Dosing Unit; Packet Dosing Unit; Patch Dosing Unit; Syringe Dosing Unit; Tablet Dosing Unit; Tube Dosing Unit; Vial Dosing Unit | Prompt | |||
RETAMT_DAPERF | 7 | Was returned amount collected? | Returned Amount Collected | Indicate whether or not returned drug accountability was performed. | Text | DASTAT | If N (No), then DASTAT = "NOT DONE" where DATESTCD = "RETAMT". If Y (Yes), then NOT SUBMITTED. | (NY) | Yes; No | ||||
RETAMT_DADAT | 8 | What was the date the drug accountability assessment was performed? | Date Returned | Record the exact date the study treatment was returned, using this format (DD-MON- YYYY). | Date | DADTC | DADTC where DATESTCD = "RETAMT". | ||||||
RETAMT_DACAT | 9 | What was the type of treatment returned? | Treatment Type | Record the type of study treatment returned (e.g., STUDY MEDICATION, RESCUE MEDICATION, COMPARATOR, PLACEBO). | Text | DACAT | STUDY MEDICATION | Prompt | Yes | ||||
RETAMT_DAREFID | 10 | What was the returned treatment label identifier? | Returned Treatment Label Identifier | Record returned treatment label identifier. | Text | DAREFID | DAREFID where DATESTCD = "RETAMT". | Prompt | |||||
RETAMT_DAORRES | 11 | What is the amount returned? | Returned Amount | Record the actual amount of study treatment returned. | Text | DAORRES; DATEST; DATESTCD | DAORRES where DATESTCD = "RETAMT". | Prompt | |||||
RETAMT_DAORRESU | 12 | What was the unit? | Unit | Record or select the original units in which these data were collected, if not preprinted on CRF. | Text | DAORRESU | DAORRESU where DATESTCD = "RETAMT". | (UNIT) | Bag Dosing Unit; Bottle Dosing Unit; Box Dosing Unit; Capsule Dosing Unit; Container Dosing Unit; Disk Dosing Unit; Gram; Milligram; Milliliter; Package Dosing Unit; Packet Dosing Unit; Patch Dosing Unit; Syringe Dosing Unit; Tablet Dosing Unit; Tube Dosing Unit; Vial Dosing Unit | Prompt |
8.3.3 DD - Death Details
Description/Overview for the CDASHIG DD - Death Details Domain
The DD domain is used to collect additional data that are typically collected when a death occurs, such as the official cause of death, when the death occurred, and whether it was witnessed.
This domain is not intended to replace or collate data collected on designated CRF pages such as Severe Adverse Event details in the AE domain, or details of disposition in the DS domain. Data on the DD domain may be linked to other domains that relate to death using the appropriate identifier variables and the related records (RELREC) dataset as described in the SDTMIG.
Specification for the CDASHIG DD - Death Details Domain
Death Details Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | DD | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | DD | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | DD | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | DD | N/A | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | DD | N/A | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD- MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable DDDTC in ISO 8601 format. | N/A | N/A | The date the death details assessments were reported can be determined from the Visit Date variable (VISDAT) and applying that date to all of the observations at that visit or the collection date can be included on the Death Detail CRF using the date (DDDAT) field. |
Findings | DD | N/A | N/A | 6 | DDCAT | Category for Death Details | A grouping of topic-variable values based on user-defined characteristics. | What is the category of the death detail assessment? | [Death Detail Category]; NULL | Char | O | Record the death detail category, if not preprinted on the CRF. | DDCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. |
Findings | DD | N/A | N/A | 7 | DDSCAT | Subcategory for Death Details | A sub-division of the DDCAT values based on user-defined characteristics. | What is the subcategory of the death detail assessment? | [Death Detail Assessment Subcategory]; NULL | Char | O | Record the death detail subcategory, if not preprinted on the CRF. | DDSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header DDSCAT can only be used if there is an DDCAT and it must be a subcategorization of DDCAT. |
Findings | DD | N/A | N/A | 8 | DDYN | Any Death Detail Results | General prompt question regarding whether or not any death details were available. | Were any death detail assessments collected? | Any Death Details | Char | O | Indicate if the death details are known. If yes, include the appropriate details where indicated on the CRF. | N/A | Does not map to an SDTMIG variable. The SDTM Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification if all other fields on the CRF were deliberately left blank. |
Findings | DD | N/A | N/A | 9 | DDDAT | Death Details Date of Collection | The date of collection, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the date death detail assessments were collected? | Collection Date | Char | R/C | Record the date of collection using this format (DD- MON-YYYY). | DDDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable DDDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and, if so, a separate assessment date field is not required. |
Findings | DD | N/A | N/A | 10 | DDSPID | Death Details Sponsor- Defined Identifier | A sponsor- defined identifier. In CDASH, This is typically used for preprinted or auto-generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor-defined question] | [Sponsor defined] | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | DDSPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Since SPID is a sponsor- defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile data. May be used to record preprinted number (e.g. line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Findings | DD | N/A | N/A | 11 | DTHDAT | Death Date | Date of death for any subject who died. | What was the subject's date of death? | Death Date | Char | O | Record the date of death. | DM.DTHDTC | This field does not map directly to an SDTMIG variable. For the SDTM dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTM variable DTHDTC in ISO 8601 format. | N/A | N/A | The CDASH model defines Death Date as a timing variable; this is not included as a timing variable in the SDTMIG. It may be collected on any CRF deemed appropriate by the Sponsor, but should only be collected once. The SDTMIG variable DTHDTC is mapped to the DM domain during the SDTM submission dataset creation process. The SDTMIG variable DM.DTHFLG is not a CDASH variable, but it is typically populated during the SDTM submission dataset creation process. Death Date may be mapped to other SDTM domains, as deemed appropriate by the sponsor (e.g., DS). |
Findings | DD | N/A | N/A | 12 | DDTEST | Death Detail Assessment Name | Descriptive name for death details | What was the death detail assessment test name? | [Death Detail Assessment (Test Name] | Char | HR | Record the name of the death detail assessment if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | DDTEST; DDTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable DDTESTCD may be determined from the value collected in DDTEST. Both DDTESTCD and DDTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | (DTHDX) | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as Test can be included as the column header. |
Findings | DD | N/A | N/A | 13 | DDORRES | Death Details Result or Finding | Result of the death detail assessment, as originally received or collected. | What was the result of the death detail assessment? | (Result) | Char | HR | Record the death detail response. | DDORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | N/A |
Findings | DD | N/A | N/A | 14 | DDEVAL | Death Details Evaluator | The role of the person who provided the information. | Who provided the death detail assessment information? | [Evaluator/Reporter] | Char | O | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | DDEVAL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EVAL) | N/A | Used only for results that are subjective (e.g., assigned by a person or a group). May be a preprinted, or collected. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | DD | N/A | Horizontal- Generic | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | DD | N/A | Horizontal- Generic | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted for the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | DD | N/A | Horizontal- Generic | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | DD | N/A | Horizontal- Generic | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to Study Data Tabulation Model, Table 2.2.4. |
Findings | DD | N/A | Horizontal- Generic | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD- MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable DDDTC in ISO 8601 format. | N/A | N/A | The date the death details assessments were reported can be determined from the Visit Date variable (VISDAT) and applying that date to all of the observations at that visit or the collection date can be included on the Death Detail CRF using the date (DDDAT) field. |
Findings | DD | N/A | Horizontal- Generic | 6 | DDYN | Any Death Detail Results | General prompt question regarding whether or not any death details were available. | Were any death detail assessments collected? | Any Death Detail Assessments | Char | O | Indicate if the death details are known. If yes, include the appropriate details where indicated on the CRF. | N/A | Does not map to an SDTMIG variable. The SDTM Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification if all other fields on the CRF were deliberately left blank. |
Findings | DD | N/A | Horizontal- Generic | 7 | DDCAT | Category for Death Details | A grouping of topic-variable values based on user defined characteristics. | What is the category of the death detail assessment? | [Death Detail Assessment Category]; NULL | Char | O | Record the death detail category, if not preprinted on the CRF. | DDCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | DD | N/A | Horizontal- Generic | 8 | DDSCAT | Subcategory for Death Details | A sub-division of the DDCAT values based on user defined characteristics. | What is the subcategory of the death detail assessment? | [Death Detail Assessment Subcategory]; NULL | Char | O | Record the death detail subcategory, if not preprinted on the CRF. | DDSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header DDSCAT can only be used if there is an DDCAT and it must be a subcategorization of DDCAT. |
Findings | DD | N/A | Horizontal- Generic | 9 | [DTHDXCD]_DDDAT | Death Details Date of Collection | The date of collection represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the date [DTHDX] assessment was collected? | [DTHDX] Date | Char | R/C | Record the date of collection using this format (DD- MON-YYYY). | DDDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable DDDTC in ISO 8601 format. | N/A | N/A | A single date may be collected for all death detail assessments when they are collected on the same date. The date of collection of each assessment can also be collected using a CDASH variable [DTHDXCD]_DDDAT. The date of the assessment may be determined from a collected date of visit and in such cases a separate assessment date field is not required. |
Findings | DD | N/A | Horizontal- Generic | 10 | DTHDAT | Date of Death | Date of death for any subject who died. | What was the subject's date of death? | Death Date | Char | O | Record the date of death. | DM.DTHDTC | This field does not map directly to an SDTMIG variable. For the SDTM dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTM variable DTHDTC in ISO 8601 format. | N/A | N/A | The CDASH model defines Death Date as a timing variable; this is not included as a timing variable in the SDTMIG. It may be collected on any CRF deemed appropriate by the Sponsor, but should only be collected once. The SDTMIG variable DTHDTC is mapped to the DM domain during the SDTM submission dataset creation process. The SDTMIG variable DM.DTHFLG is not a CDASH variable, but it is typically populated during the SDTM submission dataset creation process. Death Date may be mapped to other SDTM domains, as deemed appropriate by the sponsor (e.g., DS). |
Findings | DD | N/A | Horizontal- Generic | 11 | [DTHDXCD]_DDORRES | Death Details Result or Finding | Result of the death detail assessment, as originally received or collected. | What was the death detail assessment result? | Result | Char | HR | Record the death detail response. | DDORRES; DDTEST; DDTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. In addition to the SDTMIG variable DDORRES, create DDTESTCD from the CDASH variable name and determine the value of DDTEST from DDTESTCD. The CDASH prompt may also contain the DDTEST. Use appropriate CDISC Controlled Terminology for the test and test code. | N/A | N/A | Each test may be collected using the CDASH variable [TESTCD] (e.g., PRCDTH) or [TESTCD]_DDORRES where TESTCD is the appropriate CT for the DD test code e.g., PRCDTH_DDORRES. This CDASH variable name is an example of what "variable name" can be used in a denormalized data structure. |
Findings | DD | N/A | Horizontal- Generic | 12 | DDEVAL | Death Details Evaluator | The role of the person who provided the information. | Who provided the death detail assessment information? | [Evaluator/Reporter] | Char | O | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | DDEVAL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EVAL) | N/A | Used only for results that are subjective (e.g., assigned by a person or a group). May be a preprinted, or collected. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Assumptions for the CDASHIG DD - Death Details Domain
1. This domain captures information pertaining to the death of a subject, including the causes of death and findings of an autopsy directly related to the death itself (e.g., cause of death). If more than 1 cause of death is obtained, these may be separated into primary and secondary causes and/or other appropriate designations.
2. This domain is not intended to include the details describing the autopsy (e.g., who conducted the autopsy or when). Autopsy information should be handled as per recommendations in the Procedures (PR) domain.
3. An autopsy is a procedure from which there will usually be findings. Results of the autopsy not specific to the death itself will be represented in the appropriate Findings domain(s).
Example CRF for the CDASHIG DD - Death Details Domain
Example 1
Title: Death Details
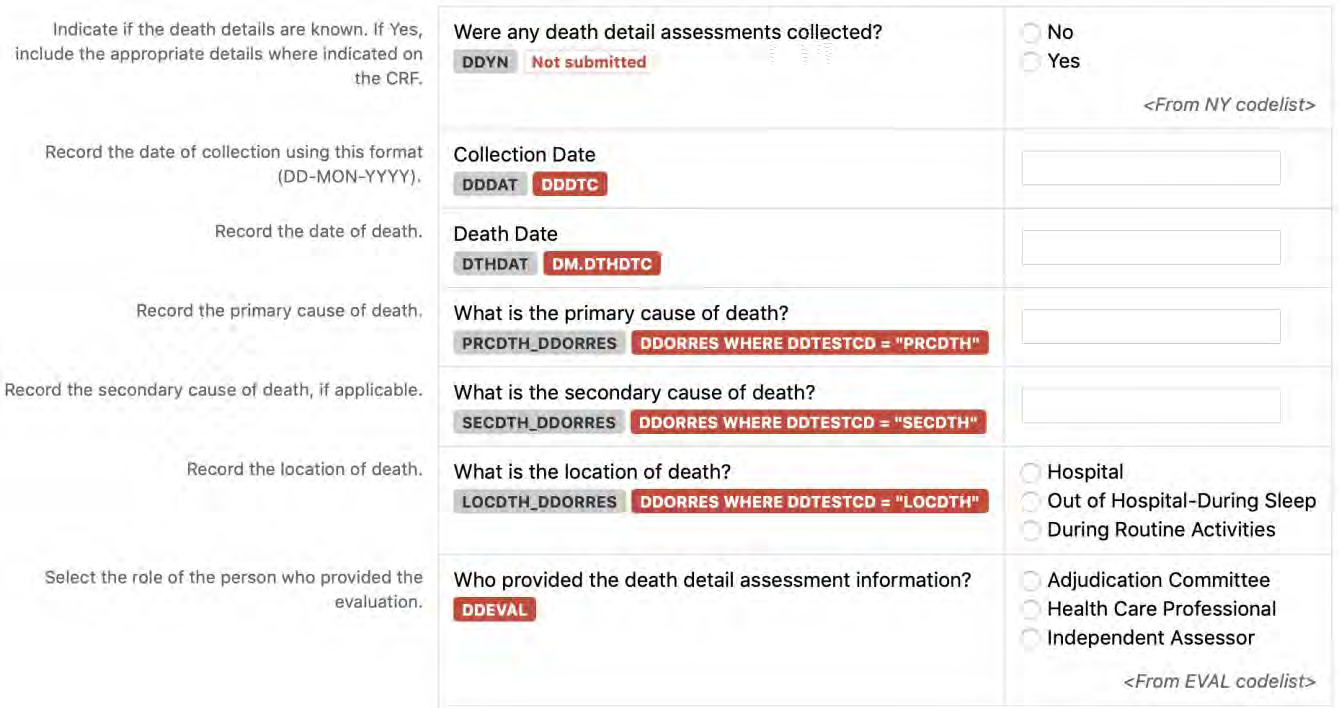
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DDYN | 1 | Were any death detail assessments collected? | Any Death Detail Assessments | Indicate if the death details are known. If Yes, include the appropriate details where indicated on the CRF. | Text | N/A | (NY) | No; Yes | |||||
DDDAT | 2 | What was the date death detail assessments were collected? | Collection Date | Record the date of collection using this format (DD- MON-YYYY). | Date | DDDTC | |||||||
DTHDAT | 3 | What was the subject’s date of death? | Death Date | Record the date of death. | Date | DM.DTHDTC | prompt | ||||||
PRCDTH_DDORRES | 4 | What is the primary cause of death? | Result | Record the primary cause of death. | Text | DDORRES; DDTEST; DDTESTCD; | DDORRES WHERE DDTESTCD = "PRCDTH" | ||||||
SECDTH_DDORRES | 5 | What is the secondary cause of death? | Result | Record the secondary cause of death, if applicable. | Text | DDORRES; DDTEST; DDTESTCD; | DDORRES WHERE DDTESTCD = "SECDTH" | ||||||
LOCDTH_DDORRES | 6 | What is the location of death? | Result | Record the location of death. | Text | DDORRES; DDTEST; DDTESTCD; | DDORRES WHERE DDTESTCD = "LOCDTH" | Hospital; Out of Hospital-During Sleep; During Routine Activities | |||||
DDEVAL | 7 | Who provided the death detail assessment information? | [Evaluator/Reporter] | Select the role of the person who provided the evaluation. | Text | DDEVAL | (EVAL) | Adjudication Committee; Health Care Professional; Independent Assessor |
8.3.4 EG - ECG Test Results
Description/Overview for the CDASHIG EG - ECG Test Results Domain
This section provides EG domain metadata for 3 different data collection scenarios.
Scenario 1: Central Reading
In this scenario, results are captured directly by an electronic device and transmitted separately or read by a central vendor, rather than recorded on the CRF. The accession number and date collected on the CRF can be used as an aid in reconciliation of the electronic data.
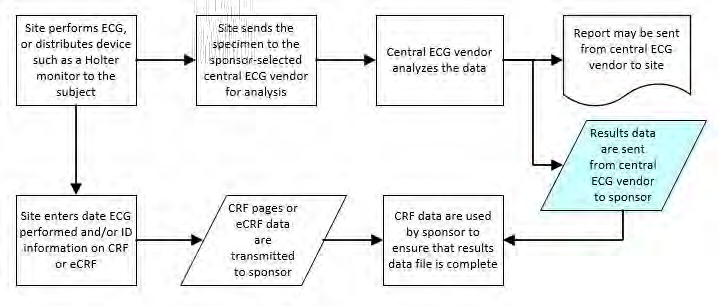
Scenario 2: Local Reading
In this scenario, subjects' ECGs are performed and analyzed, and then the results are recorded directly on the CRF.
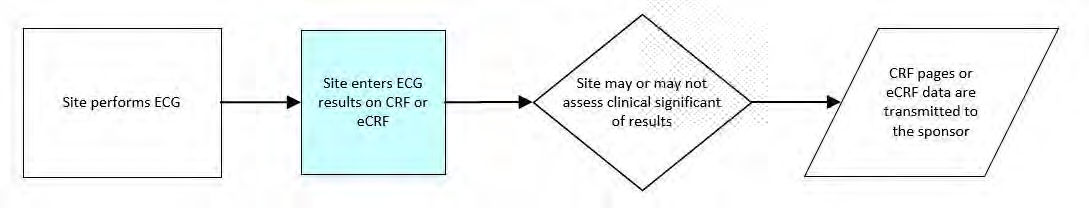
Scenario 3: Central Reading with Investigator Assessment of Clinical Significance Assessment and/or Overall Interpretation
In this scenario, results are captured directly by an electronic device by a central vendor. The results are provided in an electronic file to the sponsor. In addition, the results are provided to the investigator for assessment of clinical significance for any abnormal values and that information is provided to the sponsor on the CRF.
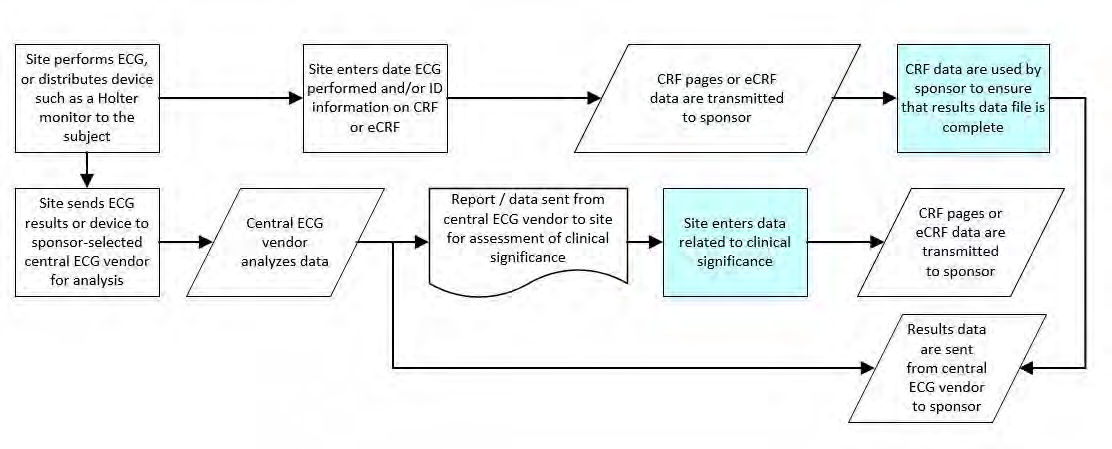
Specification for the CDASHIG EG - ECG Test Results Domain
ECG Test Results Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | EG | Central Reading | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | EG | Central Reading | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | EG | Central Reading | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/ Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | EG | Central Reading | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | EG | Central Reading | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD- MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable EGDTC in ISO 8601 format. | N/A | N/A | The date the ECG measurements were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the ECG measurements at that visit, or the collection date can be included on the ECG CRF using the date (EGDAT) field. |
Findings | EG | Central Reading | N/A | 6 | EGCAT | Category for ECG | A grouping of topic-variable values based on user-defined characteristics. | What was the category of the ECG finding? | [ECG Category]; NULL | Char | O | Record the ECG finding category, if not preprinted on the CRF. | EGCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | EG | Central Reading | N/A | 7 | EGSCAT | Subcategory for ECG | A sub-division of the EGCAT values based on user-defined characteristics. | What was the subcategory of the ECG finding? | [ECG Subcategory]; NULL | Char | O | Record the ECG finding subcategory, if not preprinted on the CRF. | EGSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header EGSCAT can only be used if there is an EGCAT and it must be a subcategorization of EGCAT. |
Findings | EG | Central Reading | N/A | 8 | EGPERF | ECG Performed | An indication whether or not a planned ECG measurement, series of ECG measurements, tests, or observations was performed | Was the ECG performed? | ECG Performed | Char | HR | Indicate whether or not an ECG or specific ECG test was done. | EGSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTMIG variable EGSTAT. If the CDASH field EGPERF= "N", the value of EGSTAT will be "NOT DONE". If EGPERF = "Y", EGSTAT should be null. A combination of SDTMIG variables (e.g., EGCAT and EGSCAT, EGTPT) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable EGTESTCD would be populated as EGALL and an appropriate test name (EGTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This may be implemented for an entire ECG, or a specific ECG test basis. General prompt question to be used as a data management tool to verify that missing results are confirmed missing. |
Findings | EG | Central Reading | N/A | 9 | EGREFID | ECG Reference ID | An internal or external identifier of the ECG (e.g., waveform number). | What was the (ECG) [reference identifier/accession number]? | (ECG) [Reference Identifier/Accession Number] | Char | O | Record the identifier number assigned. | EGREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | This can be used to confirm that the appropriate data record is present in the electronic transfer if this reference ID happens to be available to the site at the time of collection. It can also be used to link the clinical significance assessment to the proper record in the electronic data. (e.g., UUID for external waveform file, session number automatically generated by electronic equipment). |
Findings | EG | Central Reading | N/A | 10 | EGMETHOD | Method of ECG Test | Method of the test or examination. | What was the method used for the ECG? | Method | Char | O | Record the method used for the ECG. | EGMETHOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EGMETHOD) | N/A | Results may be affected by whether conditions for ECG as specified in the protocol were properly met. One possible condition is the method used to collect or calculate the ECG data. If the protocol requires this type of information, then this question may be included to confirm that the method used matches the protocol. The following are examples of when it is not necessary to collect these data on the CRF: • Method of ECG is provided as part of the electronic data. • Method of ECG is not pertinent to the protocol. • The protocol specifies only one possible method for collecting ECG measurements and the sponsor does not feel there is significant risk of the sites performing the ECG using the incorrect method. |
Findings | EG | Central Reading | N/A | 11 | EGPOS | ECG Position of Subject | The position of the subject during the ECG measurement. | What was the position of the subject during the ECG measurement? | Position | Char | O | Record the position of the subject during the ECG. | EGPOS | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (POSITION) | N/A | Results may be affected by whether conditions for ECG as specified in the protocol were properly met. One common condition is the subject's position. If the protocol requires this type of information, then this question may be included to confirm that the subject's position matches the protocol. The following are examples of when it is not necessary to collect these data on the CRF: |
Findings | EG | Central Reading | N/A | 12 | EGDAT | ECG Date | The date the ECG was performed, represented in an unambiguous date format (e.g., DD-MON-YYYY). | What was the date of the ECG? | ECG Date | Char | R/C | Record the date the ECG was done using this format (DD-MON-YYYY). | EGDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable EGDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and if so, a separate assessment date field is not required. |
Findings | EG | Central Reading | N/A | 13 | EGTPT | ECG Planned Time Point Name | A text description of planned time point when measurements should be taken as defined in the protocol. | What was the planned time point of the ECG measurement? | [Planned Time Point Name] | Char | R/C | Record the time point labels for when the ECG test should be taken, if not preprinted on the CRF. | EGTPT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. See SDTMIG for additional information on representing time points. SDTMIG time point anchors EGTPTREF (text description) and EGRFTDTC (date/time) may be needed, as well as SDTMIG variables EGTPTNUM, EGELTM. | N/A | N/A | Planned time points are needed to differentiate multiple sequential assessments. It is recommended that time points should be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Planned Time Point" can be included as the column header. |
Findings | EG | Central Reading | N/A | 14 | EGTIM | ECG Time | Time of ECG, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the time the ECG was collected? | ECG Time | Char | R/C | Record the time the ECG was done (as complete as possible). | EGDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable EGDTC in ISO 8601 format. | N/A | N/A | May be required when multiple assessments are done on 1 day or when the timing in relationship to study treatment is required for analysis. |
Findings | EG | Local Reading | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | EG | Local Reading | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | EG | Local Reading | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | EG | Local Reading | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | EG | Local Reading | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD- MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable EGDTC in ISO 8601 format. | N/A | N/A | The date the ECG measurements were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the ECG measurements at that visit, or the collection date can be included on the ECG CRF using the date (EGDAT) field. |
Findings | EG | Local Reading | N/A | 6 | EGCAT | Category for ECG | A grouping of topic-variable values based on user-defined characteristics. | What was the category of the ECG finding? | [ECG Category]; NULL | Char | O | Record the ECG finding category, if not preprinted on the CRF. | EGCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | EG | Local Reading | N/A | 7 | EGSCAT | Subcategory for ECG | A sub-division of the EGCAT values based on user-defined characteristics. | What was the subcategory of the ECG finding? | [ECG Subcategory]; NULL | Char | O | Record the ECG finding subcategory, if not preprinted on the CRF. | EGSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header EGSCAT can only be used if there is an EGCAT and it must be a subcategorization of EGCAT. |
Findings | EG | Local Reading | N/A | 8 | EGPERF | ECG Performed | An indication of whether or not a planned measurement, series of measurements, test, or observation was performed. | Was the ECG performed? | ECG Performed | Char | HR | Indicate whether or not ECG or specific ECG test was done. | EGSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTMIG variable EGSTAT. If the CDASH field EGPERF="N", the value of EGSTAT will be "NOT DONE". If EGPERF="Y", EGSTAT should be null. A combination of SDTMIG variables ( e.g., EGCAT and EGSCAT, EGTPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable EGTESTCD would be populated as EGALL and an appropriate test name (EGTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This may be implemented for an entire ECG, or a specific ECG test basis. General prompt question to be used as a data management tool to verify that missing results are confirmed missing. |
Findings | EG | Local Reading | N/A | 9 | EGMETHOD | Method of ECG Test | Method of the test or examination. | What was the method used for the ECG? | Method | Char | O | Record the method used for the ECG. | EGMETHOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EGMETHOD) | N/A | Results may be affected by whether conditions for ECG as specified in the protocol were properly met. One possible condition is the method used to collect or calculate the ECG data. If the protocol requires this type of information, then this question may be included to confirm that the method used matches the protocol. The following are examples of when it is not necessary to collect these data on the CRF: • Method of ECG is provided as part of the electronic data. • Method of ECG is not pertinent to the protocol. • The protocol specifies only one possible method for collecting ECG measurements and the sponsor does not feel there is significant risk of the sites performing the ECG using the incorrect method. |
Findings | EG | Local Reading | N/A | 10 | EGPOS | ECG Position of Subject | The position of the subject during the ECG measurement. | What was the position of the subject during the ECG measurement? | Position | Char | O | Record the position of the subject during the ECG. | EGPOS | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (POSITION) | N/A | Results may be affected by whether conditions for ECG as specified in the protocol were properly met. One common condition is the subject's position). If the protocol requires this type of information, then this question may be included to confirm that the subject's position matches the protocol. The following are examples of when it is not necessary to collect these data on the CRF: • Position of the subject is provided as part of the electronic data. • Position of the subject is not pertinent to the protocol. • The protocol specifies only one possible position and the sponsor does not feel there is significant risk of the sites performing the ECG with the subject in the wrong position. |
Findings | EG | Local Reading | N/A | 11 | EGDAT | Date of ECG | The date the ECG was performed, represented in an unambiguous date format (e.g., DD-MON-YYYY). | What was the date of the ECG? | ECG Date | Char | R/C | Record the date ECG was done using this format (DD-MON-YYYY). | EGDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable EGDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and if so, a separate assessment date field is not required. |
Findings | EG | Local Reading | N/A | 12 | EGTPT | ECG Planned Time Point Name | A text description of planned time point when measurements should be taken as defined in the protocol. | What was the planned time point of the ECG measurement? | [Planned Time Point Name] | Char | R/C | Record the time point labels for when the ECG test should be taken, if not preprinted on the CRF. | EGTPT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. See SDTMIG for additional information on representing time points. SDTMIG time point anchors EGTPTREF (text description) and EGRFTDTC (date/time) may be needed, as well as SDTMIG variables EGTPTNUM, EGELTM. | N/A | N/A | Planned time point are needed to differentiate multiple sequential assessments. It is recommended that time points should be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Planned Time Point" can be included as the column header. |
Findings | EG | Local Reading | N/A | 13 | EGTIM | Time of ECG | Time of ECG, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the time the ECG was collected? | ECG Time | Char | R/C | Record the time the ECG was done (as complete as possible). | EGDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable EGDTC in ISO 8601 format. | N/A | N/A | May be required when multiple assessments are done on 1 day or when the timing in relationship to study treatment is required for analysis. |
Findings | EG | Local Reading | N/A | 14 | EGTEST | ECG Test or Examination Name | Descriptive name of the measurement or finding. | What was the ECG test name? | [ECG Test Name] | Char | HR | Record the name of the ECG measurement or finding, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | EGTEST; EGTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable EGTESTCD may be determined from the value collected in EGTEST. The SDTMIG variables EGTESTCD and EGTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | (EGTEST) | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | EG | Local Reading | N/A | 15 | EGORRES | ECG Result or Finding in Original Units | Result of the measurement or finding as originally received or collected. | What was the result of the ECG? | (Result) | Char | HR | Record test results, interpretations or findings. | EGORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Both quantitative results and interpretive findings or summaries may be recorded here. |
Findings | EG | Local Reading | N/A | 16 | EGORRESU | ECG Original Units | The unit of the result as originally received or collected. | What was the unit of the ECG results? | Unit | Char | R/C | Record or select the original unit in which these data were collected, if not preprinted on CRF. | EGORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | (EGORRESU) | May be included if quantitative results are recorded. Because units for quantitative ECG results are typically limited, units should be preprinted on the CRF with the associated test when possible, rather than having the sites record the units. This item is not necessary for qualitative results. |
Findings | EG | Local Reading | N/A | 17 | EGCLSIG | ECG Clinical Significance | An indication whether the ECG results were clinically significant. | Was the ECG clinically significant? | Clinically Significant | Char | O | Record whether ECG results were clinically significant. | SUPPEG.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPEG dataset as the value of SUPPEG.QVAL where SUPPEG.QNAM ="EGCLSIG" and SUPPEG.QLABEL="Clinically Significant". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | Could apply to specific measurements or to overall interpretation. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD- MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable EGDTC in ISO 8601 format. | N/A | N/A | The date the ECG measurements were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the ECG measurements at that visit, or the collection date can be included on the ECG CRF using the date (EGDAT) field. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 6 | EGCAT | Category for ECG | A grouping of topic-variable values based on user-defined characteristics. | What was the category of the ECG finding? | [ECG Category]; NULL | Char | O | Record the ECG finding category, if not preprinted on the CRF. | EGCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 7 | EGSCAT | Subcategory for ECG | A sub-division of the EGCAT values based on user-defined characteristics. | What was the subcategory of the ECG finding? | [ECG Subcategory]; NULL | Char | O | Record the ECG finding subcategory, if not preprinted on the CRF. | EGSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header EGSCAT can only be used if there is an EGCAT and it must be a subcategorization of EGCAT. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 8 | EGPERF | ECG Performed | An indication of whether or not a planned measurement, series of measurements, test, or observation was performed. | Was the ECG performed? | ECG Performed | Char | HR | Indicate whether or not an ECG or specific ECG test was done. | EGSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTMIG variable EGSTAT. If the CDASH field EGPERF="N", the value of EGSTAT will be "NOT DONE". If EGPERF="Y", EGSTAT should be null. A combination of SDTMIG variables (e.g., EGCAT and EGSCAT, EGTPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable EGTESTCD would be populated as EGALL and an appropriate test name (EGTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This may be implemented for an entire ECG, or a specific ECG test basis. General prompt question to be used as a data management tool to verify that missing results are confirmed missing. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 9 | EGREFID | ECG Reference ID | An internal or external identifier of the ECG (e.g., waveform number). | What was the (ECG) [reference identifier/accession number]? | (ECG) [Reference Identifier/Accession Number] | Char | O | Record the identifier number assigned. | EGREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | This can be used to confirm that the appropriate data record is present in the electronic transfer if this reference ID happens to be available to the site at the time of collection. (e.g., Universally Unique Identifier (UUID) for external waveform file, session number automatically generated by electronic equipment). This can also be used to link. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 10 | EGMETHOD | Method of ECG Test | Method of the test or examination. | What was the method used for the ECG? | Method | Char | O | Record the method used for the ECG. | EGMETHOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EGMETHOD) | N/A | Results may be affected by whether conditions for ECG as specified in the protocol were properly met. One possible condition is the method used to collect or calculate the ECG data. If the protocol requires this type of information, then this question may be included to confirm that the method used matches the protocol. The following are examples of when it is not necessary to collect these data on the CRF: • Method of ECG is provided as part of the electronic data. • Method of ECG is not pertinent to the protocol. • The protocol specifies only one possible method for collecting ECG measurements and the sponsor does not feel there is significant risk of the sites performing the ECG using the incorrect method. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 11 | EGPOS | ECG Position of Subject | The position of the subject during the ECG measurement. | What was the position of the subject during the ECG measurement? | Position | Char | O | Record the position of the subject during the ECG. | EGPOS | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (POSITION) | N/A | Results may be affected by whether conditions for ECG as specified in the protocol were properly met. One common condition is the subject's position. If the protocol requires this type of information, then this question may be included to confirm that the subject's position matches the protocol. The following are examples of when it is not necessary to collect these data on the CRF: • Position of the subject is provided as part of the electronic data. • Position of the subject is not pertinent to the protocol. • The protocol specifies only one possible position and the sponsor does not feel there is significant risk of the sites performing the ECG with the subject in the wrong position. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 12 | EGDAT | Date of ECG | The date the ECG was performed, represented in an unambiguous date format (e.g., DD-MON-YYYY). | What was the date of the ECG? | ECG Date | Char | R/C | Record the date ECG was done using this format (DD-MON-YYYY). | EGDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable EGDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and if so, a separate assessment date field is not required. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 13 | EGTPT | ECG Planned Time Point Name | A text description of planned time point when measurements should be taken as defined in the protocol. | What was the planned time point of the ECG measurement? | [Planned Time Point Name] | Char | R/C | Record the time point labels for when the ECG test should be taken, if not preprinted on the CRF. | EGTPT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. See SDTMIG for additional information on representing time points. SDTMIG time point anchors EGTPTREF (text description) and EGRFTDTC (date/time) may be needed, as well as SDTMIG variables EGTPTNUM, EGELTM. | N/A | N/A | Planned time points are needed to differentiate multiple sequential assessments. It is recommended that time points should be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Planned Time Point" can be included as the column header. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 14 | EGTIM | Time of ECG | Time of ECG, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the time the ECG was collected? | ECG Time | Char | R/C | Record the time the ECG was done (as complete as possible). | EGDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable EGDTC in ISO 8601 format. | N/A | N/A | May be required when multiple assessments are done on 1 day or when the timing in relationship to study treatment is required for analysis. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 15 | EGEVAL | ECG Evaluator | The role of the person who provided the evaluation. | Who provided the information?; Who was the evaluator? | [Evaluator/Reporter] | Char | O | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | EGEVAL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EVAL) | N/A | Used only for results that are subjective (e.g., assigned by a person or a group). May be preprinted or collected. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 16 | INTP_EGORRES | ECG Interpretation | Overall interpretation of the result of the measurement or finding. | What was the interpretation of the ECG? | Interpretation | Char | O | Record overall interpretations of the ECG. | EGORRES | This does not map directly to an SDTM variable. For the SDTM submission dataset, the recorded interpretation is populated into the SDTMIG variable EGORRES where EGTEST= "Interpretation", and EGTESTCD="INTP". | N/A | N/A | The overall interpretation of an ECG is mapped into the appropriate SDTM test and result variables. See the SDTMIG EG Domain for details. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 17 | EGCLSIG | ECG Clinical Significance | An indication whether the ECG results were clinically significant. | Was the ECG clinically significant? | Clinically Significant | Char | HR | Record whether ECG results were clinically significant. | SUPPEG.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPEG dataset as the value of SUPPEG.QVAL where SUPPEG.QNAM = "EGCLSIG" and SUPPEG.QLABEL="Clinically Significant". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | Could apply to specific measurements or to overall interpretation. In this scenario, clinical significance could be provided by the investigator. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 18 | EGMHNO | Related Medical History Event ID | Identifier for the medical history event that was reported as a clinically significant ECG finding. | What was the identifier for the medical history event that was reported as a clinically significant ECG finding? | Medical History Event Identifier | Char | O | Identifier for the medical history event that was reported as a clinically significant ECG finding. | N/A | This does not map directly to an SDTMIG variable. For the SDTM submission datasets, may be used to create RELREC to link this record with a record in the Medical History domain. | N/A | N/A | Intent is to establish a link between the clinically significant ECG finding and the medical history event that was reported. EGMHNO can be used in RELREC to identify a relationship between records in EG dataset and records in the MH dataset. See SDTMIG v3.2 Section 8.2 for information on RELREC. |
Findings | EG | Central Reading with Investigator Assessment | N/A | 19 | EGAENO | Related Adverse Event ID | Identifier for the adverse event that was reported as a clinically significant ECG finding. | What was the identifier for the adverse event(s) that was reported as a clinically significant ECG finding? | Adverse Event Identifier | Char | O | Identifier for the adverse event that was the reported as a clinically significant ECG finding. | N/A | This does not map directly to an SDTMIG variable. For the SDTM submission datasets, may be used to create RELREC to link this record with a record in the Adverse Events domain. | N/A | N/A | Intent is to establish a link between the clinically significant ECG finding and the AE that was reported. EGAENO can be used to identify a relationship between records in EG dataset and records in the AE dataset. See SDTMIG v3.2 Section 8.2 for information on RELREC. |
Assumptions for the CDASHIG EG - ECG Test Results Domain
1. The ECG tests that should be collected are not specified by CDASH; this is a medical and scientific decision that should be based on the needs of the protocol.
2. Sponsors should decide which scenario is appropriate for each protocol.
3. As required or defined by the study protocol, clinically significant results may need to be reported on the Adverse Event CRF.
4. As required or defined by the study protocol, changes that are worsening may need to be reported on the AE CRF.
5. As depicted in Scenario 3, where CRF includes site assessment of clinical significance and/or overall interpretation, results are returned to the sites, and the sites complete a CRF page of clinical significance for any abnormal/unexpected values and/or record an overall interpretation of the results. As in Scenario 1, the actual testing results are transmitted electronically, but the CRF includes data necessary to identify and rate the clinical significance of the abnormal results.
Example CRFs for the CDASHIG EG - ECG Test Results Domain
Example 1
This example shows a CRF that collects electrocardiogram data utilizing a central reader.
Title: ECG Central Reading
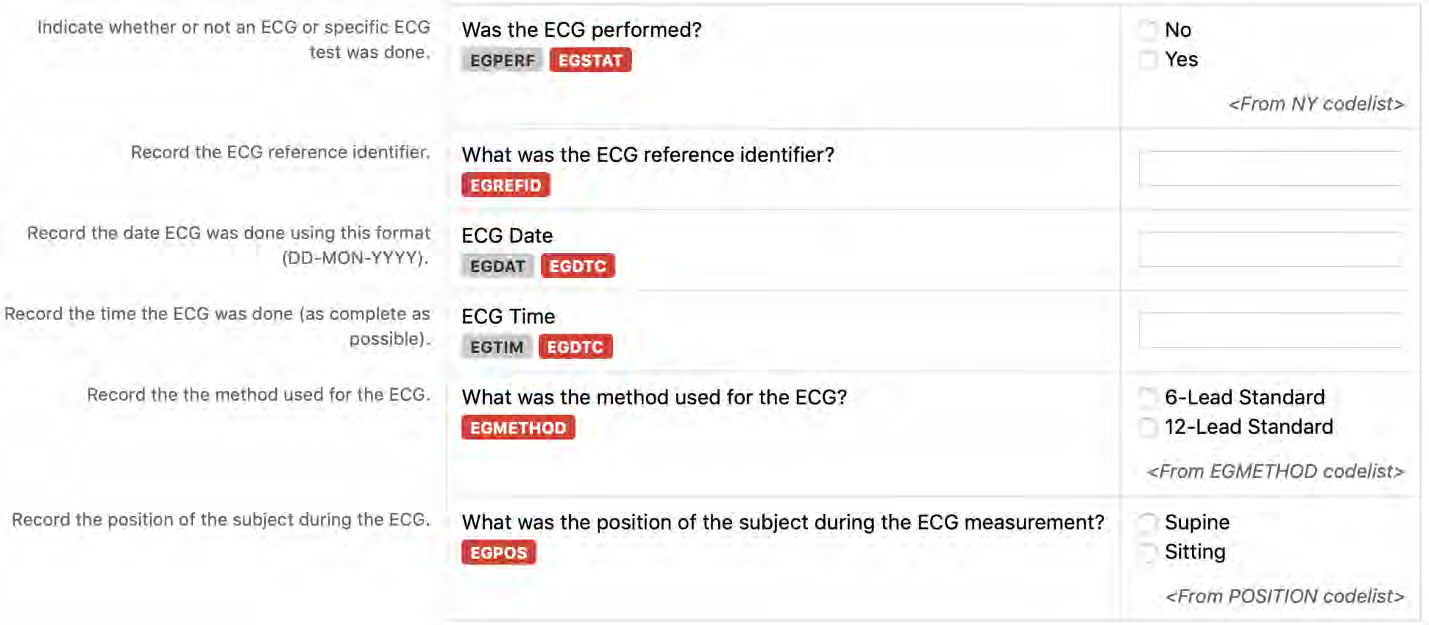
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTM Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
EGPERF | 1 | Was the ECG performed? | ECG Performed | Indicate whether or not an ECG or specific ECG test was done. | Text | EGSTAT | (NY) | No; Yes | |||||
EGREFID | 2 | What was the ECG reference identifier? | ECG Reference Identifier | Record the ECG reference identifier. | Text | EGREFID | |||||||
EGDAT | 3 | What was date of the ECG? | ECG Date | Record the date ECG was done using this format (DD-MON-YYYY). | Date | EGDTC | |||||||
EGTIM | 4 | What was the time the ECG was collected? | ECG Time | Record the time the ECG was done (as complete as possible). | Time | EGDTC | |||||||
EGMETHOD | 5 | What was the method used for the ECG? | ECG Method | Record the the method used for the ECG. | Text | EGMETHOD | (EGMETHOD) | 6-Lead Standard; 12-Lead Standard | |||||
EGPOS | 6 | What was the position of the subject during the ECG measurement? | Position | Record the position of the subject during the ECG. | Text | EGPOS | (POSITION) | Supine; Sitting |
Example 2
This example shows a CRF that collects electrocardiogram data utilizing a local reader.
Title: ECG Local Reading
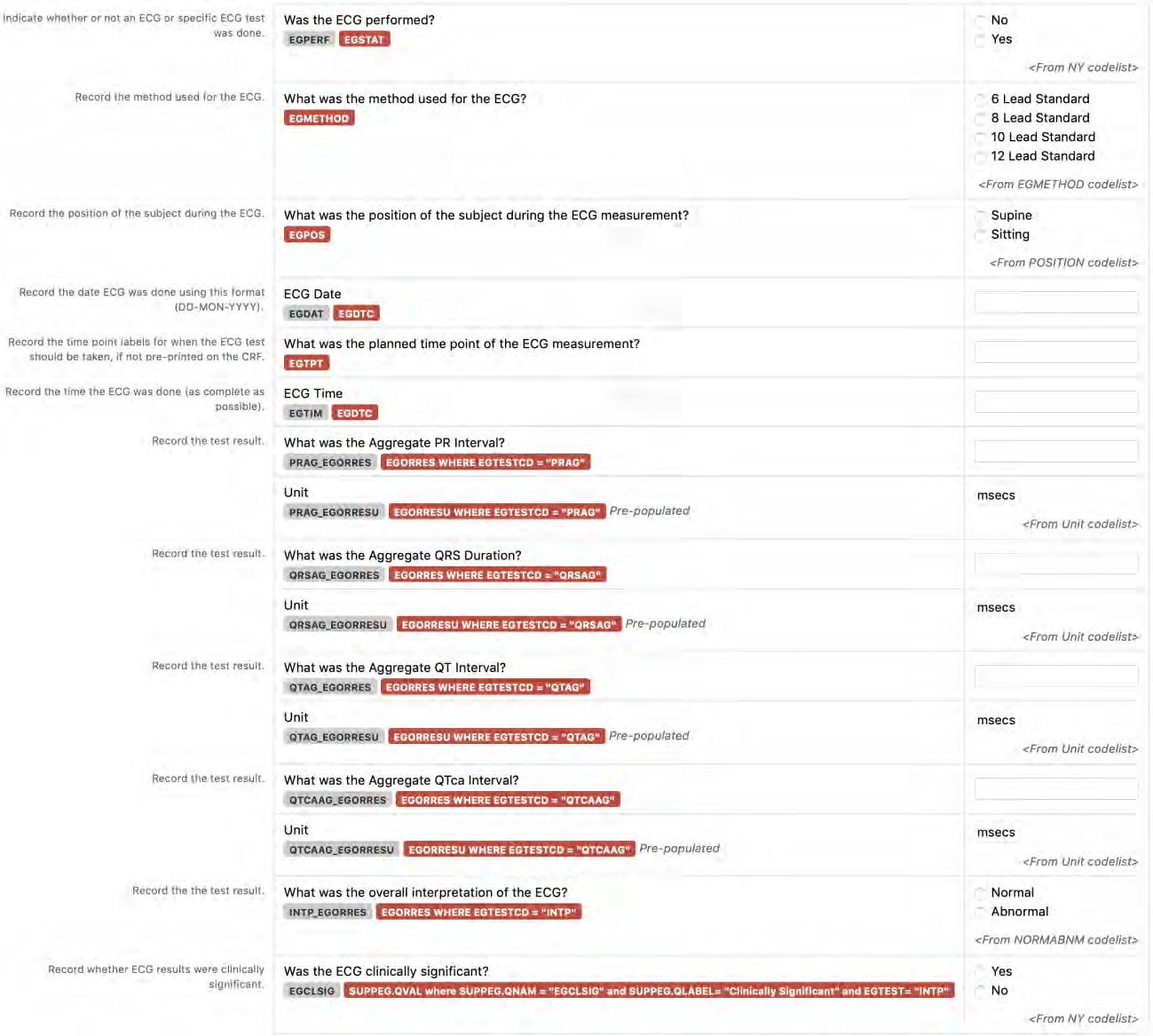
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTM Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
EGPERF | 1 | Was the ECG performed? | ECG Performed | Indicate whether or not an ECG or specific ECG test was done. | Text | EGSTAT | (NY) | No; Yes | |||||
EGMETHOD | 2 | What was the method used for the ECG? | Method | Record the method used for the ECG. | Text | EGMETHOD | (EGMETHOD) | 6 Lead Standard; 8 Lead Standard; 10 Lead Standard; 12 Lead Standard |
| ||||
EGPOS | 3 | What was the position of the subject during the ECG measurement? | Position | Record the position of the subject during the ECG. | Text | EGPOS | (POSITION) | Supine; Sitting |
| ||||
EGDAT | 4 | What was date of the ECG? | ECG Date | Record the date ECG was done using this format (DD-MON-YYYY). | Date | EGDTC |
|
| |||||
EGTPT | 5 | What was the planned time point of the ECG measurement? | [Planned Time Point Name] | Record the time point labels for when the ECG test should be taken, if not pre-printed on the CRF. | Text | EGTPT |
|
| |||||
EGTIM | 6 | What was the time the ECG was collected? | ECG Time | Record the time the ECG was done (as complete as possible). | Time | EGDTC |
|
| |||||
PRAG_EGORRES | 7 | What was the Aggregate PR Interval? | PR Interval, Aggregate | Record the test result. | Text | EGORRES | EGORRES WHERE EGTESTCD = "PRAG" |
| |||||
PRAG_EGORRESU | 8 | What was the unit of the Aggregate PR Interval? | Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | Text | EGORRES | EGORRESU WHERE EGTESTCD = "PRAG" | (Unit) | msecs | ||||
QRSAG_EGORRES | 9 | What was the Aggregate QRS Duration? | QRS Duration, Aggregate | Record the test result. | Text | EGORRES | EGORRES WHERE EGTESTCD = "QRSAG" |
| |||||
QRSAG_EGORRESU | 10 | What was the unit of the Aggregate QRS Duration? | Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | Text | EGORRES | EGORRESU WHERE EGTESTCD = "QRSAG" | (Unit) | msecs | ||||
QTAG_EGORRES | 11 | What was the Aggregate QT Interval? | QT Interval, Aggregate | Record the test result. | Text | EGORRES | EGORRES WHERE EGTESTCD = "QTAG" | ||||||
QTAG_EGORRESU | 12 | What was the unit of the Aggregate QT Interval result? | Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | Text | EGORRES | EGORRESU WHERE EGTESTCD = "QTAG" | (Unit) | msecs | ||||
QTCAAG_EGORRES | 13 | What was the Aggregate QTca Interval? | QTca Interval, Aggregate | Record the test result. | Text | EGORRES | EGORRES WHERE EGTESTCD = "QTCAAG" | ||||||
QTCAAG_EGORRESU | 14 | What was the unit of the Aggregate QTca Interval? | Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | Text | EGORRES | EGORRESU WHERE EGTESTCD = "QTCAAG" | (Unit) | msecs | ||||
INTP_EGORRES | 15 | What was the overall interpretation of the ECG? | Interpretation | Record the the test result. | Text | EGORRES | EGORRES WHERE EGTESTCD = "INTP" | (NORMABNM) | Normal; Abnormal | ||||
EGCLSIG | 16 | Was the ECG clinically significant? | Clinically Significant | Record whether ECG results were clinically significant. | Text | SUPPEG.QVAL | SUPPEG.QVAL where SUPPEG.QNAM = "EGCLSIG" and SUPPEG.QLABEL= "Clinically Significant" and EGTEST= "INTP" | (NY) | Yes;No |
Example 3
This example shows a CRF that collects electrocardiogram data which is centrally processed.
Title: ECG Central Reading with Investigator Assessment
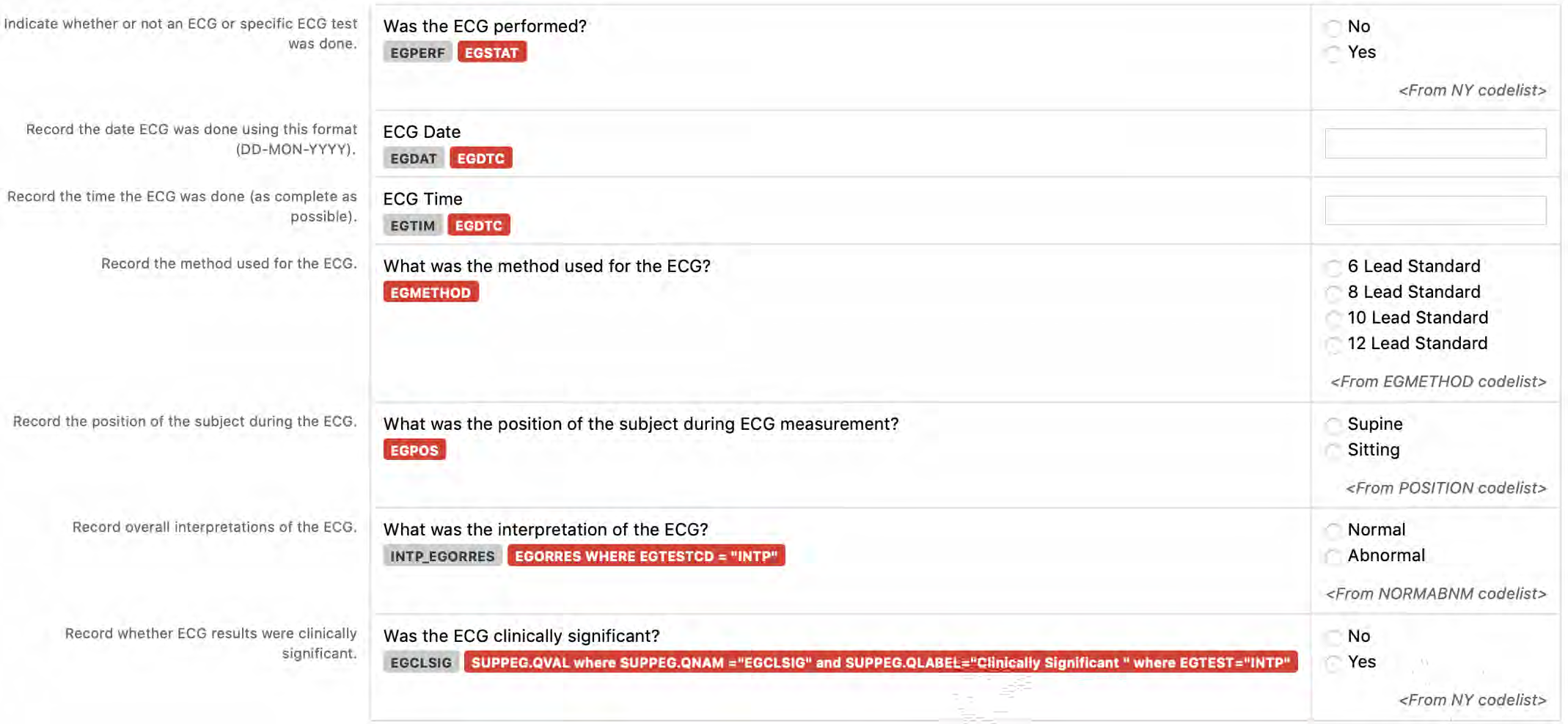
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTM Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
EGPERF | 1 | Was the ECG performed? | ECG Performed | Indicate whether or not an ECG or specific ECG test was done. | Text | EGSTAT | (NY) | No; Yes | |||||
EGMETHOD | 4 | What was the method used for the ECG? | Method | Record the method used for the ECG. | Text | EGMETHOD | (EGMETHOD) | 6 Lead Standard; 8 Lead Standard; 10 Lead Standard; 12 Lead Standard | |||||
EGPOS | 5 | What was the position of the subject during ECG measurement? | Position | Record the position of the subject during the ECG. | Text | EGPOS | (POSITION) | Supine; Sitting | |||||
EGDAT | 2 | What was the ECG date? | ECG Date | Record the date ECG was done using this format (DD- MON-YYYY). | Date | EGDTC | PROMPT | ||||||
EGTIM | 3 | What was the time the ECG was collected? | ECG Time | Record the time the ECG was done (as complete as possible). | Time | EGDTC | PROMPT | ||||||
INTP_EGORRES | 7 | What was the interpretation of the ECG? | Interpretation | Record overall interpretations of the ECG. | Text | EGORRES | EGORRES WHERE EGTESTCD = "INTP" | (NORMABNM) | Normal; Abnormal | ||||
EGCLSIG | 8 | Was the ECG clinically significant? | Clinically Significant | Record whether ECG results were clinically significant. | Text | SUPPEG.QVAL | SUPPEG.QVAL where SUPPEG.QNAM ="EGCLSIG" and SUPPEG.QLABEL="Clinically Significant " where EGTEST="INTP" | (NY) | No; Yes |
8.3.5 IE - Inclusion/Exclusion Criteria Not Met
Description/Overview for the CDASHIG IE - Inclusion/Exclusion Criteria Not Met Domain
The CDASHIG IE domain is recommended for collecting only inclusion/exclusion exceptions. In other words, those criteria that are "not met"; these are the data that are required to be in the SDTMIG IE domain. The CDASHIG IE domain is used to collect failures on or exceptions to the inclusion/exclusion criteria during the screening process before a subject is enrolled in a study. It is not intended to collect protocol deviations or violations that occur after enrollment; protocol deviations are collected using the DV domain.
The recommendation is that sites be given an entry criteria worksheet to be used for each subject to record the results of eligibility review. This worksheet should be considered a source document, used in monitoring activities, and maintained with the subject’s site files. The worksheet should identify each criterion using a unique identifier, which can be easily recorded on the CRF if a subject does not meet that criterion. If criteria lists are numbered the same for both inclusion and exclusion criteria (e.g., inclusion 001-100, exclusion 001-100), then this identifier could include a means of identifying the TYPE of criterion (e.g., I001-I100, E001-E100). Alternatively, the criteria could be collected in 2 separate sections on the CRF labeled "Inclusion" and "Exclusion" and the output records could include the values of Inclusion or Exclusion on each record. These are only examples; an organization’s numbering scheme may be different, but some method that captures both the Inclusion or Exclusion category and the unique criterion identifier should be used.
The recommended collection method has been simplified to require the site to record a single "Y/N" value in the IEYN variable to indicate whether or not the subject met all of the criteria. If any criteria are not met, the site then records only those "unmet" criteria in the CRF. The result value for each unmet criterion may then be derived in the SDTMIG IE domain from the collection of the specific criterion that was not met. In other words, if the collected criterion is an inclusion criterion that was not met, the value of "N" can be derived into IEORRES and IESTRESC for that record in the SDTMIG IE domain. If it is an exclusion criterion, then "Y" can be derived into IEORRES and IESTRESC to indicate that the subject met the conditions for that exclusion record in IE.
The rationale for the recommended collection method is that what is being collected in the IE CRF is aligned with the data that would be in the SDTMIG IE domain.
This design allows criteria to change over the life of a study or project (e.g., when adaptive trial designs are used or protocol amendments result in changes to the inclusion or exclusion criteria). If inclusion/exclusion criteria were amended during the trial, then each complete set of criteria must be included in the TI domain. TIVERS is used to distinguish between the versions of the eligibility criteria.
CDASH recommends the use of uniquely numbered entry criteria within a study to manage effectively protocol changes and to facilitate both the collection and submission of IE data. See the current SDTMIG for more details. The Inclusion/Exclusion worksheet may need to be updated and re-numbered/re-lettered whenever a protocol amendment changes one or more criteria. For example, if new versions of a criterion have not been given new numbers, separate values of IETESTCD might be created by appending letters (e.g., INCL003A, INCL003B).
A field could be added to the CRF to capture the version number of the criteria being used; this can be mapped to the SDTMIG variable TI.TIVERS. This enables the retrieval of the full text of the criterion from the code used on the CRF.
Alternatively, an implementer may choose to include the full text of each criterion (IETEST) with a result field (IEORRES) in the CRF and request the site to record explicitly the "Y" or the "N" for each criterion, but only the recommended, simplified method is presented in the IE example CRF.
Specification for the CDASHIG IE - Inclusion/Exclusion Criteria Not Met Domain
Inclusion/Exclusion Criteria Not Met Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | IE | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | IE | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | IE | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | IE | N/A | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | IE | N/A | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD- MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable IEDTC in ISO 8601 format. | N/A | N/A | The date the inclusion and exclusion assessments were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the observations at that visit or the collection date can be included on the Inclusion/Exclusion CRF using the date (IEDAT) field. |
Findings | IE | N/A | N/A | 6 | IEYN | Any Incl/Excl Criteria Findings | Indication whether the subject met all the eligibility requirements for this study at the time the subject was enrolled. | Were all eligibility criteria met? | Met Criteria | Char | HR | Record Yes if all eligibility criteria were met at the time the subject was enrolled. Record No if subject did not meet all criteria at the time the subject was enrolled. | N/A | Does not map to an SDTMIG variable. The SDTM aCRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification if all other fields on the CRF were deliberately left blank. |
Findings | IE | N/A | N/A | 7 | IEDAT | Inclusion/Exclusion Collection Date | The date of collection of the inclusion/exclusion criteria represented in an unambiguous date format. (e.g., DD-MON- YYYY). | What was the date the eligibility criteria assessment was performed? | Date | Char | O | Record complete date when the eligibility assessment was performed using this format (DD- MON-YYYY). | IEDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable IEDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and if so, a separate assessment date field is not required. |
Findings | IE | N/A | N/A | 8 | IECAT | Inclusion/Exclusion Category | A grouping category to denote whether the protocol entry criterion being assessed is Inclusion Criteria or Exclusion Criteria | What was the category of the criterion? | Criterion Type | Char | R/C | Record whether the criterion exception was Inclusion or Exclusion. Checkbox: Check Inclusion or Exclusion. | IECAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (IECAT) | N/A | These categories have been defined in SDTM and have controlled terminology that must be used. Only records for criteria that are not met appear in the IE SDTMIG domain. IECAT must be populated. This criterion category may be collected on the CRF in a checkbox format or it may be included as part of the Criterion Identification and mapped when the SDTM submission datasets are created. |
Findings | IE | N/A | N/A | 9 | IESCAT | Inclusion/Exclusion Subcategory | A sub-division of the IECAT values based on user-defined characteristics. | What was the subcategory of the criterion? | [Criterion Subtype]; NULL | Char | O | If collected on the CRF, the sponsor provides instructions to ensure the data is entered as intended. | IESCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header This can be used to distinguish criteria for a sub- study or to categorize the criteria as a major or minor exception. |
Findings | IE | N/A | N/A | 10 | IETESTCD | Inclusion/Exclusion Criterion Short Name | The unique identifier associated with the criterion that was the exception. | What was the identifier of the inclusion criterion the subject did not meet or the exclusion criterion the subject met ? | Exception Criterion Identifier | Char | HR | If the subject was not eligible, Record the identifying code for each criterion that was an exception. | IETESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This field is required to appear on the CRF, but may be null if there are no exceptions to the inclusion/exclusion criteria. The CRF should allow multiple exceptions to be recorded. See SDTMIG v3.2 Section 7.5.2 for assumptions regarding protocol versioning. Sponsors may provide a list of inclusion/ exclusion criteria and the unique identifying codes to the site. The list provided should be versioned/updated when the protocol changes and the criteria are changed. Sponsors should use sponsor- developed controlled terminology for IETESTCD. |
Findings | IE | N/A | N/A | 11 | IETEST | Inclusion/Exclusion Criterion | Descriptive name of the inclusion or exclusion criterion that was the exception. | What was the description of the inclusion criterion the subject did not meet or the exclusion criterion the subject met? | Exception Criterion Description | Char | O | Record the description of the criterion, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | IETEST | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable IETESTCD may be determined from the value collected in IETEST. The SDTMIG variables IETESTCD and IETEST are required in the SDTM submission datasets. | N/A | N/A | Sponsors could automatically populated the text in EDC systems when the Criterion Identifier is populated by the investigator. This can be verified by the PI to ensure the right Exception Identifier was selected. |
Findings | IE | N/A | N/A | 12 | IEORRES | I/E Criterion Original Result | An indication of which Inclusion criterion was not met or Exclusion criterion was met. | What is the result? | (Result) | Char | HR | If collected on the CRF, the sponsor provides instructions to ensure the data is entered as intended. | IEORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | (NY) | This is only a data collection field when a complete list of inclusion and exclusion criteria are included on the CRF with Yes/No response options. If the sponsor collects only the criteria that are not fulfilled then, when an Inclusion Criteria is not met, IEORRES is mapped to "N" and when an Exclusion Criteria is met, IEORRES is mapped to "Y". |
Assumptions for the CDASHIG IE - Inclusion/Exclusion Criteria Not Met Domain
1. The recommendation is for only those records for criteria that are not met to be collected on the IE CRF.
2. The complete list of inclusion/exclusion criteria and the version number of each of the criteria are provided in the SDTMIG TI dataset. The IETEST and IETESTCD values used to collect data on the IE CRF should match the values in the TI dataset.
3. Categories IECAT and IESCAT
a. The SDTMIG variable IECAT must be populated with INCLUSION or EXCLUSION. This criterion category may be collected on the CRF in a checkbox format using the CDASHIG field IECAT, or it may be included as part of the Criterion Identification and populated when the SDTM submission datasets are created.
b. IESCAT may be used by the sponsor to further categorize the exception criteria within the larger categories of Inclusion or Exclusion (e.g., Major, Minor).
c. These categories may be collected on the CRF, or they may be used as titles on the CRF and hidden/defaulted in the operational system. If these categories are not collected on the CRF or created in the operational data management system, they are added when the SDTM submission datasets are created.
4. There should be a unique IETESTCD for each unique criterion text in IETEST, and these values must match the values in the TI domain.
5. It may be useful to collect the protocol version under which a subject was screened.
6. The Collection Date (IEDAT) is the date that the IE data were recorded for the study, and not the actual date the exception occurred. The Visit Date (VISDAT) may be used, instead, to populate the SDTMIG IEDTC variable.
7. The Result (IEORRES)—"Y" or "N"—for each SDTMIG IETESTCD is derived or inferred from the collection of the specific criterion not met. IEORRES must be populated in the SDTMIG IE domain because it is a Required variable. Sponsors will populate this in the operational data management system or in the creation of the SDTMIG submission datasets. When an Inclusion Criterion is not met, the SDTMIG variable IEORRES is populated with "N"; when an Exclusion Criterion is not met, IEORRES is populated with "Y".
Example CRF for the CDASHIG IE - Inclusion/Exclusion Criteria Not Met Domain
Example 1
Title: Inclusion/Exclusion Criteria
Example CRF Completion Instructions
All procedures must be performed and the subject’s eligibility determined within (protocol-specified time period prior to study medication administration). (If applicable, include the following instructions) Complete the Inclusion/Exclusion Worksheet as source document by recording a “Yes” or “No” response to each criterion. Record the criterion identification that was an exception. |
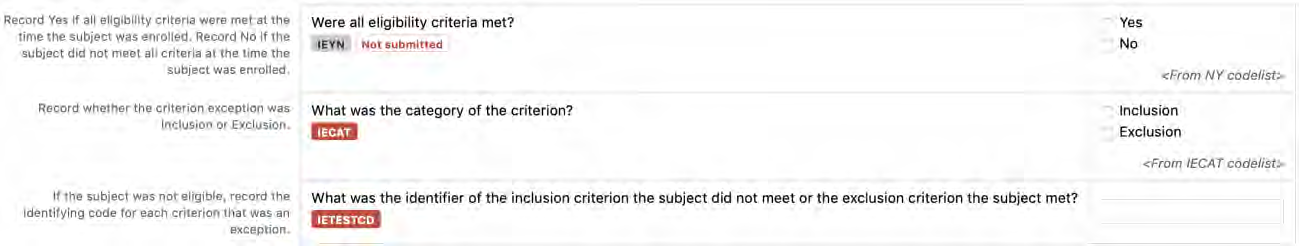
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
IEYN | 1 | Were all eligibility criteria met? | Met Criteria | Record Yes if all eligibility criteria were met at the time the subject was enrolled. Record No if the subject did not meet all criteria at the time the subject was enrolled. | Text | N/A | (NY) | Yes; No | radio | ||||
IECAT | 2 | What was the category of the criterion? | Criterion Type | Record whether the criterion exception was Inclusion or Exclusion. | Text | IECAT | (IECAT) | Inclusion; Exclusion | radio | ||||
IETESTCD | 3 | What was the identifier of the inclusion criterion the subject did not meet or the exclusion criterion the subject met? | Exception Criterion Identifier | If the subject was not eligible, record the identifying code for each criterion that was an exception. | Text | IETESTCD |
8.3.6 LB - Laboratory Test Results
Description/Overview for the CDASHIG LB - Laboratory Test Results Domain
This Findings domain includes but is not limited to hematology, clinical chemistry, and urinalysis data. The LB domain does not include microbiology or pharmacokinetic data, which are represented in separate domains. CDASH does not specify the actual lab parameters that should be collected.
This section describes 3 different data collection scenarios for laboratory test results. It is up to the sponsor to determine which data collection scenario best meets the study needs.
Scenario 1: Central Processing
In this scenario, subject specimens are taken at site and sent out for processing. Results are provided in an electronic file and the sponsor has chosen to collect reconciliation data (e.g., LBDAT, LBTIM, VISITNUM, LBREFID) on the CRF. This scenario may also apply if the central lab results are imported into the sponsor's electronic data collection (EDC) system. The fields for test results are not defined here, as these data are not part of the CRF.
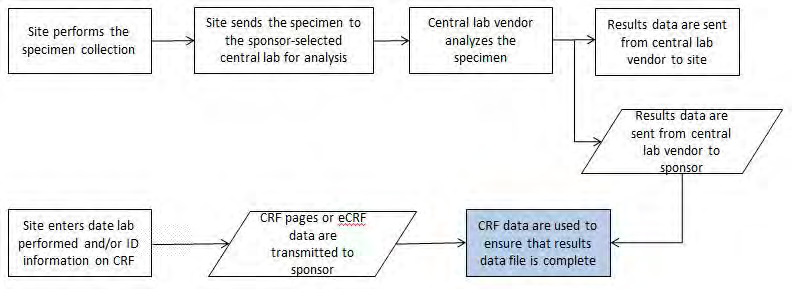
Scenario 2: Central Processing with Investigator Assessment of Clinical Significance Assessment for Abnormal Values
In this scenario, subject specimens are taken at site and sent to a central lab for processing. The results are provided in an electronic file to the sponsor. In addition, the results are provided to the investigator for assessment of clinical significance for any abnormal values and that information is provided to the sponsor on the CRF.
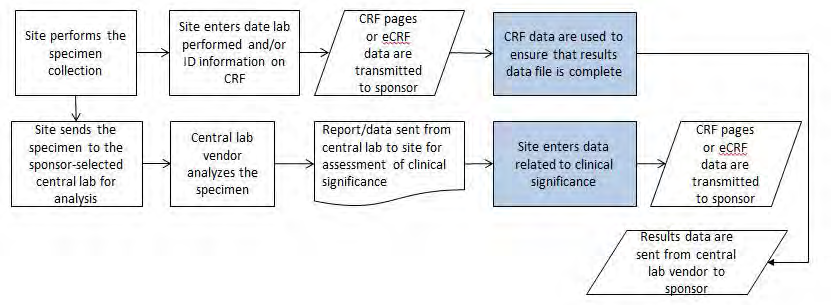
Scenario 3: Local Processing
In this scenario, subject specimens are taken and analyzed, and then the results are recorded directly on the CRF.
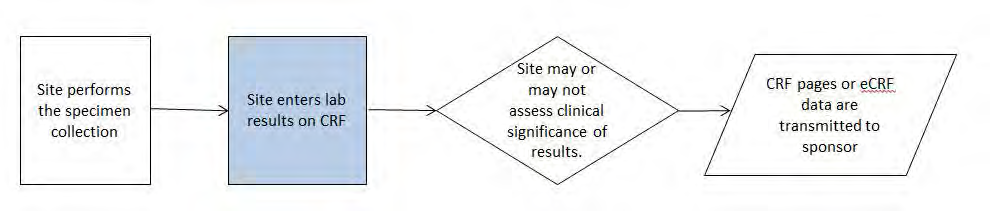
Specification for the CDASHIG LB - Laboratory Test Results Domain
Laboratory Test Results Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | LB | Central Processing | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM- based dataset creation before submission. |
Findings | LB | Central Processing | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | LB | Central Processing | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | LB | Central Processing | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | LB | Central Processing | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable LBDTC in ISO 8601 format. | N/A | N/A | The date the laboratory tests were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the laboratory tests at that visit, or the collection date can be collected on the Laboratory CRF using the date (LBDAT) field. |
Findings | LB | Central Processing | N/A | 6 | LBPERF | Lab Performed | An indication of whether or not a planned lab measurement, series of lab measurements, tests, observations or was performed or specimens collected. | Was the sample collected?; Was the lab performed? | Lab Performed; Sample Collected | Char | HR | Indicate whether or not lab specimen was collected or measurement performed. | LBSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTMIG variable LBSTAT. If the CDASH variable LBPERF = "N", the value of LBSTAT will be "NOT DONE". If LBPERF = "Y", LBSTAT should be null. A combination of SDTMIG variables ( e.g., LBCAT and LBSCAT, LBTPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable LBTESTCD would be populated as LBALL and an appropriate test name (LBTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This may be implemented for an Lab entire panel, or on a specific lab test basis. General prompt question to be used as a data management tool to verify that missing results are confirmed missing. |
Findings | LB | Central Processing | N/A | 7 | LBDAT | Specimen Collection Date | The date of specimen collection, represented in an unambiguous date format (e.g., DD-MON-YYYY). | What was the date of the lab specimen collection? | Collection Date | Char | R/C | Record the date when specimen collection was done using this format (DD- MON-YYYY). | LBDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable LBDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and if so, a separate assessment date field is not required. The SDTMIG LBDTC variable contains either a date/time when a specimen is collected at a point in time or the start date/time, when a specimen is collected over time. |
Findings | LB | Central Processing | N/A | 8 | LBTIM | Specimen Collection Time | The time of specimen collection, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the (start) time of the lab specimen collection? | [Start] Collection Time | Char | R/C | Record time of collection (as complete as possible). | LBDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable LBDTC in ISO 8601 format. | N/A | N/A | May be required when multiple assessments are done on 1 day or when the timing in relationship to study treatment is required for analysis or a specimen is collected over an extended time period. |
Findings | LB | Central Processing | N/A | 9 | LBCAT | Category for Lab Test | A grouping of topic-variable values based on user-defined characteristics. | What was the name of the lab panel? | [Lab Panel Name]; NULL | Char | R/C | Record the lab test category, if not preprinted on the CRF. | LBCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. Laboratory tests have commonly recognized categories and subcategories that should be used whenever appropriate. LBCAT and or LBSCAT should be included if lab status (NOT DONE) is collected for each lab category/subcategory (e.g., HEMATOLOGY, CHEMISTRY, URINALYSIS). |
Findings | LB | Central Processing | N/A | 10 | LBSCAT | Subcategory for Lab Test | A sub-division of the LBCAT values based on user-defined characteristics. | What was the name of the lab sub-panel? | [Lab Sub-Panel Name]; NULL | Char | R/C | Record the lab test subcategory, if not preprinted on the CRF. | LBSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology (e.g. Electrolytes, Liver Function). This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. Laboratory tests have commonly recognized categories and subcategories that should be used whenever appropriate. LBSCAT can only be used if there is an LBCAT and it must be a subcategorization of LBCAT. |
Findings | LB | Central Processing | N/A | 11 | LBTPT | Lab Planned Time Point Name | A text description of planned time point when measurements should be taken as defined in the protocol. | What was the planned time point of the lab? | [Planned Time Point Name] | Char | R/C | Record the planned time point labels for the lab test, if not preprinted on the CRF. | LBTPT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. See the SDTMIG for additional information on representing time points. SDTMIG time point anchors LBTPTREF (text description) and LBRFTDTC (date/time) may be needed, as well as SDTMIG variables LBTPTNUM, LBELTM. | N/A | N/A | Planned time points are needed to differentiate multiple sequential assessments. It is recommended that time points should be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as Planned Time Point can be included as the column header. |
Findings | LB | Central Processing | N/A | 12 | LBCOND | Lab Test Condition Met | Indication whether the testing condition defined in the protocol were met (e.g., Low fat diet). | Were the protocol-defined testing conditions met? | Test Condition Met | Char | R/C | Record whether protocol defined testing conditions were met. | SUPPLB.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPLB dataset as the value of SUPPLB.QVAL where SUPPLB.QNAM ="LBCOND" and SUPPLB.LABEL="Test Condition Met". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | This information is collected when the laboratory test results may be affected by whether conditions for testing were properly met. The specific testing conditions required should be preprinted on the CRF. This may not be relevant for all tests. |
Findings | LB | Central Processing | N/A | 13 | LBFAST | Lab Fasting Status | An indication that the subject has abstained from food/water for the specified amount of time. | Was the subject fasting? | Fasting | Char | R/C | Record whether the subject was fasting prior to the test being performed. | LBFAST | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | Results may be affected by whether the subject was fasting. This may not be relevant for all tests. |
Findings | LB | Central Processing | N/A | 14 | LBREFID | Lab Specimen ID | An internal or external identifier such as specimen identifier. | What was the (laboratory test) [reference identifier/accession number]? | (Laboratory) [Reference identifier/Accession Number] | Char | R/C | Record the specimen or accession number assigned. | LBREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | This can be used to confirm that the appropriate data record is present in the electronic transfer. May be included for linking back to specimens (e.g., Specimen ID). |
Findings | LB | Central Processing with CS | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM- based dataset creation before submission. |
Findings | LB | Central Processing with CS | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | LB | Central Processing with CS | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | LB | Central Processing with CS | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | LB | Central Processing with CS | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable LBDTC in ISO 8601 format. | N/A | N/A | The date of the laboratory tests were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the laboratory tests at that visit, or the collection date can be collected on the Laboratory CRF using the date (LBDAT) field. |
Findings | LB | Central Processing with CS | N/A | 6 | LBPERF | Lab Performed | An indication of whether or not a planned lab measurement, series of lab measurements, test, or observation was performed or specimens collected. | Was the sample collected?; Was the lab performed? | Lab Performed; Sample Collected | Char | HR | Indicate whether or not lab specimen was collected or measurement performed. | LBSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTMIG variable LBSTAT. If the CDASH variable LBPERF="N", the value of LBSTAT will be "NOT DONE". If LBPERF="Y", LBSTAT should be null. A combination of SDTMIG variables (e.g., LBCAT and LBSCAT, LBTPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable LBTESTCD would be populated as LBALL and an appropriate test name (LBTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This may be implemented for an entire lab panel, or on a specific lab test basis. This general prompt question is used as a data management tool to verify that missing results are confirmed missing. |
Findings | LB | Central Processing with CS | N/A | 7 | LBDAT | Specimen Collection Date | The date of specimen collection, represented in an unambiguous date format (e.g., DD-MON-YYYY). | What was the date of the lab specimen collection | Collection Date | Char | R/C | Record the date when the specimen collection was done using this format (DD- MON-YYYY). | LBDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable LBDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and if so, a separate assessment date field is not required. The SDTMIG LBDTC variable contains either a date/time when a specimen is collected at a point in time or the start date/time, when a specimen is collected over time. |
Findings | LB | Central Processing with CS | N/A | 8 | LBTIM | Specimen Collection Time | The time of specimen collection, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the (start) time of the lab specimen collection? | [Start] Collection Time | Char | R/C | Record time of collection (as complete as possible). | LBDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable LBDTC in ISO 8601 format. | N/A | N/A | May be required when multiple assessments are done on one day or when the timing in relationship to study treatment is required for analysis or a specimen is collected over an extended time period. |
Findings | LB | Central Processing with CS | N/A | 9 | LBCAT | Category for Lab Test | A grouping of topic-variable values based on user-defined characteristics. | What was the name of the lab panel? | [Lab Panel Name]; NULL | Char | R/C | Record the lab test category, if not preprinted on the CRF. | LBCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. Laboratory tests have commonly recognized categories and subcategories that should be used whenever appropriate. LBCAT and or LBSCAT should be included if lab status (NOT DONE) is collected for each lab category (e.g., HEMATOLOGY, CHEMISTRY, URINALYSIS). |
Findings | LB | Central Processing with CS | N/A | 10 | LBSCAT | Subcategory for Lab Test | A sub-division of the LBCAT values based on user-defined characteristics. | What was the name of the lab sub-panel? | [Lab Sub-Panel Name]; NULL | Char | R/C | Record the lab test subcategory, if not preprinted on the CRF. | LBSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology (e.g. Electrolytes, Liver Function). This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. Laboratory tests have commonly recognized categories and subcategories that should be used whenever appropriate. LBSCAT can only be used if there is an LBCAT and it must be a subcategorization of LBCAT. |
Findings | LB | Central Processing with CS | N/A | 11 | LBTPT | Lab Planned Time Point Name | A text description of planned time point when measurements should be taken as defined in the protocol. | What was the planned time point of the lab? | [Planned Time Point Name] | Char | R/C | Record the planned time point labels for the lab test, if not preprinted on the CRF. | LBTPT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. See SDTMIG for additional information on representing time points. SDTMIG time point anchors LBTPTREF (text description) and LBRFTDTC (date/time) may be needed, as well as SDTMIG variables LBTPTNUM, LBELTM. | N/A | N/A | Planned time points are needed to differentiate multiple sequential assessments. It is recommended that time points should be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as Planned Time Point can be included as the column header. |
Findings | LB | Central Processing with CS | N/A | 12 | LBCOND | Lab Test Condition Met | Indication whether the testing condition defined in the protocol were met (e.g., Low fat diet). | Were the protocol-defined testing conditions met? | Test Condition Met | Char | O | Record whether protocol defined testing conditions were met. | SUPPLB.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPLB dataset as the value of SUPPLB.QVAL where SUPPLB.QNAM ="LBCOND" and SUPPLB.LABEL="Test Condition Met". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | This information is collected when the laboratory test results may be affected by whether conditions for testing were properly met. The specific testing conditions required should be preprinted on the CRF (e.g., "Did subject meet diet requirements?"). This may not be relevant for all tests. |
Findings | LB | Central Processing with CS | N/A | 13 | LBFAST | Lab Fasting Status | An indication that the subject has abstained from food/water for the specified amount of time. | Was the subject fasting? | Fasting | Char | R/C | Record whether the subject was fasting prior to the test being performed. | LBFAST | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | Results may be affected by whether the subject was fasting. This may not be relevant for all tests. |
Findings | LB | Central Processing with CS | N/A | 14 | LBTEST | Lab Test or Examination Name | Descriptive name of the lab test or examination used to obtain the measurement or finding. Any test normally performed by a clinical laboratory is considered a lab test. | What was the lab test name? | [Laboratory Test Name] | Char | HR | Record the name of the Lab measurement or finding, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | LBTEST; LBTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable LBTESTCD may be determined from the value collected in LBTEST. The SDTMIG variables LBTESTCD and LBTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | (LBTEST) | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | LB | Central Processing with CS | N/A | 15 | LBORRES | Lab Result or Finding in Original Units | Result of the measurement or finding as originally received or collected. | What was the result of the lab test? | (Result) | Char | HR | Record laboratory test result. | LBORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Optional if already provided from central lab. |
Findings | LB | Central Processing with CS | N/A | 16 | LBORRESU | Lab Original Units | The unit of the result as originally received or collected. | What was the unit of the lab result? | Unit | Char | O | Record or select the original unit in which these data were collected. | LBORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | N/A | Optional if already provided from central lab or a sponsor stored units separately. |
Findings | LB | Central Processing with CS | N/A | 17 | LBCLSIG | Lab Clinical Significance | An indication whether lab test results were clinically significant. | Was this result clinically significant? | Clinically Significant | Char | HR | Record whether laboratory test results were clinically significant. | SUPPLB.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPLB dataset as the value of SUPPLB.QVAL where SUPPLB.QNAM ="LBCLSIG" and SUPPLB.QLABEL="Clinically Significant". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | Key data collected in this scenario. |
Findings | LB | Central Processing with CS | N/A | 18 | LBREFID | Lab Specimen ID | An internal or external identifier (e.g., specimen identifier). | What was the (laboratory test) [reference identifier/accession number]? | (Laboratory test) [Reference identifier/Accession Number] | Char | R/C | Record the specimen or accession number assigned. | LBREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | This can be used to confirm that the appropriate data record is present in the electronic transfer. May be included for linking back to specimens (e.g., Specimen ID). |
Findings | LB | Central Processing with CS | N/A | 19 | LBMETHOD | Lab Method of Test or Examination | Method of the test or examination. | What was the method used for the lab test or examination? | Method of Test or Examination | Char | O | Record the method of Test or Examination. | LBMETHOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (METHOD) | N/A | This information may be collected when more than 1 method is possible, and collecting the method used is necessary. |
Findings | LB | Local Processing | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM- based dataset creation before submission. |
Findings | LB | Local Processing | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | LB | Local Processing | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | LB | Local Processing | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | LB | Local Processing | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable LBDTC in ISO 8601 format. | N/A | N/A | The date of the laboratory tests were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the laboratory tests at that visit, or the collection date can be collected on the Laboratory CRF using the date (LBDAT) field. |
Findings | LB | Local Processing | N/A | 6 | LBPERF | Lab Performed | An indication of whether or not a planned lab measurement, series of lab measurements, test, or observation was performed or specimens collected. | Was the sample collected?; Was the lab performed? | Sample Collected; Lab Performed | Char | HR | Indicate whether or not lab specimen was collected or measurement performed. | LBSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTMIG variable LBSTAT. If the CDASH variable LBPERF = "N", the value of LBSTAT will be "NOT DONE". If LBPERF = "Y", LBSTAT should be null. A combination of SDTMIG variables (e.g., LBCAT and LBSCAT, LBTPT) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable LBTESTCD would be populated as LBALL and an appropriate test name (LBTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This may be implemented for an entire panel, or on a specific test basis. General prompt question to be used as a data management tool to verify that missing results are confirmed missing. |
Findings | LB | Local Processing | N/A | 7 | LBDAT | Specimen Collection Date | The date of specimen collection, represented in an unambiguous date format (e.g., DD-MON-YYYY). | What was the date of the lab specimen collection? | Collection Date | Char | R/C | Record the date of specimen collection using this format (DD- MON-YYYY). | LBDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable LBDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and if so, a separate assessment date field is not required. The SDTMIG LBDTC variable contains either a date/time when a specimen is collected at a point in time or the start date/time, when a specimen is collected over time. |
Findings | LB | Local Processing | N/A | 8 | LBTIM | Specimen Collection Time | The time of specimen collection, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the (start) time of the lab specimen collection? | [Start] Collection Time | Char | R/C | Record time of collection (as complete as possible). | LBDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable LBDTC in ISO 8601 format. | N/A | N/A | May be required when multiple assessments are done on one day or when the timing in relationship to study treatment is required for analysis or a specimen is collected over an extended time period. |
Findings | LB | Local Processing | N/A | 9 | LBCAT | Category for Lab Test | A grouping of topic-variable values based on user-defined characteristics. | What was the name of the lab panel? | [Lab Panel Name]; NULL | Char | R/C | Record the lab test category, if not preprinted on the CRF. | LBCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. Laboratory tests have commonly recognized categories and subcategories that should be used whenever appropriate. LBCAT and or LBSCAT should be included if lab status (NOT DONE) is collected for each lab category (e.g., HEMATOLOGY, CHEMISTRY, URINALYSIS). |
Findings | LB | Local Processing | N/A | 10 | LBSCAT | Subcategory for Lab Test | A sub-division of the LBCAT values based on user defined characteristics. | What was the name of the lab sub-panel? | [Lab Sub-Panel Name]; NULL | Char | R/C | Record the lab test subcategory, if not preprinted on the CRF. | LBSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. Laboratory tests have commonly recognized categories and subcategories that should be used whenever appropriate. LBSCAT can only be used if there is an LBCAT and it must be a subcategorization of LBCAT. |
Findings | LB | Local Processing | N/A | 11 | LBTPT | Lab Planned Time Point Name | A text description of planned time point when measurements should be taken as defined in the protocol. | What was the planned time point of the lab? | [Planned Time Point Name] | Char | R/C | Record the planned time point labels for the lab test, if not preprinted on the CRF. | LBTPT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. See SDTMIG for additional information on representing time points. SDTMIG time point anchors LBTPTREF (text description) and LBRFTDTC (date/time) may be needed, as well as SDTMIG variables LBTPTNUM, LBELTM. | N/A | N/A | Planned time points are needed to differentiate multiple sequential assessments. It is recommended that time points should be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Planned Time Point" can be included as the column header. |
Findings | LB | Local Processing | N/A | 12 | LBFAST | Lab Fasting Status | An indication that the subject has abstained from food/water for the specified amount of time. | Was the subject fasting? | Fasting | Char | R/C | Record whether the subject was fasting prior to the test being performed. | LBFAST | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | Results may be affected by whether the subject was fasting. This may not be relevant for all tests. |
Findings | LB | Local Processing | N/A | 13 | LBCOND | Lab Test Condition Met | Indication whether the testing condition defined in the protocol were met (e.g., Low fat diet). | Were the protocol-defined testing conditions met? | Test Condition Met | Char | R/C | Record whether protocol defined testing conditions were met. | SUPPLB.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPLB dataset as the value of SUPPLB.QVAL where SUPPLB.QNAM = "LBCOND" and SUPPLB.LABEL="Test Condition Met". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | This information is collected when the laboratory test results may be affected by whether conditions for testing were properly met. The specific testing conditions required should be preprinted on the CRF (e.g., "Did subject meet diet requirements?"). This may not be relevant for all tests. |
Findings | LB | Local Processing | N/A | 14 | LBSPCCND | Lab Specimen Condition | Description of the condition of the specimen. | What was the condition of the specimen? | Specimen Condition | Char | O | Record condition of specimen. | LBSPCCND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECCOND) | N/A | May be collected using free or standardized text. Results may be affected by whether conditions for specimen were properly met. When local processing is used, sponsors may not routinely collect specimen condition. |
Findings | LB | Local Processing | N/A | 15 | LBTEST | Lab Test or Examination Name | Descriptive name of the lab test or examination used to obtain the measurement or finding. Any test normally performed by a clinical laboratory is considered a lab test. | What was the lab test name? | [Laboratory Test Name] | Char | HR | Record the name of the Lab measurement or finding, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | LBTEST; LBTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable LBTESTCD may be determined from the value collected in LBTEST. The SDTMIG variables LBTESTCD and LBTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | (LBTEST) | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | LB | Local Processing | N/A | 16 | LBORRES | Lab Result or Finding in Original Units | Result of the measurement or finding as originally received or collected. | What was the result of the lab test? | (Result) | Char | HR | Record laboratory test result. | LBORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Both quantitative results and interpretive Findings or summaries may be recorded here. |
Findings | LB | Local Processing | N/A | 17 | LBMETHOD | Lab Method of Test or Examination | Method of the test or examination. | What was the method used for the lab test or examination? | Method of [Test/Examination] | Char | O | Record the method of Test or Examination. | LBMETHOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (METHOD) | N/A | This information may be collected when more than one method is possible, and collecting the method used is necessary. |
Findings | LB | Local Processing | N/A | 18 | LBORRESU | Lab Original Units | The unit of the result as originally received or collected. | What was the unit of the lab result? | Unit | Char | R/C | Record or select the original unit in which these data were collected, if not preprinted on CRF. | LBORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | N/A | Should be preprinted on the CRF with the associated test when possible, rather than collected in a field that requires the site to enter text. Should be included if applicable and not available elsewhere. For some lab tests the units may not be applicable (e.g., urine color). |
Findings | LB | Local Processing | N/A | 19 | LBCRESU | Lab Collected Non- Standard Unit | The unit of the result as originally received if it were collected as a non-standard unit. | What was the unit of the lab result? | Unit | Char | O | Record or select the original unit in which these data were collected, if not preprinted on CRF. | SUPPLB.QVAL | This does not map directly to an SDTMIG variable. The collected, non-standard unit(s) may be submitted in a supplemental qualifier dataset. | N/A | N/A | The collected, non-standard unit(s) should be reported as an equivalent standard unit in LBORRESU. |
Findings | LB | Local Processing | N/A | 20 | LBTOXGR | Lab Standard Toxicity Grade | The toxicity grade using a standard toxicity scale (e.g., NCI CTCAE). | What is the Toxicity Grade? | Toxicity Grade | Char | O | Record the Toxicity Grade. | LBTOXGR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the toxicity scale name and version used to map the terms utilizing the Define-XML external code list attributes. | N/A | N/A | This is commonly used in oncology trials but sponsors may not collect these toxicity grades on CRFs. Terminology codes lists (TOXGRV3) and (TOXGRV4) are available for use. |
Findings | LB | Local Processing | N/A | 21 | LBTOX | Lab Toxicity | A description of toxicity quantified by LBTOXGR (e.g., NCI CTCAE Short Name). | What is the description of the toxicity? | Toxicity | Char | O | Record the description of the toxicity. | LBTOX | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The sponsor is expected to provide the toxicity scale name and version used to map the terms utilizing the Define-XML external code list attributes. | N/A | N/A | This would typically be the text description quantified by LBTOXGR (e.g., HYPERCALCEMIA, HYPOCALCEMIA) |
Findings | LB | Local Processing | N/A | 22 | LBORNRLO | Lab Ref Range Lower Limit in Orig Unit | The lower end of normal range or reference range for continuous results stored in LBORRES. | What was the lower limit of the reference range for this lab test? | Normal Range Lower Limit | Char | R/C | Record the lower limit of the reference range of the lab test. | LBORNRLO | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | LBORNRLO and LBORNRHI should be populated only for continuous results; LBSTNRC should be populated only for non- continuous results. These data may be obtained from the lab or the electronic equipment. These data could be determined from a site or lab specific set of normal ranges stored in a look up table. See the SDTMIG for details on mapping and selecting the proper variable name. |
Findings | LB | Local Processing | N/A | 23 | LBORNRHI | Lab Ref Range Upper Limit in Orig Unit | The upper end of normal range or reference range for continuous results stored in LBORRES. | What was the high limit of the reference range for this lab test? | Normal Range Upper Limit | Char | R/C | Record the upper limit of the reference range of the lab test. | LBORNRHI | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | LBORNRLO and LBORNRHI should be populated only for continuous results; LBSTNRC should be populated only for non- continuous results. These data may be obtained from the lab or the electronic equipment. These data could be determined from a site or lab specific set of normal ranges stored in a look up table. See the SDTMIG for details on mapping and selecting the proper variable name. |
Findings | LB | Local Processing | N/A | 24 | LBNRIND | Lab Reference Range Indicator | An indication or description about how the value compares to the normal range or reference range. | How [did/do] the reported values compare within the [reference/normal/expected] range? | Comparison to [Reference/Expected/Normal] Range | Char | R/C | Record where the lab result fell with respect to the reference range (e.g. HIGH, LOW, ABNORMAL). | LBNRIND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NRIND) | N/A | Reference ranges may be defined by LBORNRLO and LBORNRHI or other objective criteria. Typically for local processing "Reference Range Indicator" may be derived or determined programmatically and is not collected on the CRF. Should not be used to indicate clinical significance. |
Findings | LB | Local Processing | N/A | 25 | LBCLSIG | Lab Clinical Significance | An indication whether lab test results were clinically significant. | Was this result clinically significant? | Clinically Significant | Char | O | Record whether lab results were clinically significant. | SUPPLB.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPLB dataset as the value of SUPPLB.QVAL where SUPPLB.QNAM= "LBCLSIG" and SUPPLB.QLABEL="Clinically Significant". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | May be included if required by the protocol. |
Findings | LB | Local Processing | N/A | 26 | LBNAM | Vendor Name | The name or identifier of the vendor (e.g., laboratory) that provided the test results. | What was the name of the laboratory used? | Laboratory Name | Char | R/C | Record the laboratory name. | LBNAM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Recommended to collect on the CRF if lab names was not collected at the site/study level or if multiple labs are used by a site. |
Assumptions for the CDASHIG LB - Laboratory Test Results Domain
1. The lab parameters that should be collected are not specified by CDASH, as this is a medical and scientific decision that is based on the needs of the protocol.
2. Sponsors should decide which scenario is appropriate for each protocol.
3. As required or defined by the study protocol, clinically significant results may need to be reported on the Medical History or Adverse Event CRF.
4. As required or defined by the study protocol, changes that are worsening may need to be reported on the AE CRF.
5. All pertinent laboratory normal ranges/units and laboratory certification for all laboratories used during the study will be provided to the sponsor. This is required for regulatory and database purposes.
Example CRFs for the CDASHIG LB - Laboratory Test Results Domain
Example 1
Title: Laboratory Findings Scenario 1: Central Processing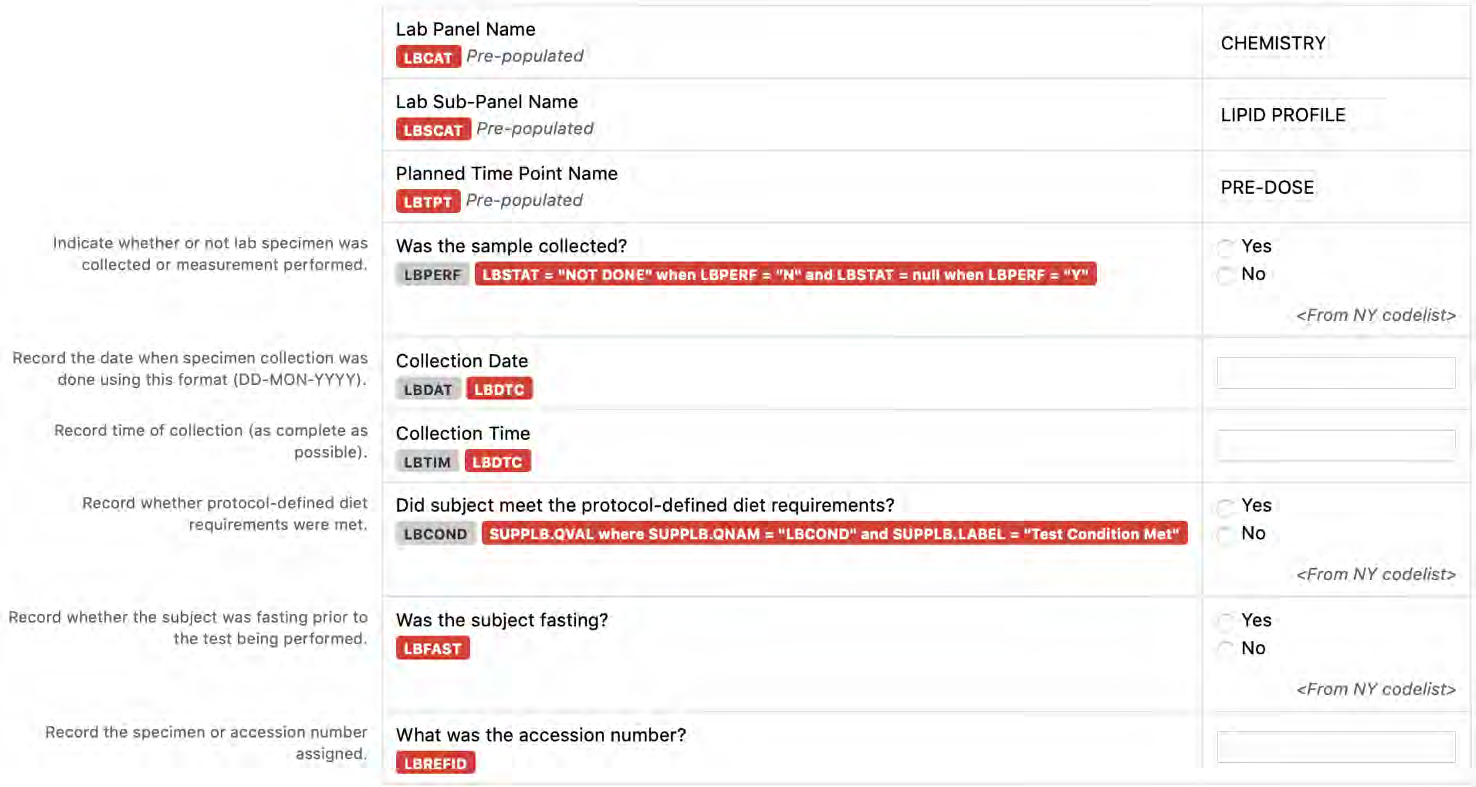
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTM Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
LBCAT | 1 | What was the name of the lab panel? | Lab Panel Name | Record the lab test category, if not pre- printed on the CRF. | Text | LBCAT | CHEMISTRY | Prompt | |||||
LBSCAT | 2 | What was name of the lab sub- panel? | Lab Sub- Panel Name | Record the lab test subcategory, if not pre- printed on the CRF. | Text | LBSCAT | LIPID PROFILE Prompt | ||||||
LBTPT | 3 | What was the planned time point of the lab? | Planned Time Point Name | Record the planned time point labels for the lab test, if not pre- printed on the CRF. | Text | LBTPT | PRE-DOSE | Prompt | |||||
LBPERF | 4 | Was the sample collected? | Sample Collected | Indicate whether or not lab specimen was collected or measurement performed. | Text | LBSTAT | LBSTAT = "NOT DONE" when LBPERF = "N" and LBSTAT = null when LBPERF = "Y" | (NY) | Yes; No | radio | |||
LBDAT | 5 | What was the date of the lab specimen collection? | Collection Date | Record the date when specimen collection was done using this format (DD-MON- YYYY). | Date | LBDTC | Prompt | ||||||
LBTIM | 6 | What was the time of the lab specimen collection? | Collection Time | Record time of collection (as complete as possible). | Time | LBDTC | Prompt | ||||||
LBCOND | 7 | Did subject meet the protocol-defined diet requirements? | Diet Requirements Met | Record whether protocol-defined diet requirements were met. | Text | SUPPLB.QVAL | SUPPLB.QVAL where SUPPLB.QNAM = "LBCOND" and SUPPLB.LABEL = "Test Condition Met" | (NY) | Yes; No | radio | |||
LBFAST | 8 | Was the subject fasting? | Fasting | Record whether the subject was fasting prior to the test being performed. | Text | LBFAST | (NY) | Yes; No | radio | ||||
LBREFID | 9 | What was the accession number? | Accession Number | Record the specimen or accession number assigned. | Text | LBREFID |
|
Example 2
This CRF is used when lab data is received electronically, and the site will assess clinical significance for any abnormal values from the lab data.
Title: Laboratory Findings Scenario 2: Central Processing with Investigator Assessment of Clinical Significance Assessment for Abnormal Values
Example CRF Completion Instructions/ For each abnormal finding on the lab report, please enter that lab test identification here and indicate whether the finding is clinically significant. |
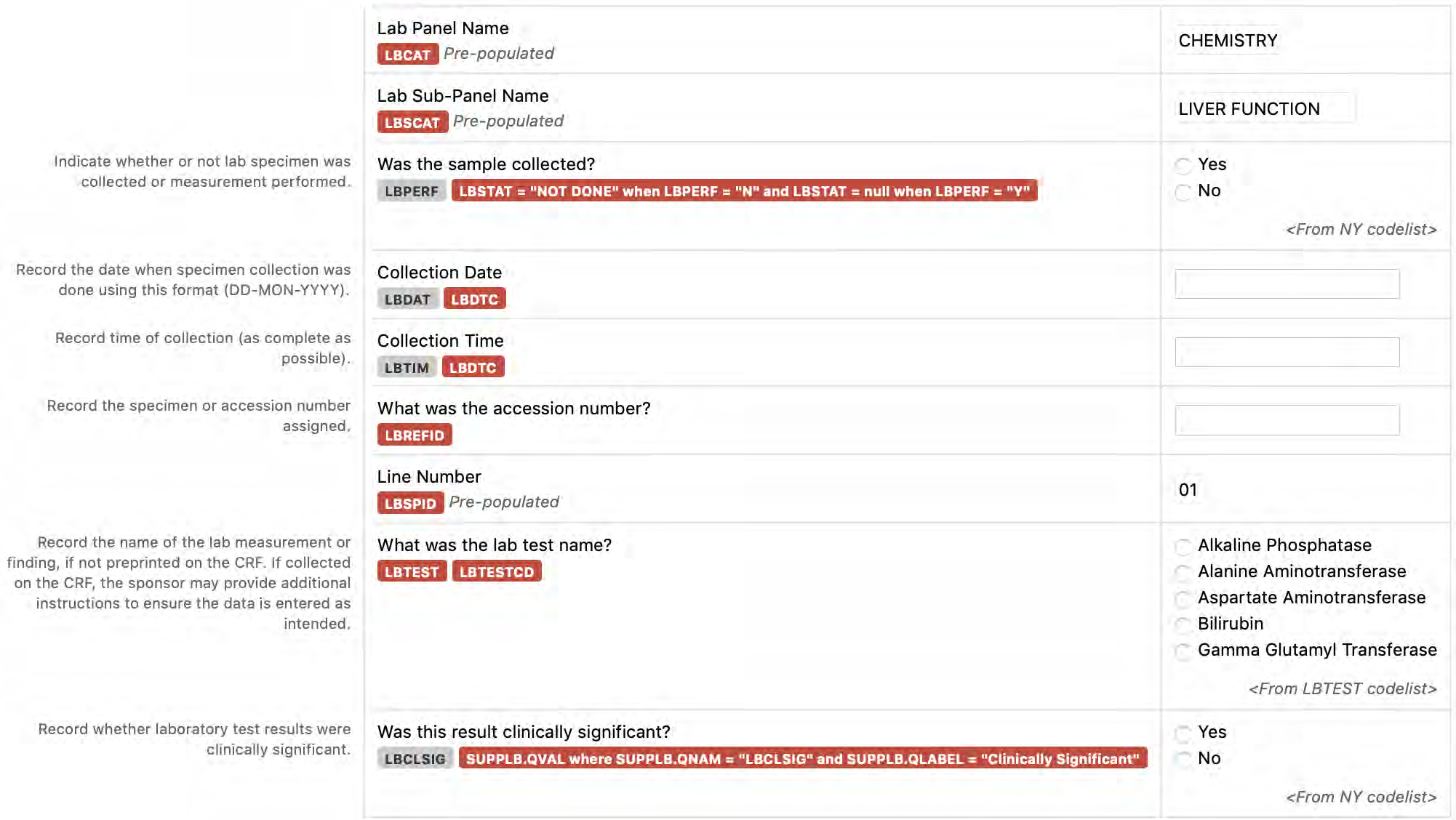
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
LBCAT | 1 | What was the name of the lab panel? | Lab Panel Name | Record the lab test category, if not pre- printed on the CRF. | Text | LBCAT | CHEMISTRY | Prompt | |||||
LBSCAT | 2 | What was name of the lab sub-panel? | Lab Sub- Panel Name | Record the lab test subcategory, if not pre-printed on the CRF. | Text | LBSCAT | LIVER FUNCTION | Prompt | |||||
LBPERF | 3 | Was the sample collected? | Sample Collected | Indicate whether or not lab specimen was collected or measurement performed. | Text | LBSTAT | LBSTAT = "NOT DONE" when LBPERF = "N" and LBSTAT = null when LBPERF = "Y" | (NY) | Yes; No | radio | |||
LBDAT | 4 | What was the date of the lab specimen collection? | Collection Date | Record the date when specimen collection was done using this format (DD-MON-YYYY). | Date | LBDTC | Prompt | ||||||
LBTIM | 5 | What was the time of the lab specimen collection? | Collection Time | Record time of collection (as complete as possible). | Time | LBDTC | Prompt | ||||||
LBREFID | 6 | What was the accession number? | Accession Number | Record the specimen or accession number assigned. | Text | LBREFID |
|
| |||||
LBSPID | 7 | What is the lab test identifier? | Line Number | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | Text | LBSPID | 01 | Prompt | |||||
LBTEST | 8 | What was the lab test name? | Lab Test Name | Record the name of the lab measurement or finding, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | Text | LBTEST; LBTESTCD | (LBTEST) | Alkaline Phosphatase; Alanine Aminotransferase; Aspartate Aminotransferase; Bilirubin; Gamma Glutamyl Transferase | radio | ||||
LBCLSIG | 9 | Was this result clinically significant? | Clinically Significant | Record whether laboratory test results were clinically significant. | Text | SUPPLB.QVAL | SUPPLB.QVAL where SUPPLB.QNAM = "LBCLSIG" and SUPPLB.QLABEL = "Clinically Significant" | (NY) | Yes; No | radio |
Example 3
Title: Laboratory Findings Scenario 3: Local Processing

CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
LBCAT | 1 | What was the name of the lab panel? | Lab Panel Name | Record the lab test category, if not pre-printed on the CRF. | Text | LBCAT | HEMATOLOGY | Prompt | |||||
LBSCAT | 2 | What was name of the lab sub-panel? | Lab Sub-Panel Name | Record the lab test subcategory, if not pre- printed on the CRF. | Text | LBSCAT | COAGULATION | Prompt | |||||
LBPERF | 3 | Was the lab performed? | Lab Performed | Indicate whether or not lab specimen was collected or measurement performed. | Text | LBSTAT | LBSTAT = "NOT DONE" when LBPERF = "N" and LBSTAT = null when LBPERF = "Y" | (NY) | Yes; No | Radio | |||
LBDAT | 4 | What was the date of the lab specimen collection? | Collection Date | Record the date of specimen collection using this format (DD-MON-YYYY). | Date | LBDTC | Prompt | ||||||
LBTIM | 5 | What was the time of the lab specimen collection? | Collection Time | Record time of collection (as complete as possible). | Time | LBDTC | Prompt | ||||||
LBSPCCND | 6 | What was the condition of the specimen? | Specimen Condition | Record condition of specimen. | Text | LBSPCCND | (SPECCOND) | FRESH; FROZEN; REFRIGERATED | Radio | ||||
LBNAM | 7 | What was the name of the laboratory used? | Laboratory Name | Record the laboratory name. | Text | LBNAM | |||||||
LBTEST | 8 | What is the lab test name? | Lab Test Name | Record the name of the lab measurement or finding, if not pre-printed on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | Text | LBTEST; LBTESTCD | LBTEST and LBTESTCD | (LBTEST) | Prothrombin Intl. Normalized Ratio; Activated Partial Thromboplastin Time; Prothrombin Time; Thrombin Time; Fibrinogen; D-Dimer; Factor V; Factor VIII | Prompt | Radio | ||
LBMETHOD | 9 | What was the method used for the lab test or examination? | Method of Test | Record the method of the test or examination. | Text | LBMETHOD | (METHOD) | CLAUSS METHOD; CLOT DETECTION; MANUAL CLOT DETECTION; MECHANICAL CLOT DETECTION; PHOTOMETRIC CLOT DETECTION | Radio | ||||
LBORRES | 10 | What was the result of the lab test? | Result | Record laboratory test result. | Text | LBORRES | Prompt | ||||||
LBORRESU | 11 | What was the unit of the lab result? | Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | Text | LBORRESU | (UNIT) | g/L; mg/dL; mg/L; U/mL; ug/L; sec; RATIO | Prompt | Radio | |||
LBORNRLO | 12 | What was the lower limit of the reference range for this lab test? | Normal Range Lower Limit | Record the lower limit of the reference range of the lab test. | Text | LBORNRLO | Prompt | ||||||
LBORNRHI | 13 | What was the high limit of the reference range for this lab test? | Normal Range Upper Limit | Record the upper limit of the reference range of the lab test. | Text | LBORNRHI | Prompt | ||||||
LBNRIND | 14 | How do the reported values compare within the reference range? | Comparison to Reference Range | Record where the lab result fell with respect to the reference range (i.e., HIGH, NORMAL, LOW). | Text | LBNRIND |
| (NRIND) | High; Normal; Low |
| Prompt | Radio |
|
LBCLSIG | 15 | Was this result clinically significant? | Clinically Significant | Record whether lab results were clinically significant. | Text | SUPPLB.QVAL | SUPPLB.QVAL where SUPPLB.QNAM = "LBCLSIG" and SUPPLB.QLABEL = "Clinically Significant" | (NY) | Yes; No | Radio | |||
LBTOXGR | 16 | What is the Toxicity Grade? | Toxicity Grade | Record the toxicity grade. | Text | LBTOXGR | 0; 1; 2; 3; 4 | Radio | |||||
LBTOX | 17 | What is the description of the toxicity? | Toxicity | Record the description of the toxicity. | Text | LBTOX |
|
8.3.7 MB - Microbiology Specimen
Description/Overview for the CDASHIG MB - Microbiology Domain
The MB (Microbiology Specimen) and MS (Microbiology Susceptibility) domains are a pair of domains used to represent microbiology findings. The MB domain is used to represent findings such as the identification, quantification, and other characterizations of microorganisms in subject samples, with the exception of drug susceptibility testing. The MB domain typically includes organisms found (e.g., non-host organisms identified including bacteria, viruses, parasites, protozoa, and fungi), gram stain results, and organism growth status. Culture characteristics may also be included (e.g., concepts such as growth/no growth, colony quantification measures, colony color, colony morphology).
Newer releases of the SDTMIG (e.g., SDTMIG v3.3) provide more information on how microbiology specimen and results are submitted. For example, the variable MBTSTDTL can be used as a further description of MBTESTCD and MBTEST (e.g., MBTSTDTL="VIRAL LOAD" when MBTESTCD represents viral genetic material such as "HCRNA"; MBTSTDTL ="QUANTIFICATION" when MBTESTCD represents any organism being quantified; MBTSTDTL="DETECTION" when MBTEST equals the name of the organism/antigen targeted by the identification assay).
The Therapeutic Area User Guides (TAUGs) for TB (Tuberculosis) and CDAD (Clostridium Difficile Associated Diarrhea) also provide MB examples that are modeled after SDTMIG v3.3. SDTMIG v3.3 provides recommendation on how to represent (1) tests that target an organism, group of organisms, or antigen for identification, (2) tests that are non-targeted identification of organisms (i.e., tests that have the ability to identify a range of organisms without specifically targeting any), and (3) tests about organisms being characterized.
Specification for the CDASHIG MB - Microbiology Specimen Domain
Microbiology Specimen Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | MB | Central Processing | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | MB | Central Processing | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted for the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | MB | Central Processing | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | MB | Central Processing | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | MB | Central Processing | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable MBDTC in ISO 8601 format. | N/A | N/A | The date that the Microbiology Findings were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the Microbiology Findings at that visit, or the collection date can be included on the Microbiology Findings CRF using the date (MBDAT) field. |
Findings | MB | Central Processing | N/A | 6 | MBPERF | Microbiology Sampling Performed | An indication of whether or not a planned measurement, series of measurements, tests or observations was performed or specimen was collected. | Was [specimen type] collected or [test topic] performed? | [Specimen/Sample] Collected; [Test topic] Performed | Char | O | Indicate whether or not microbiology specimen was collected | MBSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTM variable MBSTAT. If MBPERF = "N", the value of MBSTAT will be "NOT DONE". If MBPERF = "Y", MBSTAT should be null. A combination of SDTMIG variables (e.g., MBCAT and MBSCAT, MBTPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable MBTESTCD would be populated as MBALL and an appropriate test name (MBTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This may be implemented for an entire panel. General prompt question to be used as a data management tool to verify that missing results are confirmed missing. For the SDTM submission dataset, the SDTMIG variable MBSTAT is populated using the CDASH field MBPERF. The question text used might be reflected in the reason not done (MBREASND). |
Findings | MB | Central Processing | N/A | 7 | MBREFID | MB Reference ID | An internal or external identifier such as specimen identifier. | What was the (microbiology test) [reference identifier/accession number]? | (Microbiology Test) [Reference Identifier/ Accession Number] | Char | O | Record the specimen [accession/reference] number assigned. | MBREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | This can be used to confirm that the appropriate data record is present in the electronic transfer. May be included for linking back to specimens (e.g., Specimen ID). |
Findings | MB | Central Processing | N/A | 8 | MBDAT | MB Specimen Collection Date | The date of specimen collection ,represented in an unambiguous date format (e.g., DD- MON-YYYY). | What was the (start) date of the (microbiology) specimen collection? | Collection Date | Char | R/C | Record the date when specimen collection occurred using this format (DD-MON- YYYY). | MBDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable MBDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and, if so, a separate assessment date field is not required. |
Findings | MB | Central Processing | N/A | 9 | MBTIM | MB Specimen Collection Time | The time of specimen collection, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the (start) time of the (microbiology) specimen collection? | Collection Time | Char | R/C | Record time of collection (as complete as possible). | MBDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable MBDTC in ISO 8601 format. | N/A | N/A | May be required when multiple assessments are done on one day or when the timing in relationship to study treatment is required for analysis. |
Findings | MB | Central Processing | N/A | 10 | MBCAT | MB Category for Microbiology Finding | A grouping of topic- variable values based on user-defined characteristics. | What was the category of the microbiology finding? | [Microbiology Category]; NULL | Char | O | Record the microbiology finding category, if not preprinted on the CRF. | MBCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | MB | Central Processing | N/A | 11 | MBSCAT | MB Subcategory for Microbiology Finding | A sub-division of the MBCAT values based on user-defined characteristics. | What was the subcategory of the microbiology finding? | [Microbiology Subcategory]; NULL | Char | O | Record the microbiology finding subcategory, if not preprinted on the CRF. | MBSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. MBSCAT can only be used if there is an MBCAT and it must be a subcategorization of MBCAT. |
Findings | MB | Central Processing | N/A | 12 | MBSPEC | MB Specimen Type | Defines the type of specimen used for a measurement. | What is the specimen material type? | Specimen Type | Char | R/C | Record the specimen material type. | MBSPEC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECTYPE) | N/A | The type of specimen used for a measure. Should be collected if not available elsewhere, or if required to differentiate multiple specimens. |
Findings | MB | Central Processing | N/A | 13 | MBSPCCND | MB Specimen Condition | Description of the condition of the specimen. | What was the condition of the specimen? | Specimen Condition | Char | R/C | Record condition of specimen. | MBSPCCND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECCOND) | N/A | May be collected using free or standardized text. This can be used when results may be affected by whether conditions for specimen were properly met. |
Findings | MB | Central Processing | N/A | 14 | MBLOC | MB Specimen Collection Location | A description of the anatomical location of the subject relevant to the collection of specimen. | What was the anatomical location where the specimen was collected? | Anatomical Location | Char | O | Record or select the anatomical location of specimen collection. | MBLOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. LAT, DIR, PORTOT are used to further describe the anatomical location. |
Findings | MB | Central Processing | N/A | 15 | MBLAT | MB Specimen Collection Laterality | Qualifier for anatomical location further detailing the side of the body. | What was the side of the anatomical location of the specimen collection? | Side | Char | O | Record the side of the anatomical location of the specimen collection. | MBLAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | MB | Central Processing | N/A | 16 | MBDIR | MB Specimen Collection Directionality | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What was the directionality of the anatomical location of the specimen collection? | Directionality | Char | O | Record the directionality of the anatomical location of the specimen collection. | MBDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | MB | Local Processing | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | MB | Local Processing | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | MB | Local Processing | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | MB | Local Processing | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | MB | Local Processing | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable MBDTC in ISO 8601 format. | N/A | N/A | The date that the Microbiology Specimen was collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the Microbiology Findings at that visit, or the collection date can be included on the Microbiology Findings CRF using the date (MBDAT) field. |
Findings | MB | Local Processing | N/A | 6 | MBPERF | Microbiology Sampling Performed | An indication of whether or not a planned measurement, series of measurements, test, or observation was performed or specimen was collected. | Was the microbiology examination performed? | Microbiology Examination Performed | Char | O | Indicate whether or not microbiology examination performed. | MBSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTM variable MBSTAT. If MBPERF="N", the value of MBSTAT will be "NOT DONE". If MBPERF="Y", MBSTAT should be null. A combination of SDTMIG variables (e.g., MBCAT and MBSCAT, MBTPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable MBTESTCD would be populated as MBALL and an appropriate test name (MBTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This may be implemented for an entire panel. General prompt question to be used as a data management tool to verify that missing results are confirmed missing. |
Findings | MB | Local Processing | N/A | 7 | MBREFID | MB Reference ID | An internal or external identifier such as specimen identifier. | What was the (microbiology test) [reference identifier/accession number]? | (Microbiology Test) [Reference identifier/ Accession Number] | Char | O | Record the specimen [accession/reference] number assigned. | MBREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | This can be used to reconcile CRF data. May be included for linking back to specimens (e.g., Specimen ID). |
Findings | MB | Local Processing | N/A | 8 | MBSPID | MB Sponsor- Defined Identifier | A sponsor-defined identifier. In CDASH, This is typically used for preprinted or auto- generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor-defined question] | [Sponsor defined] | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | MBSPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Because SPID is a sponsor- defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile data. May be used to record preprinted number (e.g., line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Findings | MB | Local Processing | N/A | 9 | MBGRPID | MB Group ID | A group identifier used to link together a block of related records within a subject in a domain. | What [is/was] the [test/procedure/observation] group identifier? | [Test/Procedure/ Observation] Group Identifier | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each microbiology specimen or result record has a unique identifier that will link records across domains. | MBGRPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | SDTMIG v3.2 uses --GRPID as the variable that links the Microbiology Specimen/Result domain (MB) with the Microbiology Susceptibility domain (MS). In RELREC, this one-to-many relationship may be represented with a simple 2-record dataset-to-dataset RELREC. |
Findings | MB | Local Processing | N/A | 10 | MBLNKID | MB Link ID | An identifier used to link related records across domains. | What [is/was] the [test/procedure/observation] link identifier? | [Domain/Observation] Link Identifier | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each microbiology specimen or result record has a unique identifier that will link records across domains. | MBLNKID | Maps directly to the SDTM variable listed in the column with the heading "SDTM Target". May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | This may be a one-to-one or a one-to-many relationship. For example, a single microbiology specimen or result may have multiple measurements/assessments performed (e.g., susceptibility testing). |
Findings | MB | Local Processing | N/A | 11 | MBDAT | MB Specimen Collection Date | The date of specimen collection, represented in an unambiguous date format (e.g., DD- MON-YYYY). | What was the (start) date of the (microbiology) specimen collection? | Collection Date | Char | R/C | Record the date of specimen collection using this format (DD- MON-YYYY). | MBDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable MBDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and, if so, a separate assessment date field is not required. |
Findings | MB | Local Processing | N/A | 12 | MBTIM | MB Specimen Collection Time | The time of specimen collection, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the (start) time of the (microbiology) specimen collection? | Collection Time | Char | R/C | Record time of collection (as complete as possible). | MBDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable MBDTC in ISO 8601 format. | N/A | N/A | May be required when multiple assessments are done on one day or when the timing in relationship to study treatment is required for analysis. |
Findings | MB | Local Processing | N/A | 13 | MBCAT | MB Category for Microbiology Finding | A grouping of topic- variable values based on user-defined characteristics. | What was the category of the Microbiology finding? | [Microbiology Category]; NULL | Char | O | Record the microscopic finding category, if not preprinted on the CRF. | MBCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | MB | Local Processing | N/A | 14 | MBSCAT | MB Subcategory for Microbiology Finding | A sub-division of the MBCAT values based on user-defined characteristics. | What was the subcategory of the Microbiology finding? | [Microbiology Subcategory]; NULL | Char | O | Record the microscopic finding subcategory, if not preprinted on the CRF. | MBSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. MBSCAT can only be used if there is an MBCAT and it must be a subcategorization of MBCAT. |
Findings | MB | Local Processing | N/A | 15 | MBTEST | Microbiology Test or Finding Name | Descriptive name of the Microbiology test or examination used to obtain the measurement or finding. | What was the Microbiology examination test name? | [Microbiology Test Name] | Char | HR | Record the type or name of the microscopic examination, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | MBTEST; MBTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable MBTESTCD may be determined from the value collected in MBTEST. Both MBTESTCD and MBTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | (MBTEST) | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | MB | Local Processing | N/A | 16 | MBTSTDTL | Microbiology Examination Detail | Detail of the Microbiology examination used to obtain the measurement or finding. | What was the Microbiology examination detail? | [Examination Name Detail] | Char | O | Record the detail of the microbiology examination, if not preprinted on the CRF. | MBTSTDTL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Provides additional details for the microbiology examination. It is recommended that the test detail be preprinted on the CRF. If the form is laid out as a grid, then words such as "Test Detail" can be included as the column header. |
Findings | MB | Local Processing | N/A | 17 | MBORRES | MB Result or Finding in Original Units | Result of the measurement or finding as originally received or collected. | What was the result of the examination? | (Result) | Char | HR | Record test result, interpretation or finding. | MBORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Both quantitative results and interpretive findings or summaries may be recorded here. |
Findings | MB | Local Processing | N/A | 18 | MBORRESU | MB Original Units | The unit of the result as originally received or collected. | What was the unit of the result? | Unit | Char | R/C | Record or select the original unit in which these data were collected, if not preprinted on CRF. | MBORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | N/A | Should be preprinted on the CRF with the associated test when possible, rather than collected in a field that requires the site to enter text. Should be included if applicable and not available elsewhere. For some tests the units may not be applicable (e.g., where MBTEST="Microbial Organism Identification"). |
Findings | MB | Local Processing | N/A | 19 | MBCLSIG | MB Clinical Significance | An indication whether the test results were clinically significant. | Was the result clinically significant? | Clinically Significant | Char | O | Indicate whether the results were clinically significant. | SUPPMB.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPMB dataset as the value of SUPPMB.QVAL where SUPPMB.QNAM = "MBCLSIG" and SUPPMB.QLABEL="Clinically Significant". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | N/A |
Findings | MB | Local Processing | N/A | 20 | MBRESCAT | MB Result Category | Used to categorize the result of a finding. | What was the result category? | Result Category | Char | O | Record the category of the test results. | MBRESCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Used to categorize the results of a finding (e.g., INFECTING, COLONIZER). |
Findings | MB | Local Processing | N/A | 21 | MBNAM | MB Vendor Name | The name or identifier of the vendor (e.g., laboratory) that provided the test results. | What was the name of the vendor used? | Vendor Name | Char | O | Record the laboratory name. | MBNAM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Recommended to collect on the CRF if lab names was not collected at the site/study level or if multiple labs are used by a site. |
Findings | MB | Local Processing | N/A | 22 | MBSPEC | MB Specimen Type | The type of specimen used for a measurement. | What is the specimen material type? | Specimen Type | Char | O | Record the specimen material type. | MBSPEC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECTYPE) | N/A | The type of specimen used for a measure. Should be collected if not available elsewhere, or if required to differentiate multiple specimens. |
Findings | MB | Local Processing | N/A | 23 | MBSPCCND | MB Specimen Condition | Describes the condition of the specimen. | What was the condition of the specimen? | Specimen Condition | Char | O | Record condition of specimen. | MBSPCCND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECCOND) | N/A | May be collected using free or standardized text. This can be used when results may be affected by whether conditions for specimen were properly met. |
Findings | MB | Local Processing | N/A | 24 | MBLOC | MB Specimen Collection Location | A description of the anatomical location of the subject relevant to the collection of specimen. | What was the anatomical location where the specimen was collected? | Anatomical Location | Char | O | Record or select the anatomical location of specimen collection. | MBLOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location. Sponsors may collect the data using a subset list of Controlled Terminology on the CRF. LAT, DIR, and PORTOT are used to further describe the anatomical location. |
Findings | MB | Local Processing | N/A | 25 | MBLAT | MB Specimen Collection Laterality | Qualifier for anatomical location further detailing the side of the body. | What was the side of the anatomical location of the specimen collection? | Side | Char | O | Record the side of the anatomical location of the specimen collection. | MBLAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | MB | Local Processing | N/A | 26 | MBDIR | MB Specimen Collection Directionality | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What was the directionality of the anatomical location of the specimen collection? | Directionality | Char | O | Record the directionality of the anatomical location of the specimen collection. | MBDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | MB | Local Processing | N/A | 27 | MBMETHOD | MB Method of Test or Examination | Method of the test or examination. | What was the method used for the test or examination? | Method | Char | O | Record the method of test or examination. | MBMETHOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (METHOD) | N/A | This information may be collected when more than one method is possible, and collecting the method used is necessary. This could include technique or type of staining used for the slides. |
Findings | MB | Local Processing | N/A | 28 | MBEVAL | MB Evaluator | The role of the person who provided the evaluation. | Who was the evaluator? | [Evaluator/Reporter] | Char | O | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | MBEVAL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EVAL) | N/A | Used only for results that are subjective (e.g., assigned by a person or a group). May be a preprinted, or collected. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Assumptions for the CDASHIG MB - Microbiology Specimen Domain
1. The MB domain is used to represent identification, quantification, and other characterizations of microorganisms in subject samples. Culture characteristics such as growth/no growth, colony quantification measures, colony color, and colony morphology are also represented in this domain.
2. Drug susceptibility testing is not represented in the MB domain.
3. Data about actions taken that affect or may affect a specimen, such as specimen collection, freezing and thawing, aliquoting, and transportation are not included in this domain, but are included in the BE (Biospecimen Events) domain. See BE domain assumptions in the SDTMIG for Pharmacogenomic/Genetics (SDTMIG-PGx).
4. MBDTC represents the date the specimen was collected.
Example CRFs for the CDASHIG MB - Microbiology Specimen Domain
Example 1
Title: Acute Respiratory Infection-Pathogen Testing CRF
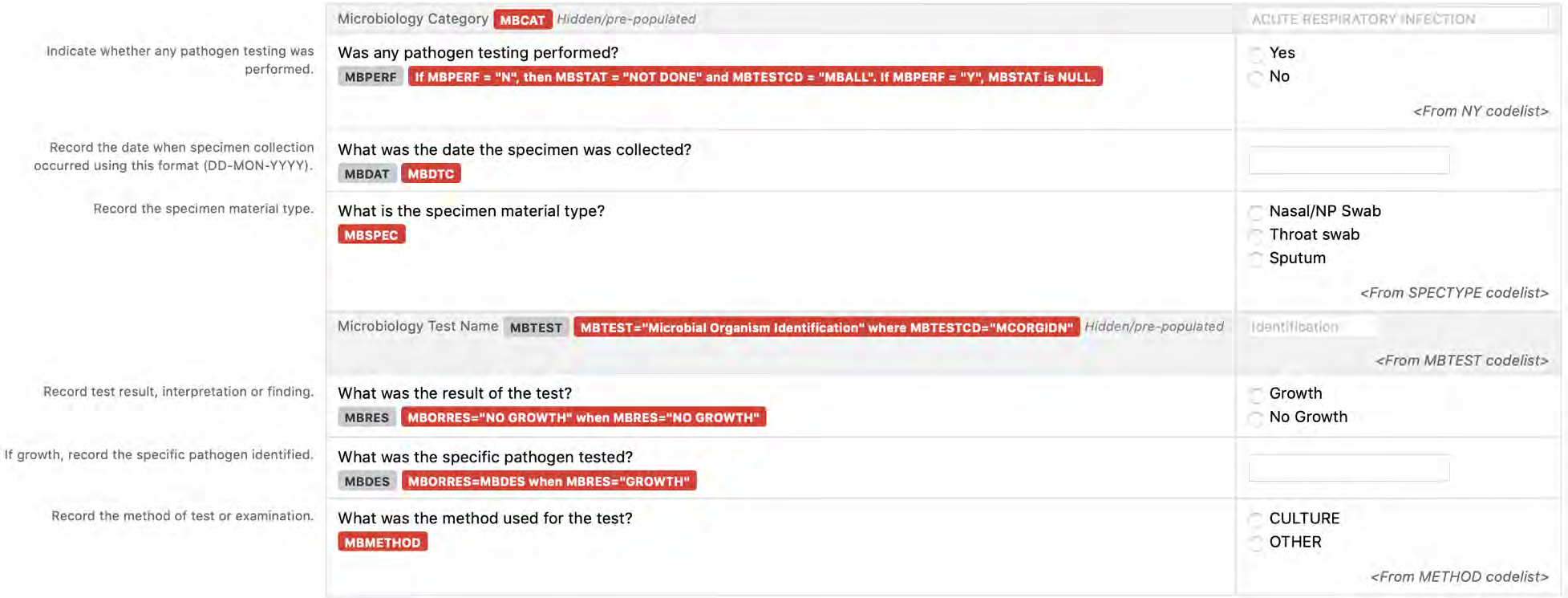
CRF Metadata
| Order Number | CDASH Variable | Question Text | Prompt | Type | CRF Completion Instructions | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Codelist Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | MBCAT | What was the category of the microbiology finding? | Microbiology Category | Text | Record the microbiology finding category, if not pre-printed on the CRF. | MBCAT | N/A | ACUTE RESPIRATORY INFECTION | Y | ||||
2 | MBPERF | Was any pathogen testing performed? | Pathogen Testing Performed | Text | Indicate whether any pathogen testing was performed. | MBSTAT | If MBPERF = "N", then MBSTAT = "NOT DONE" and MBTESTCD = "MBALL". If MBPERF = "Y", MBSTAT is NULL. | (NY) | Yes;No | ||||
3 | MBDAT | What was the date the specimen was collected? | Collection Date | Text | Record the date when specimen collection occurred using this format (DD- MON-YYYY). | MBDTC | N/A | ||||||
4 | MBSPEC | What is the specimen material type? | Specimen Type | Text | Record the specimen material type. | MBSPEC | (SPECTYPE) | Nasal/NP Swab;Throat swab;Sputum | |||||
5 | MBTEST | What was the Microbiology examination test name? | Microbiology Test Name | Text | Record the type or name of the microscopic examination, if not pre-printed on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | MBTEST; MBTESTCD | MBTEST="Microbial Organism Identification" where MBTESTCD="MCORGIDN" | (MBTEST) | Identification | Y | |||
6 | MBRES | What was the result of the test? | Result | Text | Record test result, interpretation or finding. | MBORRES | MBORRES="NO GROWTH" when MBRES="NO GROWTH" | N/A | Growth;No Growth | ||||
7 | MBDES | What was the specific pathogen tested? | Pathogen Tested | Text | If growth, record the specific pathogen identified. | MBORRES | MBORRES=MBDES when MBRES="GROWTH" | N/A | |||||
8 | MBMETHOD | What was the method used for the test? | Method | Text | Record the method of test or examination. | MBMETHOD | (METHOD) | CULTURE;OTHER |
Example 2
Title: Tuberculosis Pathogen Testing
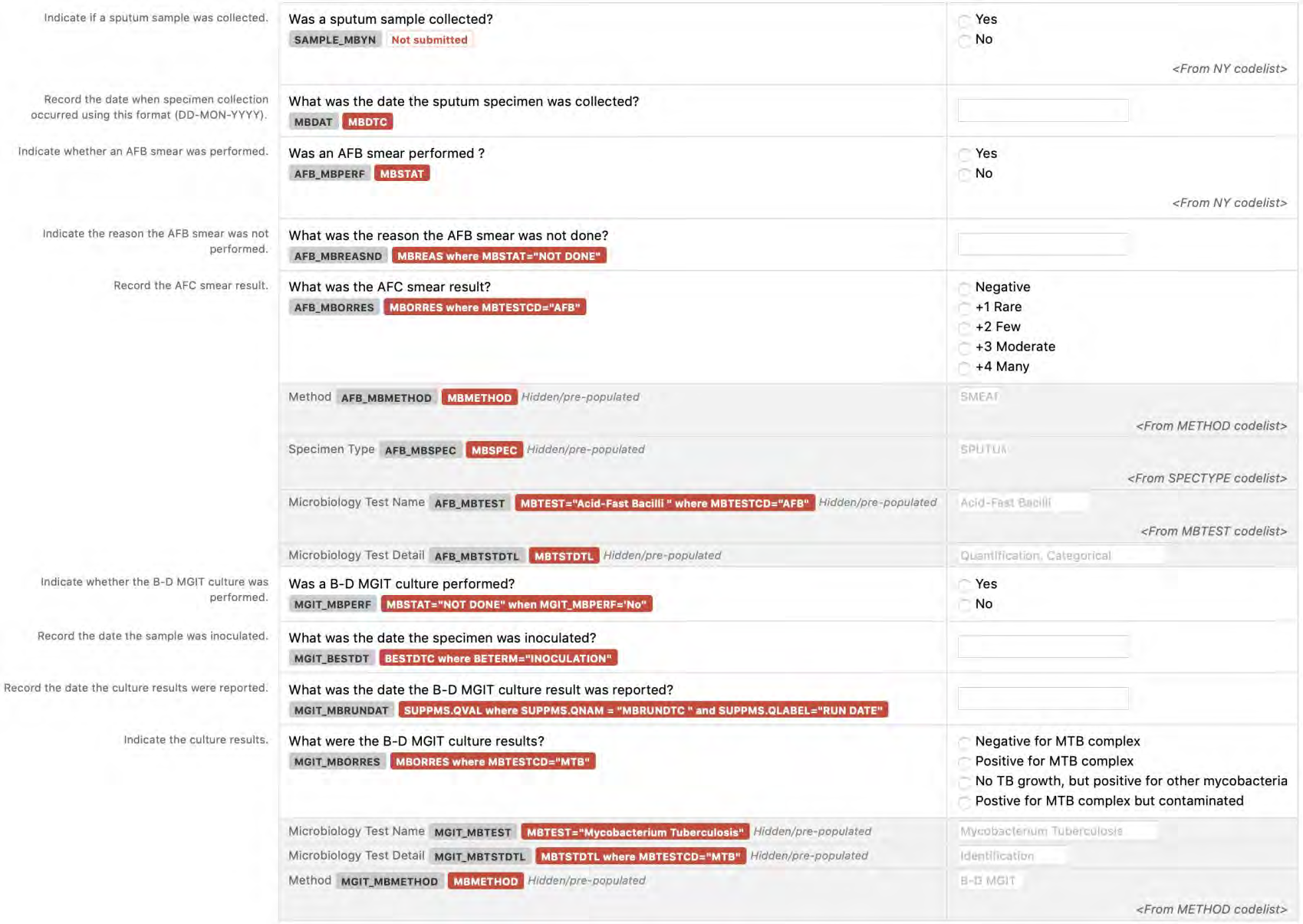
CRF Metadata
| Order Number | CDASHIG Variable | Question Text | Prompt | Type | CRF Completion Instructions | SDTMIG Target | SDTMIG Variable Mapping | Controlled Terminology Codelist Name | CRF Implementation Notes | Permissible Values | Pre- specified Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | SAMPLE_MBYN | Was a sputum sample collected? | Sample Collected | Text | Indicate if a sputum sample was collected. | (NY) | Yes;No | |||||||
2 | MBDAT | What was the date the sputum specimen was collected? | Collection Date | Text | Record the date when specimen collection occurred using this format (DD-MON-YYYY). | MBDTC | N/A | |||||||
3 | AFB_MBPERF | Was an AFB smear performed ? | AFB Smear | Text | Indicate whether an AFB smear was performed. | MBSTAT | (NY) | Yes;No | ||||||
5 | AFB_MBREASND | What was the reason the AFB smear was not done? | Reason | Text | Indicate the reason the AFB smear was not performed. | MBREAS | MBREAS where MBSTAT="NOT DONE" | |||||||
6 | AFB_MBORRES | What was the AFC smear result? | Smear Result | Text | Record the AFC smear result. | MBORRES | MBORRES where MBTESTCD="AFB" | N/A | Negative; +1 Rare; +2 Few; +3 Moderate; +4 Many |
| ||||
4 | AFB_MBMETHOD | What was the method? | Method | Text | Indicate the method used for the smear. | MBMETHOD | (METHOD) | SMEAR | Y | |||||
6 | AFB_MBSPEC | What is the specimen material type? | Specimen Type | Text | Record the specimen material type. | MBSPEC | (SPECTYPE) | SPUTUM | Y | |||||
7 | AFB_MBTEST | What was the Microbiology examination test name? | Microbiology Test Name | Text | Record the type or name of the microscopic examination, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | MBTEST; MBTESTCD | MBTEST="Acid-Fast Bacilli" where MBTESTCD="AFB" | (MBTEST) | Acid-Fast Bacilli | Y | ||||
AFB_MBTSTDTL | What was the test detail? | Microbiology Test Detail | Text | N/A | MBTSTDTL | Quantification, Categorical | Y | |||||||
7 | MGIT_MBPERF | Was a B-D MGIT culture performed? | B-D MGIT Performed | Text | Indicate whether the B-D MGIT culture was performed. | MBSTAT | MBSTAT="NOT DONE" when MGIT_MBPERF='No" | Yes;No | ||||||
8 | MGIT_BESTDT | What was the date the specimen was inoculated? | Innoulation Date | Text | Record the date the sample was inoculated. | BESTDTC where BETERM="INOCULATION" |
| |||||||
MGIT_MBRUNDAT | What was the date the B-D MGIT culture result was reported? | Culture Result Date | Text | Record the date the culture results were reported. | SUPPMB.QVAL | SUPPMS.QVAL where SUPPMS.QNAM = "MBRUNDTC " and SUPPMS.QLABEL="RUN DATE" |
| |||||||
| MGIT_MBORRES | What were the B-D MGIT culture results? | Result | Text | Indicate the culture results. | MBORRES | MBORRES where MBTESTCD="MTB" | Negative for MTB complex;Positive for MTB complex;No TB growth, but positive for other mycobacteria;Postive for MTB complex but contaminated | ||||||
7 | MGIT_MBTEST | What was the Microbiology examination test name? | Microbiology Test Name | Text | Record test result, interpretation or finding. | MBTEST;MBTESTCD; | MBTEST="Mycobacterium Tuberculosis" | N/A | Mycobacterium Tuberculosis | Y | ||||
| MGIT_MBTSTDTL | What was the test detail? | Microbiology Test Detail | Text | N/A | MBTSTDTL | MBTSTDTL where MBTESTCD="MTB" | Identification | Y | |||||
8 | MGIT_MBMETHOD | What was the method used for the test? | Method | Text | Record the method of test or examination. | MBMETHOD | (METHOD) | B-D MGIT | Y |
8.3.8 MS - Microbiology Susceptibility
Description/Overview for the CDASHIG MS - Microbiology Susceptibility Domain
The MB (Microbiology Specimen) and MS (Microbiology Susceptibility) domains are a pair of domains used to represent microbiology findings. The MS domain is used to represent data from drug susceptibility testing.This includes phenotypic testing (where drug is added directly to a culture of organisms) and genotypic tests that provide results in terms of susceptible or resistant. Drug susceptibility testing may occur on a wide variety of non-host organisms, including bacteria, viruses, fungi, protozoa, and parasites. Phenotypic drug susceptibility testing may involve determining susceptibility/resistance (qualitative) at a pre-defined concentration of drug, or may involve determining a specific dose (quantitative) at which a drug inhibits organism growth or some other process associated with virulence. The MS domain is appropriate for both of these types of drug susceptibility tests.
Newer releases of the SDTMIG (e.g., SDTMIG 3.3) provide more information on how Microbiology Susceptibility results may be submitted. Sponsors can continue to show the "agent" with which the organism is being evaluated against.for susceptibility (as in examples in the SDTMIG v3.2) or can follow the newer examples used in SDTMIG 3.3 and several therapeutic area user guides (e.g., TAUG-Tuberculosis, TAUG- Clostridium Difficile Associated Diarrhea). In particular, several new variables—Non-host Organism ID (NHOID), Agent Name (MSAGENT), Agent Concentration (MSCONC), and Agent Concentration Unit (MSCONCU)—were added.
When data is submitted to a health authority using an older version of the SDTMIG (e.g., version prior to 3.3), sponsors may elect to use this newer approach for representing drug susceptibility testing. Using this approach, sponsors may define non-standard variables as described in the SDTMIG for NHOID, MSAGENT, MSCONC, and MSCONCU.
The current controlled terminology for MSTESTCD assumes that the "agent" used is not included in the MSTESTCD as is shown in SDTMIG 3.2.
CDASHIG v2.1 has included these new variables in the CDASH Domain Metadata table and CRF examples, when appropriate, as non-standard variables.
Specification for the CDASHIG MS - Microbiology Susceptibility Domain
Microbiology Susceptibility Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | MS | Central Processing | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | MS | Central Processing | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | MS | Central Processing | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/ Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | MS | Central Processing | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | MS | Central Processing | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable MIDTC in ISO 8601 format. | N/A | N/A | The date the Microscopic Findings were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the Microscopic Findings at that visit, or the collection date can be included on the Microscopic Findings CRF using the date (MIDAT) field. |
Findings | MS | Central Processing | N/A | 6 | MSPERF | MS Test Performed | An indication of whether or not a planned measurement, series of measurements, tests or observations was performed. | Was the Microbiology Susceptibility test performed? | Microbiology Susceptibility Performed | Char | O | Indicate whether or not a microbiology susceptibility test was performed. | MSSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTM variable MSSTAT. If MSPERF="N", the value of MSSTAT will be "NOT DONE". If MSPERF="Y", MSSTAT should be null. A combination of SDTMIG variables (e.g., MSCAT and MSSCAT, MSTPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable MSTESTCD would be populated as MSALL and an appropriate test name (MSTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This may be implemented for an entire panel. General prompt question to be used as a data management tool to verify that missing results are confirmed missing. For the SDTM submission dataset, the SDTMIG variable MSSTAT is populated using the CDASH field MSPERF. The question text used might be reflected in the reason not done (MSREASND). |
Findings | MS | Central Processing | N/A | 7 | MSREFID | MS Reference ID | An internal or external identifier such as specimen identifier. | What was the (microbiology susceptibility test) [reference identifier/accession number]? | (Microbiology Susceptibility Test) [Reference Identifier/Accession Number] | Char | O | Record the specimen [accession/reference] number assigned. | MSREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | This can be used to confirm that the appropriate data record is present in the electronic transfer. May be included for linking back to specimens (e.g., Specimen ID). |
Findings | MS | Central Processing | N/A | 8 | MSDAT | MS Specimen Collection Date | The date of specimen collection, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the (start) date of the (microbiology) specimen collection? | Test Date | Char | R/C | Record the date when specimen collection occurred using this format (DD-MON- YYYY). | MSDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable MSDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and if so, a separate assessment date field is not required. |
Findings | MS | Central Processing | N/A | 9 | MSTIM | MS Specimen Collection Time | The time of specimen collection, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the (start) time of the (microbiology) specimen collection? | Susceptibility Test Time | Char | R/C | Record time of susceptibility test (as complete as possible). | MSDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable MSDTC in ISO 8601 format. | N/A | N/A | May be required when multiple assessments are done on one day or when the timing in relationship to study treatment is required for analysis. |
Findings | MS | Central Processing | N/A | 10 | MSCAT | MS Category for Organism Findings | A grouping of topic-variable values based on user-defined characteristics. | What was the category of the microbiology susceptibility test? | [Microbiology Susceptibility Category]; NULL | Char | O | Record the microbiology susceptibility category, if not preprinted on the CRF. | MSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | MS | Central Processing | N/A | 11 | MSSCAT | MS Subcategory for Organism Findings | A sub-division of the MSCAT values based on user-defined characteristics. | What was the subcategory of the microbiology susceptibility finding? | [Microbiology Susceptibility Subcategory]; NULL | Char | O | Record the microbiology susceptibility subcategory, if not preprinted on the CRF. | MSSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. MSSCAT can only be used if there is an MSCAT and it must be a subcategorization of MSCAT. |
Findings | MS | Central Processing | N/A | 12 | MSSPEC | MS Specimen Type | Defines the type of specimen used for a measurement. | What is the specimen material type? | Specimen Type | Char | R/C | Record the specimen material type. | MSSPEC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECTYPE) | N/A | The type of specimen used for a measure. Should be collected if not available elsewhere, or if required to differentiate multiple specimens. |
Findings | MS | Central Processing | N/A | 13 | MSSPCCND | MS Specimen Condition | Description of the condition of the specimen. | What was the condition of the specimen? | Specimen Condition | Char | R/C | Record condition of specimen. | MSSPCCND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECCOND) | N/A | May be collected using free or standardized text. This can be used when results may be affected by whether conditions for specimen were properly met. |
Findings | MS | Central Processing | N/A | 14 | MSLOC | MS Specimen Collection Location | A description of the anatomical location of the subject relevant to the collection of specimen. | What was the anatomical location where the specimen was collected? | Anatomical Location | Char | O | Record or select the anatomical location of specimen collection. | MSLOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location. Sponsors may collect the data using a subset list of Controlled Terminology on the CRF. LAT, DIR, and PORTOT are used to further describe the anatomical location. |
Findings | MS | Central Processing | N/A | 15 | MSLAT | MS Specimen Collection Laterality | Qualifier for anatomical location further detailing the side of the body. | What was the side of the anatomical location of the specimen collection? | Side | Char | O | Record the side of the anatomical location of the specimen collection. | MSLAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | MS | Central Processing | N/A | 16 | MSDIR | MS Specimen Collection Directionality | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What was the directionality of the anatomical location of the specimen collection? | Directionality | Char | O | Record the directionality of the anatomical location of the specimen collection. | MSDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | MS | Local Processing | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | MS | Local Processing | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | MS | Local Processing | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | MS | Local Processing | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | MS | Local Processing | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable MIDTC in ISO 8601 format. | N/A | N/A | The date of Microscopic Findings were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the Microscopic Findings at that visit, or the collection date can be included on the Microscopic Findings CRF using the date (MSDAT) field. |
Findings | MS | Local Processing | N/A | 6 | MSPERF | MS Susceptibility Test Performed | An indication of whether or not a planned measurement, series of measurements, tests or observations was performed. | Was the microbiology susceptibility test performed? | Microbiology Susceptibility Performed | Char | O | Indicate whether or not microbiology susceptibility test was performed. | MSSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTM variable MSSTAT. If MSPERF = "N", the value of MSSTAT will be "NOT DONE". If MSPERF = "Y", MSSTAT should be null. A combination of SDTMIG variables (e.g., MSCAT and MSSCAT, MSTPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable MSTESTCD would be populated as MSALL and an appropriate test name (MSTEST) provided. See SDTMIG Section 4.1.5.1.2. | (NY) | N/A | This may be implemented for an entire panel. General prompt question to be used as a data management tool to verify that missing results are confirmed missing. |
Findings | MS | Local Processing | N/A | 7 | MSREFID | MS Reference ID | An internal or external identifier such as specimen identifier. | What was the (microbiology susceptibility test) [reference identifier/accession number]? | (Microbiology Susceptibility test) [Reference identifier/Accession Number] | Char | O | Record the specimen [accession/reference] number assigned. | MSREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | This can be used to reconcile CRF data). May be included for linking back to specimens (e.g., Specimen ID). |
Findings | MS | Local Processing | N/A | 8 | MSSPID | MS Sponsor- Defined Identifier | A sponsor- defined identifier. In CDASH, This is typically used for preprinted or auto-generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor-defined question] | [Sponsor defined] | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | MSSPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Because SPID is a sponsor- defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile data. May be used to record preprinted number (e.g. line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Findings | MS | Local Processing | N/A | 9 | MSLNKID | MS Link ID | An identifier used to link related records across domains. | What [is/was] the [test/procedure/observation] link identifier? | [Domain/Observation] Link Identifier | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each microbiology susceptibility result has an identifier that will link records across domains. | MSLNKID | Maps directly to the SDTM variable listed in the column with the heading "SDTM Target". May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | This may be a one-to- one or a one-to-many relationship. For example, a single microbiology specimen or result may have multiple measurements/assessments performed (e.g., susceptibility testing). |
Findings | MS | Local Processing | N/A | 10 | MSDAT | MS Specimen Collection Date | The date of specimen collection ,represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the (start) date of the (microbiology) specimen collection? | Collection Date | Char | R/C | Record the date of specimen collection using this format (DD- MON-YYYY). | MSDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable MSDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and if so, a separate assessment date field is not required. |
Findings | MS | Local Processing | N/A | 11 | MSTIM | MS Specimen Collection Time | The time of specimen collection, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the (start) time of the the (microbiology) collection? | Collection Time | Char | R/C | Record time of collection (as complete as possible). | MSDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable MSDTC in ISO 8601 format. | N/A | N/A | May be required when multiple assessments are done on one day or when the timing in relationship to study treatment is required for analysis. |
Findings | MS | Local Processing | N/A | 12 | MSCAT | MS Category for Organism Findings | A grouping of topic-variable values based on user-defined characteristics. | What was the category of the Microbiology Susceptibility finding? | [Microbiology Susceptibility Category]; NULL | Char | O | Record the microscopic finding category, if not preprinted on the CRF. | MBCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | MS | Local Processing | N/A | 13 | MSSCAT | MS Subcategory for Organism Findings | A sub-division of the MSCAT values based on user-defined characteristics. | What was the subcategory of the Microbiology Susceptibility finding? | [Microbiology Susceptibility Subcategory]; NULL | Char | O | Record the microscopic finding subcategory, if not preprinted on the CRF. | MSSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. MSSCAT can only be used if there is an MSCAT and it must be a subcategorization of MSCAT. |
Findings | MS | Local Processing | N/A | 14 | MSTEST | MS Organism Test or Finding Name | Descriptive name of the Microbiology Susceptibility test or examination used to obtain the measurement or finding. | What was the Microbiology Susceptibility test name? | [Microbiology Susceptibility Test Name] | Char | HR | Record the type or name of the microscopic examination, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | MSTEST; MSTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable MSTESTCD may be determined from the value collected in MSTEST. Both MSTESTCD and MSTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | (MBTEST) | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | MS | Local Processing | N/A | 15 | MSTSTDTL | Microbiology Susceptibility Test Detail | Detail of the Microbiology Susceptibility Test used to obtain the measurement or finding. Helps to establish a unque record. | What was the Microbiology Susceptibility Test detail? | Test Detail | Char | O | Record the detail of the microbiology susceptibility test, if not preprinted on the CRF. | MSTSTDTL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Provides additional details for the microbiology susceptibility test if necessary to define a unique record. It is recommended that the test detail be preprinted on the CRF. If the form is laid out as a grid, then words such as "Test Detail" can be included as the column header. |
Findings | MS | Local Processing | N/A | 16 | MSORRES | MS Result or Finding in Original Units | Result of the measurement or finding as originally received or collected. | What was the result of the examination? | (Result) | Char | HR | Record test result, interpretation or finding. | MSORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Both quantitative results and interpretive findings or summaries may be recorded here. |
Findings | MS | Local Processing | N/A | 17 | MSORRESU | MS Original Units | The unit of the result as originally received or collected. | What was the unit of the result? | Unit | Char | R/C | Record or select the original unit in which these data were collected, if not preprinted on CRF. | MSORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | N/A | Should be preprinted on the CRF with the associated test when possible, rather than collected in a field that requires the site to enter text. Should be included if applicable and not available elsewhere. For some tests the units may not be applicable (e.g., where MSTEST requires simply a qualitative result). |
Findings | MS | Local Processing | N/A | 18 | MSCLSIG | MS Clinical Significance | An indication whether the test results were clinically significant. | Was the result clinically significant? | Clinically Significant | Char | O | Indicate whether the results were clinically significant. | SUPPMS.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPMS dataset as the value of SUPPMS.QVAL where SUPPMS.QNAM="MSCLSIG" and SUPPMS.QLABEL="Clinically Significant". Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. | (NY) | N/A | N/A |
Findings | MS | Local Processing | N/A | 19 | MSRESCAT | MS Result Category | Used to categorize the result of a finding. | What was the result category? | Result Category | Char | O | Record the category of the test results. | MSRESCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Used to categorize the results of a finding (e.g., "SUSCEPTIBLE", "RESISTANT"). |
Findings | MS | Local Processing | N/A | 20 | MSNAM | MS Vendor Name | The name or identifier of the vendor (e.g., laboratory) that provided the test results. | What was the name of the vendor used? | Vendor Name | Char | O | Record the laboratory name. | MSNAM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Recommended to collect on the CRF if lab names was not collected at the site/study level or if multiple labs are used by a site. |
Findings | MS | Local Processing | N/A | 21 | MSSPEC | MS Specimen Type | Defines the type of specimen used for a measurement. | What is the specimen material type? | Specimen Type | Char | R/C | Record the specimen material type. | MSSPEC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECTYPE) | N/A | The type of specimen used for a measure. Should be collected if not available elsewhere, or if required to differentiate multiple specimens. |
Findings | MS | Local Processing | N/A | 22 | MSSPCCND | MS Specimen Condition | Describes the condition of the specimen. | What was the condition of the specimen? | Specimen Condition | Char | O | Record condition of specimen. | MSSPCCND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECCOND) | N/A | May be collected using free or standardized text. This can be used when results may be affected by whether conditions for specimen were properly met. |
Findings | MS | Local Processing | N/A | 23 | MSLOC | MS Specimen Collection Location | A description of the anatomical location of the subject relevant to the collection of specimen. | What was the anatomical location where the specimen was collected? | Anatomical Location | Char | O | Record or select the anatomical location of specimen collection. | MSLOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. LAT, DIR, and PORTOT are used to further describe the anatomical location. |
Findings | MS | Local Processing | N/A | 24 | MSLAT | MS Specimen Collection Laterality | Qualifier for anatomical location further detailing the side of the body. | What was the side of the anatomical location of the specimen collection? | Side | Char | O | Record the side of the anatomical location of the specimen collection. | MSLAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | MS | Local Processing | N/A | 25 | MSDIR | MS Specimen Collection Directionality | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What was the directionality of the anatomical location of the specimen collection? | Directionality | Char | O | Record the directionality of the anatomical location of the specimen collection. | MSDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | MS | Local Processing | N/A | 26 | MSMETHOD | MS Method of Test or Examination | Method of the test or examination. | What was the method used for the test or examination? | Method | Char | O | Record the method of test or examination. | MSMETHOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (METHOD) | N/A | This information may be collected when more than one method is possible, and collecting the method used is necessary. This could include technique or type of staining used for the slides. |
Findings | MS | Local Processing | N/A | 27 | MSEVAL | MS Evaluator | The role of the person who provided the evaluation. | Who was the evaluator? | [Evaluator/Reporter] | Char | O | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | MSEVAL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EVAL) | N/A | Used only for results that are subjective (e.g., assigned by a person or a group). May be preprinted or collected. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | MS | Local Processing | N/A | 28 | NHOID | Non-host Organism ID | The identifier for a non-host organism which should only be used when the organism is the subject of the test. | What was the Non-host Organism ID? | Non-host Organism ID | Char | O | Record the identifier for a non-host organism that is the subject of the test. | SUPPMS.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPMS dataset as the value of SUPPMS.QVAL where SUPPMS.QNAM="NHOID" and SUPPMS.QLABEL="Non-host Organism ID". Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. | N/A | N/A | Sponsor-defined identifier for a non-host organism which should only be used when the organism is the subject of the test. These variables were added as core SDTM variables in SDTMIG v3.3. |
Findings | MS | Local Processing | N/A | 29 | MSAGENT | Microbiology Susceptibility Agent | The name of the agent for which resistance is tested | What was the name of the agent for which resistance is being tested? | Microbiology Susceptibility Agent | Char | O | Record the name of the agent for which resistance is being tested. | SUPPMS.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPMS dataset as the value of SUPPMS.QVAL where SUPPMS.QNAM="MSAGENT" and SUPPMS.QLABEL= "Microbiology Susceptibility Agent". Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. | N/A | N/A | These variables were added as core SDTM variables in SDTMIG v3.3. |
Findings | MS | Local Processing | N/A | 30 | MSCONC | MS Agent Concentration | The concentration of the agent for which resistance is tested. | What was the concentration of the agent? | Agent Concentration | Char | O | Record the concentration of the agent. | SUPPMS.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPMS.QVAL where SUPPMS.QNAM="MSCONC" and SUPPMS.QLABEL="MS Agent Concentration". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | N/A | N/A | These variables were added as core SDTM variables in SDTMIG v3.3. |
Findings | MS | Local Processing | N/A | 31 | MSCONCU | MS Agent Concentration Unit | The concentration unit of the agent for which resistance is tested. | What was the agent concentration unit? | Agent Concentration Unit | Char | O | Record the concentration unit of the agent. | SUPPMS.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPMS dataset as the value of SUPPMS.QVAL where SUPPMS.QNAM="MSCONCU" and SUPPMS.QLABEL="MS Agent Concentration Unit". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | N/A | N/A | These variables were added as core SDTM variables in SDTMIG v3.3. |
Assumptions for the CDASHIG MS - Microbiology Susceptibility Domain
1. This domain is used only to represent drug susceptibility testing results.
2. MSDTC represents the date the specimen was collected.
3. Information on processing of the biospecimen, including collection and storage information (e.g., culture start and end date), are represented in the BE (Biospecimen Events) domain. See BE domain assumptions in the SDTMIG for Pharmacogenomic/Genetics (SDTMIG-PGx).
4. If used, the variable NHOID, a non-standard variable in SDTMIG versions prior to v3.3, is an intuitive name of the non-host organism being tested. It should only be populated with values representing what is known about the identity of the organism before the results of the test are determined. It should therefore never be used as a result qualifier.
5. Genotypic tests that provide results in terms of specific changes to nucleotides, codons, or amino acids of genes/gene products associated with resistance should be represented in the PF (Pharmacogenomics/genetics Findings) domain.
Example CRF for the CDASHIG MS - Microbiology Susceptibility Domain
Example 1
This sample CRF may be set up as a table with 1 row for each Microbiology Susceptibility Agent, or 1 record can be included for each Microbiology Susceptibility finding. The NSV variable NHOID (Non-Host Organism Identifier) can be included and populated with the name of the organism that is the subject of the test.
Title: Tuberculosis Drug Susceptibility
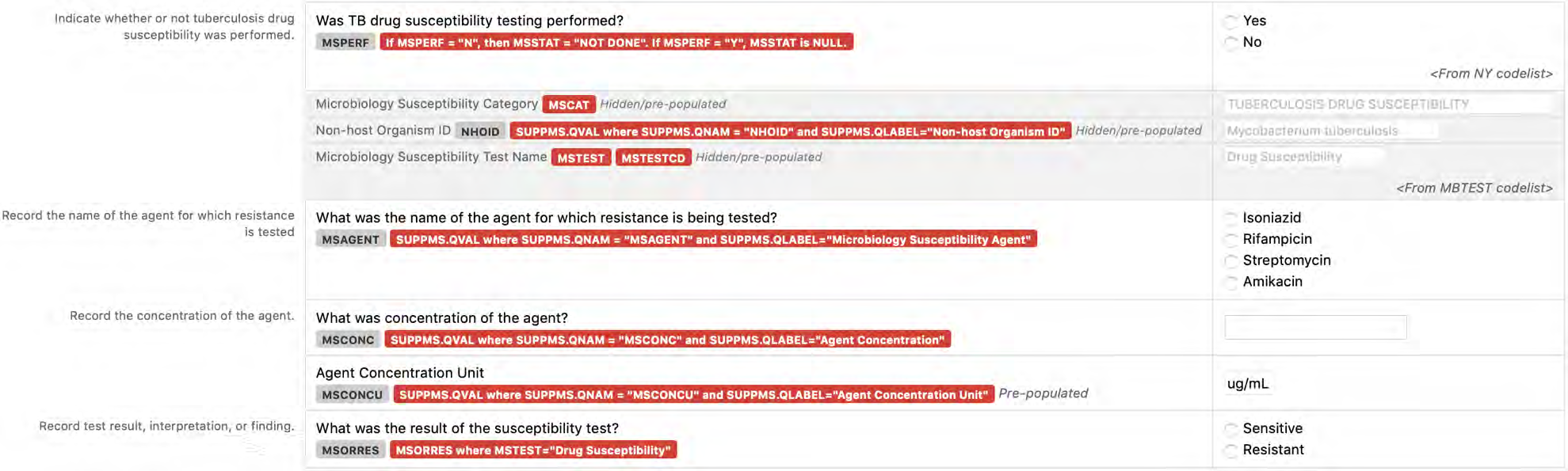
CRF Metadata
| Order Number | CDASH Variable | Question Text | Prompt | Type | Case Report Form Completion Instructions | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Codelist Name | Permissible Values | Pre-Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | MSPERF | Was TB drug susceptibility testing performed? | TB Drug Susceptibility Performed | Text | Indicate whether or not tuberculosis drug susceptibility was performed. | MSSTAT | If MSPERF = "N", then MSSTAT = "NOT DONE". If MSPERF = "Y", MSSTAT is NULL. | (NY) | Yes;No; | ||||
2 | MSCAT | What was the category of the microbiology susceptibility test? | Microbiology Susceptibility Category | Text | Record the microbiology susceptibility category, if not pre-printed on the CRF. | MSCAT | N/A | TUBERCULOSIS DRUG SUSCEPTIBILITY | Y | ||||
3 | NHOID | What was the Non- host Organism ID? | Non-host Organism ID | Text | Record the identifier for a non-host organism that is the subject of the test. | SUPPMS.QVAL | SUPPMS.QVAL where SUPPMS.QNAM = "NHOID" and SUPPMS.QLABEL="Non-host Organism ID" | Mycobacterium tuberculosis | Y | ||||
4 | MSTEST | What was the Microbiology Susceptibility test name? | Microbiology Susceptibility Test Name | Text | Record the type or name of the microscopic examination, if not pre-printed on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | MSTEST; MSTESTCD | (MBTEST) | Drug Susceptibility | Y | ||||
5 | MSAGENT | What was the name of the agent for which resistance is being tested? | Agent | Text | Record the name of the agent for which resistance is tested | SUPPMS.QVAL | SUPPMS.QVAL where SUPPMS.QNAM = "MSAGENT" and SUPPMS.QLABEL="Microbiology Susceptibility Agent" | Isoniazid; Rifampicin; Streptomycin; Amikacin |
| ||||
6 | MSCONC | What was concentration of the agent? | Agent Concentration | Text | Record the concentration of the agent. | SUPPMS.QVAL | SUPPMS.QVAL where SUPPMS.QNAM = "MSCONC" and SUPPMS.QLABEL="Agent Concentration" | ||||||
7 | MSCONCU | What was agent concentration unit? | Agent Concentration Unit | Text | Record the concentration unit of the agent. | SUPPMS.QVAL | SUPPMS.QVAL where SUPPMS.QNAM = "MSCONCU" and SUPPMS.QLABEL="Agent Concentration Unit" | ug/mL |
| ||||
8 | MSORRES | What was the result of the susceptibility test? | Result | Text | Record test result, interpretation, or finding. | MSORRES | MSORRES where MSTEST="Drug Susceptibility" | N/A | Sensitive; Resistant |
8.3.9 MI - Microscopic Findings
Description/Overview for the CDASHIG MI - Microscopic Findings Domain
The MI domain collects the histopathology findings and microscopic evaluations that do not have specialized domains (e.g., MB, MS) for their results.
MI domain metadata are provided for 2 different data collection scenarios. It is up to the sponsor to determine which data collection scenario best meets the study needs. (Sponsors may implement Scenario 3: Central Processing with Investigator Assessment of Clinical Significance Assessment for Abnormal Values following the example in Section 8.3.6, LB - Laboratory Test Results, even though such an example is not provided in the CDASHIG Metadata Table.)
• Scenario 1: Central Processing. In this scenario, subject specimens are taken at the site, sent out for processing, and results are provided directly to the sponsor and not recorded on the CRF. This scenario also applies when results are captured directly via an electronic device and not recorded on the CRF. CRF data are captured at the site for tracking/ header reconciliation. The fields for test results are not defined here, as these data are not part of the CRF.
• Scenario 2: Local Processing. In this scenario, subject specimens are taken and analyzed, and then the results are recorded directly on the CRF.
Specification for the CDASHIG MI - Microscopic Findings Domain
Microscopic Findings Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | MI | Central Processing | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM- based dataset creation before submission. |
Findings | MI | Central Processing | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | MI | Central Processing | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participa nt] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | MI | Central Processing | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | MI | Central Processing | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable MIDTC in ISO 8601 format. | N/A | N/A | The date the Microscopic Findings were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the Microscopic Findings at that visit, or the collection date can be included on the Microscopic Findings CRF using the date (MIDAT) field. |
Findings | MI | Central Processing | N/A | 6 | MIPERF | Microscopic Examination Performed | An indication of whether or not a planned microbiology measurement, series of microbiology measurements, test, or observation was performed or specimens collected. | Was the microscopic examination performed? | Microscopic Examination Performed | Char | O | Indicate whether or not microscopic examination performed. | MISTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTM variable MISTAT. If MIPERF="N", the value of MISTAT will be "NOT DONE". If MIPERF="Y", MISTAT should be null. A combination of SDTMIG variables (e.g., MICAT and MISCAT, MITPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable MITESTCD would be populated as MIALL and an appropriate test name (MITEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This may be implemented for an entire panel. This general prompt question is used as a data management tool to verify that missing results are confirmed missing. For the SDTM submission dataset, the SDTMIG variable MISTAT is populated using the CDASH field MIPERF. The question text used might be reflected in the reason not done (MIREASND). |
Findings | MI | Central Processing | N/A | 7 | MIREFID | MI Reference ID | An internal or external identifier such as specimen identifier. | What was the (microscopic test) [reference identifier/accession number]? | (Microscopic test) [Reference Identifier/ Accession Number] | Char | O | Record the specimen [accession/reference] number assigned. | MIREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | This can be used to confirm that the appropriate data record is present in the electronic transfer. May be included for linking back to specimens (e.g., Specimen ID). |
Findings | MI | Central Processing | N/A | 8 | MIDAT | Specimen Collection Date | The date of specimen collection, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the date of the specimen collection? | Collection Date | Char | R/C | Record the date when specimen collection occurred using this format (DD-MON- YYYY). | MIDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable MIDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and if so, a separate assessment date field is not required. |
Findings | MI | Central Processing | N/A | 9 | MITIM | Specimen Collection Time | The time of specimen collection, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the (start) time of the specimen collection? | Collection Time | Char | R/C | Record time of collection (as complete as possible). | MIDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable MIDTC in ISO 8601 format. | N/A | N/A | May be required when multiple assessments are done on 1 day or when the timing in relationship to study treatment is required for analysis. |
Findings | MI | Central Processing | N/A | 10 | MICAT | Category for Microscopic Finding | A grouping of topic- variable values based on user-defined characteristics. | What was the category of the microscopic finding? | [Microscopic Category]; NULL | Char | O | Record the microscopic finding category, if not preprinted on the CRF. | MICAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | MI | Central Processing | N/A | 11 | MISCAT | Subcategory for Microscopic Finding | A sub-division of the MICAT values based on user-defined characteristics. | What was the subcategory of the microscopic finding? | [Microscopic Subcategory]; NULL | Char | O | Record the microscopic finding subcategory, if not preprinted on the CRF. | MISCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. MISCAT can only be used if there is an MICAT and it must be a subcategorization of MICAT. |
Findings | MI | Central Processing | N/A | 12 | MISPEC | MI Specimen Material Type | Defines the type of specimen used for a measurement. | What is the specimen material type? | Specimen Type | Char | R/C | Record the specimen material type. | MISPEC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECTYPE) | N/A | The type of specimen used for a measure. Should be collected if not available elsewhere, or if required to differentiate multiple specimens. |
Findings | MI | Central Processing | N/A | 13 | MISPCCND | MI Specimen Condition | Describes the condition of the specimen. | What was the condition of the specimen? | Specimen Condition | Char | R/C | Record condition of specimen. | MISPCCND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECCOND) | N/A | May be collected using free or standardized text. This can be used when results may be affected by whether conditions for specimen were properly met. |
Findings | MI | Central Processing | N/A | 14 | MILOC | MI Specimen Collection Location | A description of the anatomical location of the subject relevant to the collection of specimen. | What was the anatomical location from which the specimen was collected? | Anatomical Location | Char | O | Record or select the anatomical location of specimen collection. | MILOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. LAT, DIR, and PORTOT are used to further describe the anatomical location. |
Findings | MI | Central Processing | N/A | 15 | MILAT | MI Specimen Laterality within Subject | Qualifier for anatomical location further detailing the side of the body. | What was the side of the anatomical location of the specimen collection? | Side | Char | O | Record the side of the anatomical location of the specimen collection. | MILAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | MI | Central Processing | N/A | 16 | MIDIR | MI Specimen Directionality within Subjct | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What was the directionality of the anatomical location of the specimen collection? | Directionality | Char | O | Record the directionality of the anatomical location of the specimen collection. | MIDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | MI | Local Processing | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM- based dataset creation before submission. |
Findings | MI | Local Processing | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | MI | Local Processing | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participa nt] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | MI | Local Processing | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | MI | Local Processing | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable MIDTC in ISO 8601 format. | N/A | N/A | The date the Microscopic Findings were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the Microscopic Findings at that visit, or the collection date can be included on the Microscopic Findings CRF using the date (MIDAT) field. |
Findings | MI | Local Processing | N/A | 6 | MIPERF | Microscopic Examination Performed | An indication of whether or not a planned microbiology measurement, series of microbiology measurements, test, or observation was performed or specimens collected. | Was the microscopic examination performed? | Microscopic Examination Performed | Char | O | Indicate whether or not microscopic examination performed. | MISTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTM variable MISTAT. If MIPERF="N", the value of MISTAT will be "NOT DONE". If MIPERF="Y", MISTAT should be null. A combination of SDTMIG variables (e.g., MICAT and MISCAT, MITPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable MITESTCD would be populated as MIALL and an appropriate test name (MITEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This may be implemented for an entire panel. This general prompt question is used as a data management tool to verify that missing results are confirmed missing. |
Findings | MI | Local Processing | N/A | 7 | MIREFID | MI Reference ID | An internal or external identifier such as specimen identifier. | What was the (microscopic test) [reference identifier/accession number]? | (Microscopic test) [Reference identifier/ Accession Number] | Char | O | Record the specimen [accession/reference] number assigned. | MIREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | This can be used to reconcile CRF data. May be included for linking back to specimens (e.g., Specimen ID). |
Findings | MI | Local Processing | N/A | 8 | MISPID | MI Sponsor- Defined Identifier | A sponsor-defined identifier. In CDASH, This is typically used for preprinted or auto- generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor-defined question] | [Sponsor defined] | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | MISPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Because SPID is a sponsor-defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile data. May be used to record preprinted number (e.g. line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Findings | MI | Local Processing | N/A | 9 | MIDAT | Specimen Collection Date | The date of specimen collection, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the date of the specimen collection? | Collection Date | Char | R/C | Record the date of specimen collection using this format (DD- MON-YYYY). | MIDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable MIDTC in ISO 8601 format. | N/A | N/A A | complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and if so, a separate assessment date field is not required. |
Findings | MI | Local Processing | N/A | 10 | MITIM | Specimen Collection Time | The time of specimen collection, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the (start) time of the specimen collection? | Collection Time | Char | R/C | Record time of collection (as complete as possible). | MIDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable MIDTC in ISO 8601 format. | N/A | N/A | May be required when multiple assessments are done on one day or when the timing in relationship to study treatment is required for analysis. |
Findings | MI | Local Processing | N/A | 11 | MICAT | Category for Microscopic Finding | A grouping of topic- variable values based on user-defined characteristics. | What was the category of the microscopic finding? | [Microscopic Category]; NULL | Char | O | Record the microscopic finding category, if not preprinted on the CRF. | MICAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | MI | Local Processing | N/A | 12 | MISCAT | Subcategory for Microscopic Finding | A sub-division of the MICAT values based on user-defined characteristics. | What was the subcategory of the microscopic finding? | [Microscopic Subcategory]; NULL | Char | O | Record the microscopic finding subcategory, if not preprinted on the CRF. | MISCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. MISCAT can only be used if there is an MICAT and it must be a subcategorization of MICAT. |
Findings | MI | Local Processing | N/A | 13 | MITEST | Microscopic Examination Name | Descriptive name of the microscopic test or examination used to obtain the measurement or finding. | What [is/was] the name (of the microscopic [measurement/test/exa mination])? | [Microscopic Measurement/Te st/ Examination Name] | Char | HR | Record the type or name of the microscopic examination, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | MITEST; MITESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable MITESTCD may be determined from the value collected in MITEST. Both MITESTCD and MITEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | (MITS) | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | MI | Local Processing | N/A | 14 | MITSTDTL | Microscopic Examination Detail | Detail of the microscopic examination used to obtain the measurement or finding. | What [is/was] the [microscopic measurement/test/exa mination] detail name? | [Examination Name Detail] | Char | O | Record the detail of the microscopic examination, if not preprinted on the CRF. | MITSTDTL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (MIFTSDTL) | N/A | Provides additional details for the microscopic examination. It is recommended that the test detail be preprinted on the CRF. If the form is laid out as a grid, then words such as "Test Detail" can be included as the column header. |
Findings | MI | Local Processing | N/A | 15 | MIORRES | MI Result or Finding in Original Units | Result of the measurement or finding as originally received or collected. | What was the result of the examination? | (Result) | Char | HR | Record test result, interpretation or finding. | MIORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Both quantitative results and interpretive findings or summaries may be recorded here. |
Findings | MI | Local Processing | N/A | 16 | MIORRESU | MI Original Units | The unit of the result as originally received or collected. | What was the unit of the result? | Unit | Char | R/C | Record or select the original unit in which these data were collected, if not preprinted on CRF. | MIORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | N/A | Should be preprinted on the CRF with the associated test when possible, rather than collected in a field that requires the site to enter text. Should be included if applicable and not available elsewhere. For some tests the units may not be applicable (e.g., reaction score for HER2). |
Findings | MI | Local Processing | N/A | 17 | MICLSIG | MI Clinical Significance | An indication whether the test results were clinically significant. | Was the result clinically significant? | Clinically Significant | Char | O | Indicate whether the results were clinically significant. | SUPPMI.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPMI dataset as the value of SUPPMI.QVAL where SUPPMI.QNAM="MICLSIG" and SUPPMI.QLABEL="Clinically Significant". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | N/A |
Findings | MI | Local Processing | N/A | 18 | MIRESCAT | MI Result Category | Used to categorize the result of a finding. | What was the result category? | Result Category | Char | O | Record the category of the test results. | MIRESCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Used to categorize the results of a finding (e.g., MALIGNANT or BENIGN, RESISTANCE VARIANT for genetic variation). |
Findings | MI | Local Processing | N/A | 19 | MINAM | Laboratory/Vendor Name | The name or identifier of the vendor (e.g., laboratory) that provided the test results. | What was the name of the vendor used? | Vendor Name | Char | O | Record the laboratory name. | MINAM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Recommended to collect on the CRF if lab names was not collected at the site/study level or if multiple labs are used by a site. |
Findings | MI | Local Processing | N/A | 20 | MISPEC | MI Specimen Material Type | Defines the type of specimen used for a measurement. | What is the specimen material type? | Specimen Type | Char | R/C | Record the specimen material type. | MISPEC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECTYPE) | N/A | The type of specimen used for a measure. Should be collected if not available elsewhere, or if required to differentiate multiple specimens. |
Findings | MI | Local Processing | N/A | 21 | MISPCCND | MI Specimen Condition | Describes the condition of the specimen. | What was the condition of the specimen? | Specimen Condition | Char | R/C | Record condition of specimen. | MISPCCND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECCOND) | N/A | May be collected using free or standardized text. This can be used when results may be affected by whether conditions for specimen were properly met. |
Findings | MI | Local Processing | N/A | 22 | MILOC | MI Specimen Collection Location | A description of the anatomical location of the subject relevant to the collection of specimen. | What was the anatomical location from which the specimen was collected? | Anatomical Location | Char | O | Record or select the anatomical location of specimen collection. | MILOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. LAT, DIR, and PORTOT are used to further describe the anatomical location. |
Findings | MI | Local Processing | N/A | 23 | MILAT | MI Specimen Laterality within Subject | Qualifier for anatomical location further detailing the side of the body. | What was the side of the anatomical location of the specimen collection? | Side | Char | O | Record the side of the anatomical location of the specimen collection. | MILAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | MI | Local Processing | N/A | 24 | MIDIR | MI Specimen Directionality within Subjct | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What was the directionality of the anatomical location of the specimen collection? | Directionality | Char | O | Record the directionality of the anatomical location of the specimen collection. | MIDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | MI | Local Processing | N/A | 25 | MIMETHOD | MI Method of Test or Examination | Method of the test or examination. | What was the method used for the test or examination? | Method | Char | O | Record the method of test or examination. | MIMETHOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (METHOD) | N/A | This information may be collected when more than one method is possible, and collecting the method used is necessary. This could include technique or type of staining used for the slides. |
Findings | MI | Local Processing | N/A | 26 | MIEVAL | MI Evaluator | The role of the person who provided the evaluation. | Who was the evaluator? | [Evaluator/Report er] | Char | O | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | MIEVAL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EVAL) | N/A | Used only for results that are subjective (e.g., assigned by a person or a group). May be a preprinted or collected. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Assumptions for the CDASHIG MI - Microscopic Findings Domain
1. This domain is used for findings resulting from the microscopic examination of tissue samples. These examinations are performed on a specimen which has been prepared with some type of stain. Some examinations of cells in fluid specimens (e.g., blood, urine) are classified as lab tests and should be represented in the LB domain. Tests classified as pathology or cytology should be represented in the MI domain. Biomarkers assessed by histologic or histopathological examination (by employing cytochemical/immunocytochemical stains) will be represented in the MI domain.
2. Sponsors should decide which scenario is appropriate for each protocol.
3. The variable MITSTDTL is used when biomarker tests are represented in the MI domain. It represents test parameter details descriptive of slide stain results (e.g., cells at 1+ intensity cytoplasm stain, H-Score, nuclear reaction score).
4. These CDASHIG variables are generally not used for this domain: --POS, --MODIFY, --ORNRLO, --ORNRHI, --LEAD.
Example CRFs for the CDASHIG MI - Microscopic Findings Domain
Example 1
Title: Microscopic Findings-Programmed Death Ligand
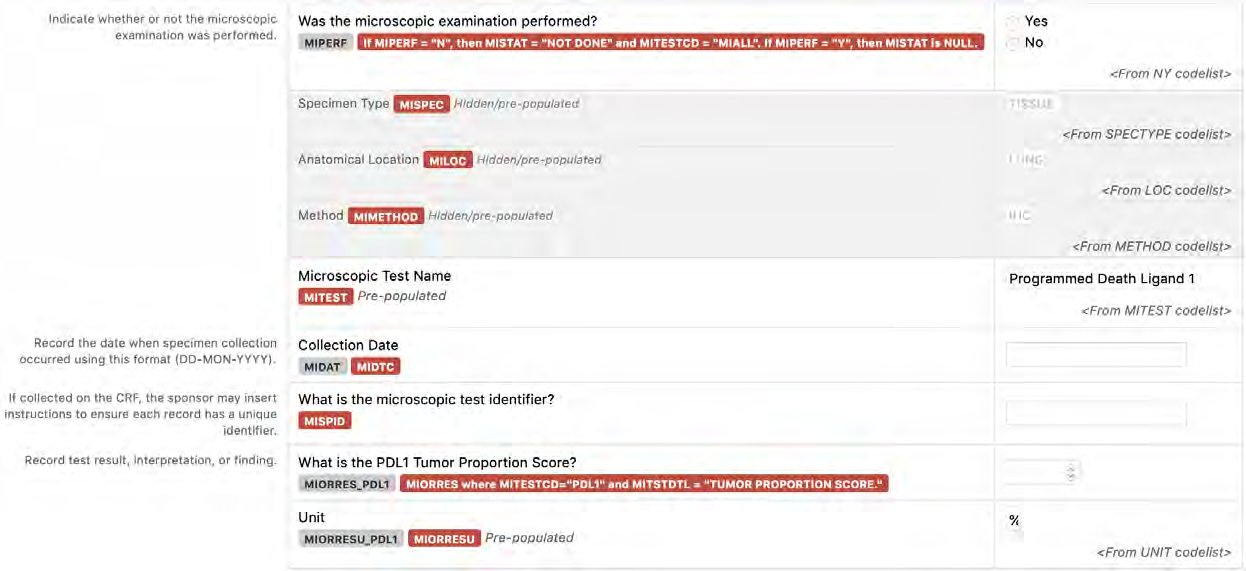
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
MIPERF | 1 | Was the microscopic examination performed? | Microscopic Examination Performed | Indicate whether or not the microscopic examination was performed. | Text | MISTAT | If MIPERF = "N", then MISTAT = "NOT DONE" and MITESTCD = "MIALL". If MIPERF = "Y", then MISTAT is NULL. | (NY) | Yes; No | Qtext | |||
MIDAT | 6 | What was the specimen collection date? | Collection Date | Record the date when specimen collection occurred using this format (DD-MON-YYYY). | Date | MIDTC |
| ||||||
MILOC | 3 | What was the anatomical location were the specimen was collection? | Anatomical Location | Record or select the anatomical location of specimen collection. | Text | MILOC | (LOC) | LUNG | Y | ||||
MIMETHOD | 4 | What was the method used for the test or examination? | Method | Record the method of test or examination. | Text | MIMETHOD | (METHOD) | IHC | Y | ||||
MITEST | 5 | What was the microscopic examination test name? | Microscopic Test Name | Record the type or name of the microscopic examination, if not pre- printed on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | Text | MITEST | (MITEST) | Programmed Death Ligand 1 | Y | ||||
MISPEC | 2 | What is the specimen material type? | Specimen Type | Record the specimen material type. | Text | MISPEC | (SPECTYPE) | TISSUE | Y | ||||
MISPID | 7 | What is the microscopic test identifier? | [Line Number/ MI Number] | If collected on the CRF, the sponsor may insert instructions to ensure each record has a unique identifier. | Text | MISPID | |||||||
MIORRES_PDL1 | 9 | What is the PDL1 Tumor Proportion Score? | Result | Record test result, interpretation, or finding. | integer | MIORRES | MIORRES where MITESTCD="PDL1" and MITSTDTL = "TUMOR PROPORTION SCORE." | ||||||
MIORRESU_PDL1 | 10 | What was the unit of the result? | Unit | Record or select the original unit in which these data were collected, if not pre- printed on CRF. | Text | MIORRESU | (UNIT) | % |
Example 2
Title: Histopatholgy-Lung Specimen Collection
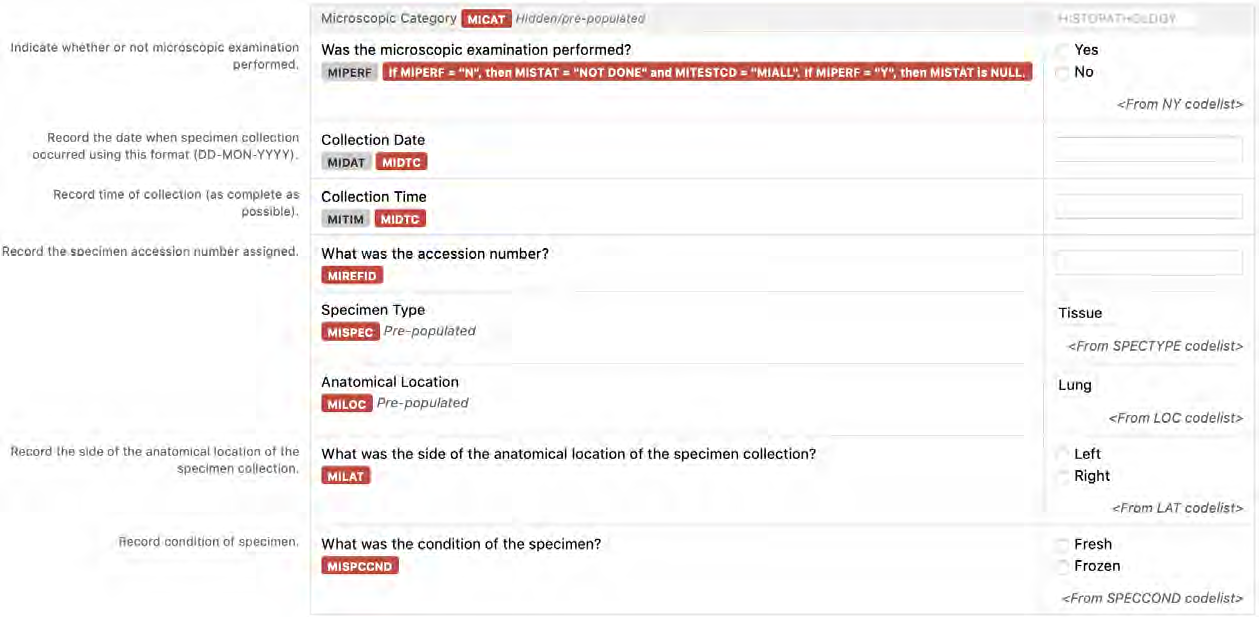
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre-Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
MICAT | 1 | What was the category of the microscopic finding? | Microscopic Category | Record the microscopic finding category, if not pre- printed on the CRF. | Text | MICAT | HISTOPATHOLOGY | Prompt | Y | ||||
MIPERF | 2 | Was the microscopic examination performed? | Microscopic Examination Performed | Indicate whether or not microscopic examination performed. | Text | MISTAT | If MIPERF = "N", then MISTAT = "NOT DONE" and MITESTCD = "MIALL". If MIPERF = "Y", then MISTAT is NULL. | (NY) | Yes; No | Radio | |||
MIDAT | 3 | What was the specimen collection date? | Collection Date | Record the date when specimen collection occurred using this format (DD-MON-YYYY). | Date | MIDTC | Prompt | ||||||
MITIM | 4 | What was the time of the specimen collection? | Collection Time | Record time of collection (as complete as possible). | Time | MIDTC | Prompt | ||||||
MIREFID | 5 | What was the accession number? | Accession Number | Record the specimen accession number assigned. | Text | MIREFID |
|
| |||||
MISPEC | 6 | What is the specimen material type? | Specimen Type | Record the specimen material type. | Text | MISPEC | (SPECTYPE) | Tissue | Radio | ||||
MISPCCND | 9 | What was the condition of the specimen? | Specimen Condition | Record condition of specimen. | Text | MISPCCND | (SPECCOND) | Fresh; Frozen | Radio | ||||
MILOC | 7 | What was the anatomical location were the specimen was collection? | Anatomical Location | Record or select the anatomical location of specimen collection. | Text | MILOC | (LOC) | Lung | Prompt | Radio | |||
MILAT | 8 | What was the side of the anatomical location of the specimen collection? | Side | Record the side of the anatomical location of the specimen collection. | Text | MILAT | (LAT) | Left; Right | Radio |
8.3.10 PC - Pharmacokinetics Sampling
Description/Overview for the CDASHIG PC - Pharmacokinetics Concentrations Domain
Information about sampling for pharmacokinetic (PK) concentration is collected on CRFs with the goal to reconcile or link sampling information (e.g., collection timing and specimen volumes) with PK concentration results provided by the laboratory. SDTMIG PC records are compiled when joining CRF sampling information and PK concentration results. This is similar to Scenario 1 in Section 8.3.6, LB - Laboratory Test Results.
The goals of the CDASHIG PC domain are:
• To standardize specimen collection details in the CRF for PK samples collected at fixed time points or over timed intervals.
• To document the data flow from the CDASHIG CRF to the SDTMIG PC dataset.
The CDASHIG PC domain defines fields for:
• The date and time of PK sample collections
o Either at fixed defined time points (e.g., 4 HRS POSTDOSE) or across a collection interval (e.g., 2-4 HRS POSTDOSE).
o How the body metabolizes and clears the analyte often determines which of these sampling approaches may be required.
• Sample properties (e.g., pH, sample volume)
Samples collected to measure drug concentration at an instant in time are generally associated with specimen types such as plasma, serum, or whole blood. Samples collected over a timed interval are generally associated with specimen types such as urine or feces.
PK Sample Collection at Fixed Time Points
In the case of fixed time points, the date (PCDAT) and time (PCTIM) of collection for each sample is recorded on the CRF. The protocol defines the time points at which samples are to be collected in relation to an intervention such as a dose of study treatment. This "reference" is depicted in Figure 1 by the longer vertical line and would correspond to a date and time in the Exposure as Collected (EC) or Exposure (EX) domain.
Figure 1. PK Sample Collection at Fixed Time Points
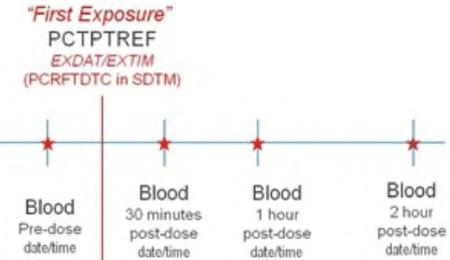
PK Sample Collection Over a Time Interval
Similarly, for PK specimens collected to measure drug excretion over a time interval, PCDAT and PCTIM capture the start date and time of the interval collection. End date (PCENDAT) and end time (PCENTIM) capture the end of the timed interval collection. As with fixed-time point collections, these timed intervals are performed in relation to an intervention such as a dose of study treatment. This "reference" is depicted in Figure 2 by the longer vertical line and would correspond to a date and time in the EC or EX domain.
Figure 2. Sample Collection Over a Time Interval
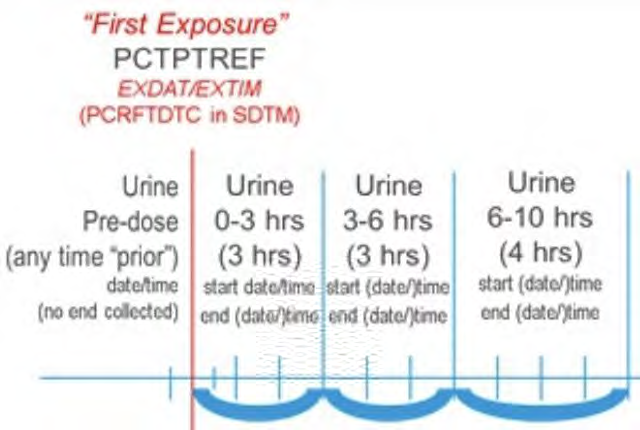
CDASHIG-SDTMIG PK Data Flow
The following concept map illustrates the data flow from PK sample collection at the site through the tabulation of both PK concentration and PK parameter results.
Concept Map. CDASHIG-SDTMIG PK Data Flow
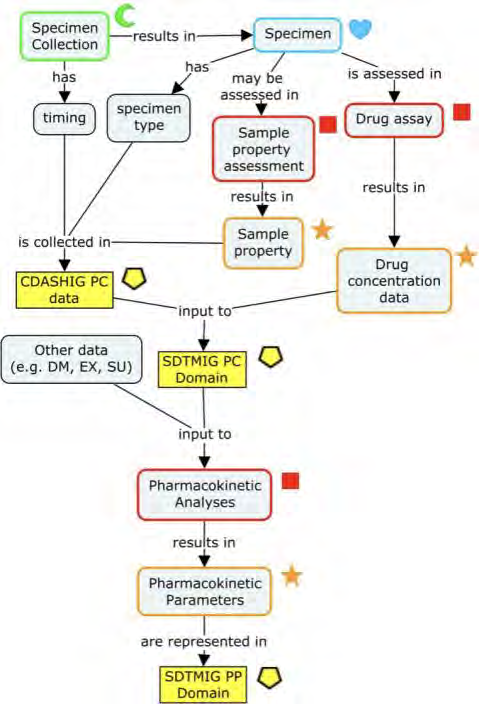
Specification for the CDASHIG PC - Pharmacokinetic Concentrations Domain
Pharmacokinetic Concentrations Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/ Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable PCDTC in ISO 8601 format. | N/A | N/A | The date the PK samples were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the PK samples at that visit, or the collection date can be collected on the PK CRF using the date (PCDAT) field. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 6 | PCPERF | PK Sampling Performed | An indication whether or not PK samples were collected. | Were PK samples collected? | Collected | Char | O | Check "No" if none of the samples were collected. | PCSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTMIG variable PCSTAT. If PCPERF="N", the value of PCSTAT will be "NOT DONE". If PCPERF="Y", PCSTAT should be null. PCTEST and PCTESTCD must reflect what tests were not done. A combination of SDTMIG variables ( e.g., PCCAT and PCSCAT, PCTPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable PCTESTCD would be populated as PCALL and an appropriate test name (PCTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | General prompt question to be used as a data management tool to verify that missing results are confirmed missing. This may be implemented at form level or sample level. These may be all samples of a particular type or all samples taken for some purpose and may need to be identified by the organization of the data on the form. Each sample collected could result in 1 or more tests performed, so there can be a one-to-one or one-to-many relationship between samples and tests/results. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 7 | PCSTAT | PK Sampling Completion Status | The variable used to indicate that data are not available by having the site recording the value as "Not Done". | Record Not Done ifthe PK sample was not collected. | Not Done | Char | HR | Indicate if the specimen was not done. | PCSTAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (ND) | N/A | A Not Done check box, which indicates the test was "NOT DONE". Typically, there would be one check box for each measurement. This field can be useful on individual sample collections to confirm that a blank result field is meant to be blank. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 8 | PCREASND | PK Sampling Reason Not Done | An explanation of why the data are not available. | What was the reason the PK sample was not collected? | Reason Not Collected | Char | O | Provide the reason why a PK sample was not collected. | PCREASND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | The reason the data are not available may be chosen from a sponsor-defined list (e.g., broken equipment, subject refused) or entered as free text. When PCREASND is used, the SDTMIG variable PCSTAT should also be populated in the SDTM-based dataset. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 9 | PCDAT | PK Sample Collection Date | The date of PK sample collection or the start date of PK sample collection over a period of time (protocol-defined time-point range), represented in an unambiguous date format (e.g., DD- MON-YYYY). | What was the date of the PK sample collection? | Collection Date | Char | HR | Record the date when PK sample collection occurred using this format (DD-MON-YYYY). If left blank, "PCDATFL" for this specimen must be populated (or "PCPERF" must be flagged to indicate this sample was not collected). | PCDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable PCDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The date of collection may be determined from the date of visit (VISDAT) and if so, a separate assessment date field is not required. The SDTMIG PCDTC variable contains either a date/time when a specimen is collected at a point in time or the start date/time, when a specimen is collected over time. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 10 | PCDATFL | PK Sampling Date Flag | Flag indicating that the PK date (or start date) is the same as the previous specimen collection date (or start date). | Was the specimen/sample collected on the same date as the [last/previous specimen/sample] [collected/collection ended]? | Same as Previous (Specimen/Sample Collection End) Date | Char | O | Select when the date of this specimen collection is the same as the date of the previous specimen collected. If left blank, "PCDAT" for this specimen must be populated. (or "PCPERF" must be flagged to indicate this sample was not collected) | N/A | Does not map to an SDTMIG variable. The SDTM aCRF is annotated to indicate that this field is NOT SUBMITTED. | N/A | N/A | When a series of specimen are taken on a single form, this field is tied to the collection date to allow for the flag to be used as a surrogate for the date field. Its selection means that the date of this specimen is the same as the date of the last specimen collected (in the series). This variable may be used when collecting PK data and re-entering dates is more cumbersome than selecting the check box. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 11 | PCTIM | PK Sample Collection Time | The time of PK sample collection or start time for a specimen collected over a period of time (protocol- defined time-point range), represented in an unambiguous time format (e.g., hh:mm:ss). | What was the time of the PK sample collection? | Collection Time | Char | HR | Record time of collection (as complete as possible). | PCDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable PCDTC in ISO 8601 format. | N/A | N/A | A complete time is expected. The SDTMIG PCDTC variable contains either a date/time when a specimen is collected at a point in time or the start date/time, when a specimen is collected over time. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 12 | PCTPT | PK Sampling Planned Time Point Name | A text description of planned time point when measurements should be taken as defined in the protocol. | What was the planned time point of the PK sample collection? | [Planned Time Point Name] | Char | R/C | Record the planned time point labels for the PK sample collection, if not preprinted on the CRF. Note: Planned time points are often described as relative to the dosing of the study drug. | PCTPT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. See SDTMIG for additional information on representing time points. The SDTMIG time point anchors PCTPTREF (text description) and PCRFTDTC (date/time) may be needed, as well as SDTMIG variables PCTPTNUM, PCELTM. | N/A | N/A | Planned time points are needed to differentiate multiple sequential assessments. It is recommended that time points should be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as Planned Time Point can be included as the column header. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 13 | PCFAST | PK Sampling Fasting Status | An indication that the subject has abstained from food/water for the specified amount of time. | Was the subject fasting? | Fasting | Char | R/C | Record whether the subject was fasting prior to the test being performed. | PCFAST | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | Results may be affected by whether the subject was fasting. Some study treatments may have a food effect and it is important to know whether the dose was taken while the subject was fasted. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 14 | PCCOND | PK Sampling Test Condition Met | Indication whether the testing conditions defined in the protocol were met (e.g., low-fat diet). | Were the protocol- defined testing conditions met? | Test Condition Met | Char | R/C | Record whether protocol defined testing conditions were met. | SUPPPC.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPPC dataset as the value of SUPPPC.QVAL where SUPPPC.QNAM ="PCCOND" and SUPP.PCLABEL= "Test Condition Met". Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. | (NY) | N/A | This information is collected when the test results may be affected by whether conditions for testing were properly met. The specific testing conditions required should be preprinted on the CRF (e.g., "Did subject meet diet requirements?"). This may not be relevant for all tests. Examples of conditions imposed may include: Calorie fast, Fluid fast, High-fat meal, Low-fat meal, or Exercise. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 15 | PCREFID | PK Sampling Reference ID | An internal or external identifier (e.g., specimen identifier). | What was the (PK) [reference identifier/accession number]? | (PK) [Reference Identifier/Accession Number] | Char | O | Record the specimen or accession number assigned. | PCREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | This can be used to reconcile CRF data. May be included for linking back to specimens (e.g., Specimen ID). |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 16 | PCSPEC | PK Sampling Specimen Type | The type of specimen used for a PK sample. | What was the specimen (material) type? | [Specimen Type] | Char | HR | Record the specimen material type, if not preprinted on the CRF. | PCSPEC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECTYPE) | N/A | The type of specimen used for a measure. Should be collected if not available elsewhere, or if required to differentiate multiple specimens. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 17 | PCTEST | PK Sampling Test Name | Descriptive name of the analyte or specimen characteristics used to obtain the PK measurement or finding. | What was the test name? | [Test Name] | Char | O | Record the name of the measurement or finding, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | PCTEST; PCTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable PCTESTCD may be determined from the value collected in PCTEST. The SDTMIG variables PCTESTCD and PCTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | N/A | N/A | Sponsors typically collect tests related to the specimen characteristics on the CRF (e.g., Volume, Ph). Results for tests on an analyte (e.g., Concentration) would typically be populated when the SDTM- based datasets are created. If the analyte test results are collected on the CRF, the test would be the analyte name. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 18 | PCORRES | PK Sampling Result in Original Units | Result of the measurement or finding as originally received or collected. | What was the result of the test? | (Result) | Char | O | Record the test result, interpretation or finding. | PCORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Should be preprinted on the CRF with the associated test when possible, rather than collected in a field that requires the site to enter text. |
Findings | PC | PK Sample Collection at Fixed Time Points | N/A | 19 | PCORRESU | PK Sampling Original Units | The unit of the result as originally received or collected. | What was the unit of the result? | Unit | Char | O | Record or select the original unit in which these data were collected, if not preprinted on CRF. | PCORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | N/A | Should be preprinted on the CRF with the associated test when possible, rather than collected in a field that requires the site to enter text. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What is the subject identifier? | Subject | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable PCDTC in ISO 8601 format. | N/A | N/A | The date the PK samples were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the PK samples at that visit, or the collection date can be collected on the PK CRF using the date (PCDAT) field. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 6 | PCPERF | PK Sampling Performed | An indication whether or not PK samples were collected. | Were PK samples collected? | Collected | Char | O | Indicate that all of the PK samples in this group were collected. | PCSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTMIG variable PCSTAT. If PCPERF="N", the value of PCSTAT will be "NOT DONE". If PCPERF="Y", PCSTAT should be null. PCTEST and PCTESTCD must reflect what tests were not done. A combination of SDTMIG variables (e.g., PCCAT and PCSCAT, PCTPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable PCTESTCD would be populated as PCALL and an appropriate test name (PCTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This general prompt question is used as a data management tool to verify that missing results are confirmed missing. This may be implemented at form level or sample level. These may be all samples of a particular type or all samples taken for some purpose and may need to be identified by the organization of the data on the form. Each sample collected could result in 1 or more tests performed, so there can be a one-to-one or one-to-many relationship between samples and tests/results. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 7 | PCREASND | PK Sampling Reason Not Done | An explanation of why the data are not available. | What was the reason the PK sample was not collected? | Reason Not Collected | Char | O | Provide the reason why a PK sample was not collected. | PCREASND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | The reason the data are not available may be chosen from a sponsor-defined list (e.g., broken equipment, subject refused) or entered as free text. When PCREASND is used, the SDTMIG variable PCSTAT should also be populated in the SDTM-based dataset. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 8 | PCDAT | PK Sample Collection Date | The date of PK sample collection or the start date of PK sample collection over a period of time (protocol-defined time-point range), represented in an unambiguous date format (e.g., DD- MON-YYYY). | What was the date of the PK sample collection? | Collection Date | Char | HR | Record the date when PK sample collection occurred using this format (DD-MON-YYYY). If left blank, "PCDATFL" for this specimen must be populated (or "PCPERF" must be flagged to indicate this sample was not collected). | PCDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable PCDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. The SDTMIG PCDTC variable contains either a date/time when a specimen is collected at a point in time or the start date/time, when a specimen is collected over time. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 9 | PCTIM | PK Sample Collection Time | The time of PK sample collection or start time for a specimen collected over a period of time (protocol- defined time-point range), represented in an unambiguous time format (e.g., hh:mm:ss). | What was the start time of the PK sample collection? | Collection Start Time | Char | HR | Record start time of collection (as complete as possible). | PCDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable PCDTC in ISO 8601 format. | N/A | N/A | A complete time is expected. In interval collection, start can be added as needed to the question text, prompt and CRF directions. The SDTMIG PCDTC variable contains either a date/time when a specimen is collected at a point in time or the start date/time, when a specimen is collected over time. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 10 | PCENDAT | PK Sample Collection End Date | The end date of the specimen collection, represented in an unambiguous date format (e.g., DD- MON-YYYY). | What was the end date of the specimen collection? | (Collection) End Date | Char | HR | Record the date when PK sample collection stopped using this format (DD-MON-YYYY). | PCENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable PCENDTC in ISO 8601 format. | N/A | N/A | The end date of specimen collection may be determined from the date of visit and if so, a separate assessment date field is not required. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 11 | PCENTIM | PK Sample Collection End Time | The end time of the specimen collection, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the specimen collection end time? | (Collection) End Time | Char | HR | Record end time of collection (as complete as possible). | PCENDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH END DATE and TIME components and populate the SDTMIG variable PCENDTC in ISO 8601 format. | N/A | N/A | A complete end time is expected. The SDTMIG variable PCENDTC variable contains the end date/time, when a specimen is collected over time. If there is no end date/time, the SDTMIG variable PCENDTC should be Null. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 12 | PCTPT | PK Sampling Planned Time Point Name | A text description of planned time point when measurements should be taken as defined in the protocol. | What was the planned time point of the PK sample collection? | [Planned Time Point Name] | Char | R/C | Record the planned time point labels for the PK sample collection, if not preprinted on the CRF. Note: Planned time points are often described as relative to the dosing of the study drug. | PCTPT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. See SDTMIG for additional information on representing time points. The SDTMIG time point anchors PCTPTREF (text description) and PCRFTDTC (date/time) may be needed, as well as SDTMIG variables PCTPTNUM, PCELTM. | N/A | N/A | Planned time points are needed to differentiate multiple sequential assessments. It is recommended that time points should be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as Planned Time Point can be included as the column header. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 13 | PCFAST | PK Sampling Fasting Status | An indication that the subject has abstained from food/water for the specified amount of time. | Was the subject fasting? | Fasting | Char | R/C | Record whether the subject was fasting prior to the test being performed. | PCFAST | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | Results may be affected by whether the subject was fasting. Some study treatments may have a food effect and it is important to know whether the dose was taken while the subject was fasted. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 14 | PCCOND | PK Sampling Test Condition Met | Indication whether the testing conditions defined in the protocol were met (e.g., low fat diet). | Were the protocol- defined testing conditions met? | Test Condition Met | Char | R/C | Record whether protocol defined testing conditions were met. | SUPPPC.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPPC dataset as the value of SUPPPC.QVAL where SUPPPC.QNAM ="PCCOND" and SUPP.PCLABEL= "Test Condition Met". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | This information is collected when the test results may be affected by whether conditions for testing were properly met. The specific testing conditions required should be preprinted on the CRF (e.g., "Did subject meet diet requirements?"). This may not be relevant for all tests. Examples of conditions imposed may include: Calorie fast, Fluid fast, High-fat meal, Low-fat meal, or Exercise. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 15 | PCREFID | PK Sampling Reference ID | An internal or external identifier (e.g., specimen identifier). | What was the (PK) [reference identifier/accession number]? | (PK) [Reference Identifier/Accession Number] | Char | O | Record the specimen or accession number assigned. | PCREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | This can be used to reconcile CRF data. May be included for linking back to specimens (e.g., Specimen ID). |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 16 | PCSPEC | PK Sampling Specimen Type | The type of specimen used for a PK sample. | What was the specimen (material) type? | Specimen Type | Char | HR | Record the specimen material type, if not preprinted on the CRF. | PCSPEC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (SPECTYPE) | N/A | The type of specimen used for a measure. Should be collected if not available elsewhere, or if required to differentiate multiple specimens. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 17 | PCTEST | PK Sampling Test Name | Descriptive name of the analyte or specimen characteristics used to obtain the PK measurement or finding. | What was the test name? | [Test Name] | Char | O | Record the name of the measurement or finding, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | PCTEST; PCTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable PCTESTCD may be determined from the value collected in PCTEST. The SDTMIG variables PCTESTCD and PCTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | N/A | N/A | Sponsors typically collect tests related to the specimen characteristics on the CRF (e.g., Volume, pH). Results for tests on an analyte (e.g., Concentration) would typically be populated when the SDTM- based datasets are created. If the analyte test results are collected on the CRF, the test would be the analyte name. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 18 | PCORRES | PK Sampling Result in Original Units | Result of the measurement or finding as originally received or collected. | What was the result of the test? | (Result) | Char | O | Record the PK sampling test result. | PCORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Both quantitative results and interpretive Findings or summaries may be recorded here. |
Findings | PC | PK Sample Collection over a Time Interval | N/A | 19 | PCORRESU | PK Sampling Original Units | The unit of the result as originally received or collected. | What was the unit of the result? | Unit | Char | O | Record the PK sampling test result. | PCORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Both quantitative results and interpretive findings or summaries may be recorded here. |
Assumptions for the CDASHIG PC - Pharmacokinetic Concentrations Domain
1. This domain contains details regarding the collection of the PK samples from subjects at the site (e.g., timing of sample collection and associated specimen properties). Typically, the CDASHIG PC domain does not include concentration results generated by the bioanalytical laboratory. However, if the sponsor has occasion to collect concentration results directly on the same CRF, variables may be created based on CDASH Model variables, using the rules previously discussed.
2. Other data may be needed for PK analysis, such as Demographics, Vital Signs, Substance Use, and Exposure. Refer to the appropriate CDASHIG sections for each of these domains.
Example CRFs for the CDASHIG PC - Pharmacokinetic Concentrations Domain
Example 1
Title: Pharmacokinetics
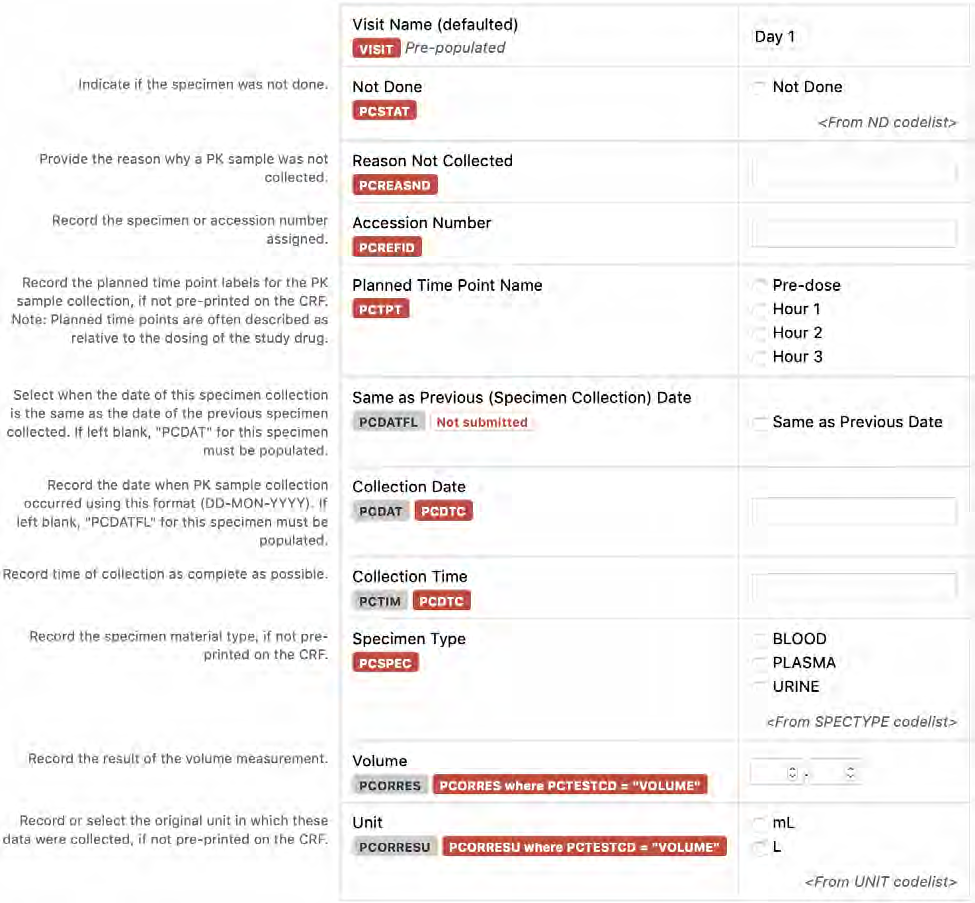
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
VISIT | 1 | What is the visit name? | Visit Name (defaulted) | N/A | Text | VISIT |
|
|
| Day 1 | Prompt | ||
PCSTAT | 2 | Indicate if the PK sample was not done. | Not Done | Indicate if the specimen was not done. | Text | PCSTAT |
| (ND) | Not Done |
| Prompt | checkbox | |
PCREASND | 3 | What was the reason the PK sample was not collected? | Reason Not Collected | Provide the reason why a PK sample was not collected. | Text | PCREASND | Prompt | ||||||
PCREFID | 4 | What was the PK accession number? | Accession Number | Record the specimen or accession number assigned. | Text | PCREFID |
|
|
|
| Prompt | ||
PCTPT | 5 | What was the planned time point of the PK sample collection? | Planned Time Point Name | Record the planned time point labels for the PK sample collection, if not pre- printed on the CRF. Note: Planned time points are often described as relative to the dosing of the study drug. | Text | PCTPT | Pre-dose; Hour 1; Hour 2; Hour 3 | Prompt | radio | ||||
PCDATFL | 6 | Check if the specimen was collected on the same date as the previous specimen collected. | Same as Previous (Specimen Collection) Date | Select when the date of this specimen collection is the same as the date of the previous specimen collected. If left blank, "PCDAT" for this specimen must be populated. | Text | N/A |
|
| Same as Previous Date |
| Prompt | checkbox | |
PCDAT | 7 | What was the date of the PK sample collection? | Collection Date | Record the date when PK sample collection occurred using this format (DD-MON-YYYY). If left blank, "PCDATFL" for this specimen must be populated. | Date | PCDTC | Prompt | ||||||
PCTIM | 8 | What was the time of the PK sample collection? | Collection Time | Record time of collection as complete as possible. | Time | PCDTC |
|
|
|
| Prompt | ||
PCSPEC | 9 | What was the specimen material type? | Specimen Type | Record the specimen material type, if not pre-printed on the CRF. | Text | PCSPEC | (SPECTYPE) | BLOOD; PLASMA; URINE | Prompt | ||||
PCORRES | 10 | What was the result of the volume measurement? | Volume | Record the result of the volume measurement. | Float | PCORRES | PCORRES where PCTESTCD = "VOLUME" |
| Prompt | ||||
PCORRESU | 11 | What was the unit of the result? | Unit | Record or select the original unit in which these data were collected, if not pre-printed on the CRF. | Text | PCORRESU | PCORRESU where PCTESTCD = "VOLUME" | (UNIT) | mL; L | Prompt | radio |
Example 2
Title: Pharmacokinetics
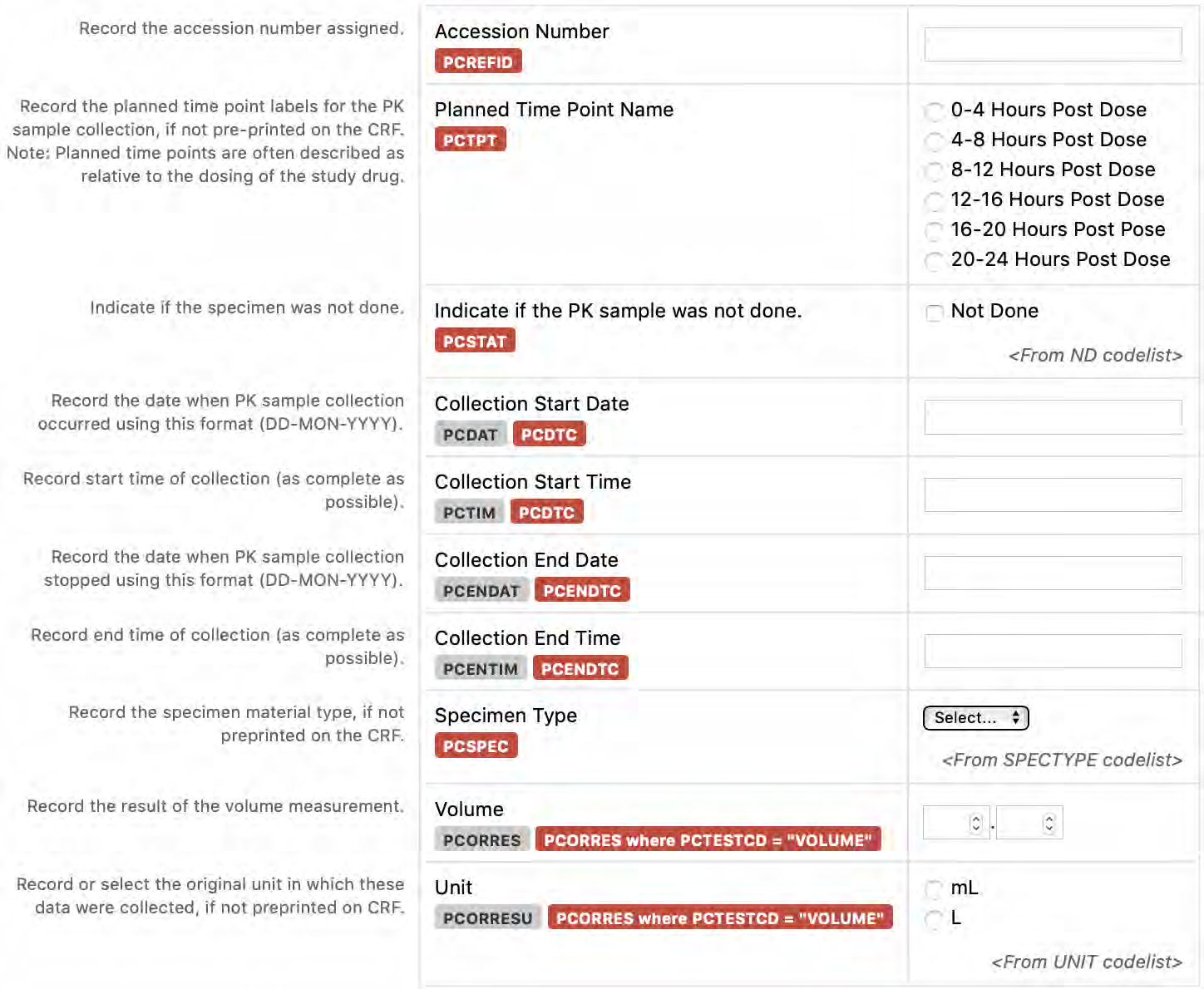
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
PCREFID | 1 | What was the (PK) [reference identifier/accession number]? | Accession Number | Record the accession number assigned. | Text | PCREFID | Prompt | ||||||
PCTPT | 2 | What was the planned time point of the PK sample collection? | Planned Time Point Name | Record the planned time point labels for the PK sample collection, if not pre-printed on the CRF. Note: Planned time points are often described as relative to the dosing of the study drug. | Text | PCTPT | 0-4 Hours Post Dose; 4-8 Hours Post Dose; 8-12 Hours Post Dose; 12-16 Hours Post Dose; 16-20 Hours Post Pose; 20-24 Hours Post Dose | Prompt | radio | ||||
PCSTAT | 3 | Indicate if the PK sample was not done. | Not Done | Indicate if the specimen was not done. | Text | PCSTAT |
| (ND) | Not Done | checkbox | |||
PCDAT | 4 | What was the date of the PK sample collection? | Collection Start Date | Record the date when PK sample collection occurred using this format (DD- MON-YYYY). | Date | PCDTC | prompt | ||||||
PCTIM | 5 | What was the (start) time of the PK sample collection? | Collection Start Time | Record start time of collection (as complete as possible). | Time | PCDTC | prompt | ||||||
PCENDAT | 6 | What was the end date of the specimen collection? | Collection End Date | Record the date when PK sample collection stopped using this format (DD- MON-YYYY). | Date | PCENDTC | prompt | ||||||
PCENTIM | 7 | What was the specimen collection end time? | Collection End Time | Record end time of collection (as complete as possible). | Time | PCENDTC | prompt | ||||||
PCSPEC | 8 | What was the specimen material type? | Specimen Type | Record the specimen material type, if not preprinted on the CRF. | Text | PCSPEC |
| (SPECTYPE) | Blood; Plasma; Urine | prompt | dropdown | ||
PCORRES | 9 | What was the result of the volume measurement? | Volume | Record the result of the volume measurement. | Float | PCORRES | PCORRES where PCTESTCD = "VOLUME" | prompt | |||||
PCORRESU | 10 | What was the unit of the result? | Unit | Record or select the original unit in which these data were collected, if not preprinted on CRF. | Text | PCORRESU | PCORRES where PCTESTCD = "VOLUME" | (UNIT) | mL; L | prompt | radio |
8.3.11 PE - Physical Examination
Description/Overview for the CDASHIG PE - Physical Examination Domain
The scope of the metadata for the PE domain is limited to general physical examinations as part of overall safety data collection. The data collection fields defined in the CDASHIG Metadata Tables may not fit the needs of targeted body system evaluations as part of therapeutic area-specific assessments. There are 3 scenarios that may be used for collecting physical exam data:
• Scenario 1 (Best Practice): The PE CRF is used only to record whether or not the exam was performed and, if so, the date of the examination. Sites are instructed to record baseline abnormalities on a medical history (MH) form, a targeted medical history form (e.g., a study-specific form requesting assessment of a predefined set of medical and/or surgical history events), or a baseline conditions form. Sites are typically instructed to record any post-baseline abnormalities or baseline conditions that worsened post-baseline on the adverse effects (AE) form.
• Scenario 2 (Traditional): The PE CRF is used at baseline and post-baseline visits.
• Scenario 3 (Traditional): The PE CRF is used at baseline, but not at post-baseline visits. Sites are instructed to record any post-baseline abnormalities or baseline conditions that worsened post-baseline on the AE form.
In Scenarios 2 and 3, similar fields are captured: date of exam, body system/code, normal/abnormal, and description of abnormality. Scenario 1 is recommended as the best practice for the following reasons:
1. It eliminates collection and reconciliation of duplicate data by capturing abnormal data in one central location. Abnormalities identified during a physical examination must also be recorded on an AE form, an MH form, or other similar form.
2. It reduces number of queries, thus reducing workload for data managers and site personnel.
3. It supports consistency and standardization for data reporting purposes. Physical examination data are rarely summarized, only tabulated in listing format. Any trend analysis or summarization of abnormalities is performed on AE data, and MH data are available for reference.
4. It reduces coding needs (if PE abnormalities are coded).
Best Practice Domain Model
Specification for the CDASHIG - Scenario 1 (Best Practice) Physical Examination
Scenario 1 is a change from the more traditional approach for the submission of physical examination data. In this approach, the SDTMIG PE domain is not submitted. The physical examination results are submitted in the appropriate SDTMIG domain using the following conventions:
• Screening/baseline results are submitted in the SDTMIG MH domain.
• Post-baseline abnormalities or baseline conditions that worsened are submitted in the SDTMIG AE domain.
• Sponsors may submit information on whether physical examinations were performed and when they were performed in the SDTMIG Procedures (PR) domain.
Note: There is no CDASH PE domain specification for this approach in the CDASHIG Metadata Table. Assumptions for the CDASHIG - Scenario 1 (Best Practice) Physical Examination
1. Because the data from a general physical examination are not required for safety or efficacy evaluations, a sponsor may decide not to collect them on a separate PE CRF. The data would be collected on other CRFs, typically the AE and MH CRFs.
2. If the sponsor chooses to create a physical examination CRF to capture only the information "Was the physical examination performed?" and Date/Time of Examination, these may be considered as optional fields intended for monitoring and data cleaning only. However, the sponsor could elect to submit this information in the SDTMIG PR domain.
Example CRF for the CDASHIG - Scenario 1 (Best Practice) Physical Examination
Example 1
The CDASH Physical Examination best practice recommends not submitting physical examination data in the SDTM PE domain. The record of a physical examination being performed may be recorded in the PR domain, as annotated below. Findings from a physical examination should be reported in the AE or MH domain, depending on whether the finding/abnormality started before or after the protocol-specified time period.
Title: Physical Examination-Scenario 1 (Best Practice)
|
Example CRF Completion Instructions If the physical finding/abnormality started prior to [protocol-specific time period], then it must be recorded as Medical History. If the physical finding started after [protocol-specific time period], it must be recorded as an Adverse Event. |
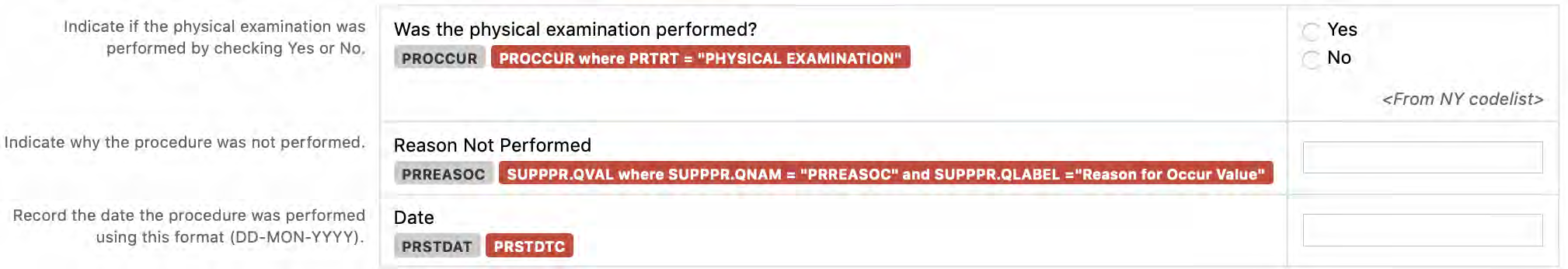
Traditional Domain Models
Specification for the CDASHIG PE -Scenarios 2 and 3 (Traditional) Physical Examination Domain
In Scenarios 2 and 3, the sponsor may elect to submit physical exam results in the SDTMIG PE domain, following the more traditional approach for data collection. The CDASHIG Metadata Table includes this traditional PE scenario.
Physical Exam (PE) Scenarios 2 and 3 (Traditional) Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | PE | PE- Traditional | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | PE | PE- Traditional | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | PE | PE- Traditional | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | PE | PE- Traditional | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | PE | PE- Traditional | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD- MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable PEDTC in ISO 8601 format. | N/A | N/A | The date of the physical examination can be determined from the Visit Date variable (VISDAT) and applying that date to all of the physical examination findings at that visit, or the collection date can be included collected on the PE CRF using the date (PEDAT) field. |
Findings | PE | PE- Traditional | N/A | 6 | PEPERF | Physical Examination Performed | An indication whether or not a planned physical examination was performed. | Was the physical examination performed? | Physical Exam Performed | Char | O | If physical examination was performed as planned then select Yes, otherwise, select No. | PESTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTMIG variable PESTAT. If PEPERF="N" the value of PESTAT="NOT DONE". If PEPERF="Y", then the actual physical exam results would be reported by body system (see PERES). PECAT, PESCAT, PETEST and PETESTCD must reflect what tests were not done. If used for an entire CRF or other set of multiple tests with PECAT and PESCAT, PETESTCD=PEALL. | (NY) | N/A | This general prompt question is used as a data management tool to verify that missing results are confirmed missing. Used to ask if the physical exam was performed at the overall subject level at the specified time point. If this field is used then the result should only be mapped to PESTAT if the overall examination, at the subject level, was not performed. If the overall examination was performed, then the value of PESTAT would be null for each examined body systems and "NOT DONE" for any body systems not examined (see PERES). |
Findings | PE | PE- Traditional | N/A | 7 | PECAT | Category for Examination | A grouping of topic- variable values based on user- defined characteristics. | What was category of the physical examination? | [PE Category]; NULL | Char | O | Record the physical examination category, if not preprinted on the CRF. | PECAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. The CRF can capture the different types of Physical Exams using PECAT (e.g., GENERAL, OPHTHAMOLOGIC, NEUROLOGICAL). This may be preprinted on the CRF. If PECAT is not collected (e.g., it is self- evident from the protocol design), it could be populated during the SDTM-based dataset creation process. |
Findings | PE | PE- Traditional | N/A | 8 | PESCAT | Subcategory for Examination | A sub-division of the PECAT values based on user- defined characteristics. | What was subcategory of the physical examination? | [PE Subcategory]; NULL | Char | O | Record the physical examination subcategory, if not preprinted on the CRF. | PESCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. The CRF can capture the different subtypes of PE categories using PESCAT. This may be preprinted on the CRF. If PESCAT is not collected (e.g. it is self-evident from the protocol design), it could be populated during the SDTM-based dataset creation process. PESCAT can only be used if there is a PECAT and it must be a subcategorization of PECAT. |
Findings | PE | PE- Traditional | N/A | 9 | PEDAT | Physical Examination Date | The date when the physical examination was performed, represented in an unambiguous date format (e.g., DD- MON-YYYY). | What was the date of the physical examination? | Exam Date | Char | R/C | Record complete date of examination using this format (DD-MON-YYYY). | PEDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable PEDTC in ISO 8601 format. | N/A | N/A | The date of examination may be determined from the date of the visit (VISDAT) and if so, a separate assessment date field is not required. |
Findings | PE | PE- Traditional | N/A | 10 | PETIM | Physical Examination Time | The time of examination, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the time of the physical examination? | Exam Time | Char | O | Record the time of examination (as complete as possible). | PEDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable PEDTC in ISO 8601 format. | N/A | N/A | Collect time if it is relevant for the analysis. |
Findings | PE | PE- Traditional | N/A | 11 | PESPID | Physical Exam Sponsor- Defined Identifier | A sponsor-defined identifier. In CDASH, This is typically used for preprinted or auto- generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor-defined question] | [Sponsor defined] | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | PESPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Because SPID is a sponsor- defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile data. May be used to record preprinted number (e.g. line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Findings | PE | PE- Traditional | N/A | 12 | PETEST | Body System Examined | Name of the body system. | What was the body system examined? | [Body System] | Char | HR | Per protocol, perform physical examinations of specified body systems. | PETEST; PETESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable PETESTCD may be determined from the value collected in PETEST. | N/A | N/A | Sponsor should pre-print all body systems to be examined on the CRF. The use of a complete list of body systems eliminates the need for another, specify category as any abnormalities identified should fall under 1 of the prespecified categories. If the form is laid out as a grid, then words such as "Body System" can be included as the column header. |
Findings | PE | PE- Traditional | N/A | 13 | PERES | Physical Exam Verbatim Finding | Overall assessment of examined body system. | Were the results normal, abnormal, or not done? | (Result) | Char | HR | Indicate the overall assessment for each exam category/body system listed. | PEORRES | This does not map directly to an SDTMIG variable. May be used to populate a value into the SDTMIG variable PEORRES. If PERES="Normal", populate PEORRES with the value of PERES. If PERES="Abnormal", populate PEORRES with the value of PEDESC. | N/A | N/A | If the examined body system is normal then the value in PEORRES should be "NORMAL". If the body system is not examined, then the value in PEORRES should be Null and the value in PESTAT should be "NOT DONE". If the examined body system is abnormal, then the value of PEORRES should contain the text of the abnormal findings (PEDESC). If the sponsor's data collection system allows for up- front recording of the abnormality and status using a single variable, then the SDTM variable name PEORRES may be used in place of CDASH variable names PERES and PEDESC. When the SDTM- based datasets are created, PESTRESC is the standardized value for PEORRES and is populated for any record where PEORRES is not null. If the abnormal findings are coded using a dictionary, then PESTRESC should be the dictionary preferred term or if not coded PEORRES. |
Findings | PE | PE- Traditional | N/A | 14 | PEDESC | Physical Exam Abnormal Findings | Text description of any abnormal findings. | If the result was abnormal, what were the findings? | Abnormal Findings | Char | HR | Record all abnormal findings for the given body system in the space provided. | PEORRES | This does not map directly to an SDTMIG variable. May be used to populate a value into the SDTMIG variable PEORRES. If PERES="Normal", populate PEORRES with the value of PERES. If PERES="Abnormal", populate PEORRES with the value of PEDESC. | N/A | N/A | If the examined body system is normal then the value in PEORRES should be "NORMAL". If the body system is not examined, then the value in PEORRES should be Null and the value in PESTAT should be "NOT DONE". If the examined body system is abnormal, then the value of PEORRES should contain the text of the abnormal findings (PEDESC). If the sponsor's data collection system allows for up front recording of the abnormality and status using a single variable then the SDTM variable name PEORRES may be used in place of CDASH variable names PERES and PEDESC. |
Findings | PE | PE- Traditional | N/A | 15 | PECLSIG | Physical Exam Clinical Significance | An indication whether the physical examination abnormality is clinically significant. | Was the physical examination result clinically significant? | Clinically Significant | Char | O | Was the physical examination result clinically significant? If Yes select Yes, otherwise, select No. | SUPPPE.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPPE dataset as the value of SUPPPE.QVAL where SUPPPE.QNAM ="PECLSIG" and SUPPPE.QLABEL="Clinically Significant". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | If this level of information is needed for reconciliation with adverse events, this field may be added to the CRF. |
Findings | PE | PE- Traditional | N/A | 16 | PEEVAL | Physical Exam Evaluator | The role of the person who provided the evaluation. | Who was the evaluator? | [Evaluator/Reporter] | Char | O | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | PEEVAL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EVAL) | N/A | Used only for results that are subjective (e.g., assigned by a person or a group). May be preprinted or collected. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | PE | PE- Traditional | N/A | 17 | PEREASND | Physical Exam Reason Not Examined | An explanation of why the data are not available. | What is the reason that data was not collected? | Reason Not Done | Char | O | Provide the reason the assessment was not done. | PEREASND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Captures the reason why the measurement or test was not done. The reason may be chosen from a sponsor-defined list (e.g., broken equipment, subject refused) or entered as free text. |
Findings | PE | PE- Traditional | N/A | 18 | PEBODSYS | Body System or Organ Class | Body System or Organ Class that is involved for a finding from the standard hierarchy for dictionary-coded results. | What is/was the [body system/ organ system]? | [Body System/Organ System] | Char | O | N/A | PEBODSYS | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | PEBODSYS should be assigned using a coding system. If included on the CRF, it is prepopulated and must be paired by the sponsor with specific prespecified verbatim terms. If not included on the CRF, PEBODSYS is assigned through the coding process. |
Findings | PE | PE- Traditional | N/A | 19 | PEMODIFY | Physical Exam Modified Reported Term | If the value for PEORRES is modified for coding purposes, then the modified text is placed here. | N/A | N/A | Char | O | N/A | PEMODIFY | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This is not a data collection field that would appear on the CRF. Sponsors will populate this through the coding process. PEMODIFY contains any modified text used for coding. Used only when the reported abnormalities in PEORRES are coded to a dictionary. This is in contrast to Events and Interventions domains where the topic variable (TERM or TRT) is modified for coding. |
Findings | PE | PE- Traditional | N/A | 20 | PELOC | Location of Physical Exam Finding | A description of the anatomical location of the subject relevant to the collection of physical examination. | What was the anatomical location of the body system examined or the finding? | Anatomical Location | Char | O | Indicate the anatomical location of the abnormal finding. | PELOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location. Sponsors may collect the data using a subset list of Controlled Terminology on the CRF. LAT, DIR, and PORTOT are used to further describe the anatomical location. |
Findings | PE | PE- Traditional | N/A | 21 | PELAT | Physical Exam Laterality | Qualifier for anatomical location further detailing the side of the body. | What was the side of the anatomical location of the body system examined or the finding? | Side | Char | O | Record the side of the anatomical location of the abnormal finding. | PELAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | PE | PE- Traditional | N/A | 22 | PEDIR | Physical Exam Directionality | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What was the directionality of the anatomical location of the of the body system examined or the finding? | Directionality | Char | O | Record the directionality of the anatomical location of the abnormal finding. | PEDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | PE | PE- Traditional | N/A | 23 | PEPORTOT | PE Location Portion or Totality | Qualifier for anatomical location further detailing the distribution, which means arrangement of, apportioning of. | What was the portion or totality of the anatomical location of the body system examined or the finding? | or Totality | Char | O | Indicate the proportionality of the anatomical location of the of the abnormal finding. | PEPORTOT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (PORTOT) | N/A | Collected when the sponsor needs to identify the specific portionality for the anatomical locations. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | PE | PE- Traditional | N/A | 24 | PEMETHOD | PE Method of Test or Examination | Method of the test or examination. | What was the method used for the test or examination? | Method | Char | O | Record the method of test or examination. | PEMETHOD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (METHOD) | N/A | This information may be collected when more than 1 method is possible, and collecting the method used is necessary. |
Assumptions for the CDASHIG PE - Scenarios 2 and 3 (Traditional) Physical Examination Domain
1. If the sponsor chooses to create a traditional PE CRF, PEYN is still considered an optional field intended for monitoring and data cleaning only. If a sponsor wants to document information about overall physical examination at the subject level that were not performed in the SDTM submission, this field is mapped to PESTAT. If PEPERF="N", then PECAT="GENERAL", PETEST="PHYSICAL EXAMINATION", PETESTCD="PEALL", and PESTAT="NOT DONE". If PEPERF="Y", then the actual physical examination results would be reported by body system (see PERES).
2. Original and Standardized Results
a. The CDASHIG variable PERES is used to collect test results or findings in the original units in character format as well as to collect the information on what specific body systems were not examined.
b. When the results of a test or examination are reported as Normal or Abnormal, a description of the abnormal finding may also be collected using the CDASHIG element PEDESC.
c. Information on what body system reviews were not done is mapped to the appropriate SDTMIG variables (i.e., PETEST, PETESTCD, PESTAT, with ORRES being NULL). The value of "NOT DONE" in CDASHIG variable PERES should not be mapped to the SDTMIG variable PEORRES.
d. The standardization of the original findings results is expected to be performed when the SDTM submission datasets are created. The SDTM mapping rules are provided in the CDASHIG Metadata Table.
3. Date of Collection is the date that the examination was performed. The SDTMIG variable PEDTC can be populated from the date of visit.
Example CRF for the CDASHIG PE - Scenarios 2 and 3 (Traditional) Physical Examination Domain
Example 1
Title: Body System Physical Examination
Example CRF Completion Instructions The physical examination must be performed by a qualified health care professional (i.e., MD, PA or NP) listed on the FDA form 1572. If the physical finding/abnormality started prior to/at [protocol-specific time period], then it must be recorded on the medical history page. If the physical finding/abnormality started after [protocol-specific time period], it must be recorded as an Adverse Event. |
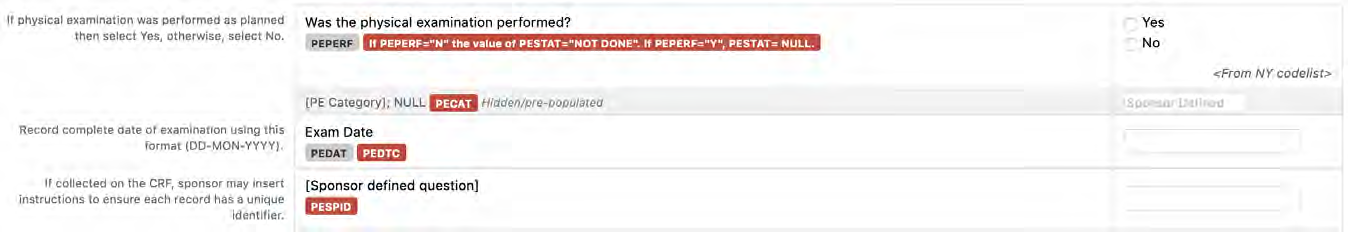
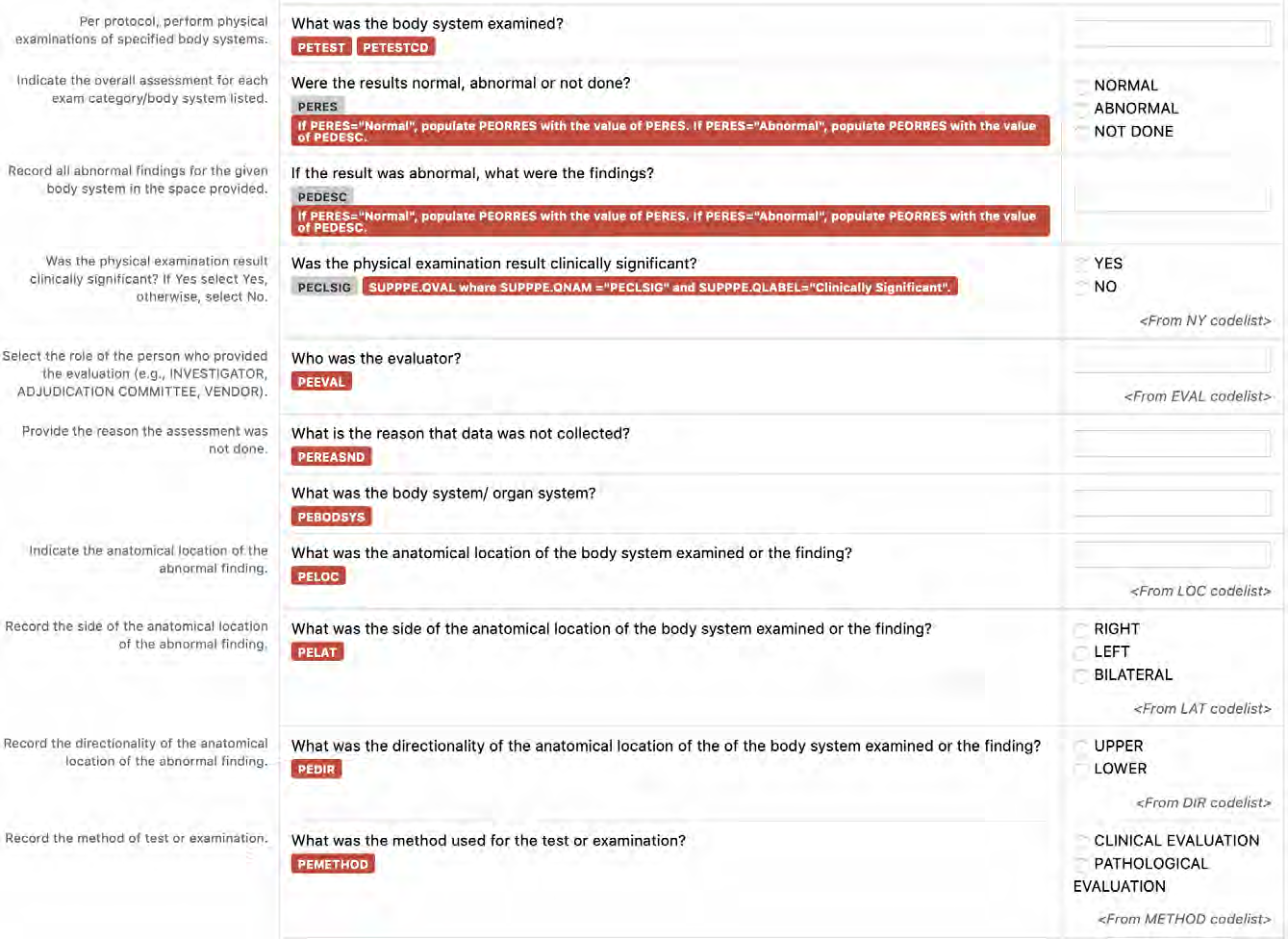
CRF Metadata
| CDASHIG Variable | Order Number | Question Text | Prompt | Case Report Form Completion Instructions | Data Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Codelist Name | CRF Implementation Notes | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
PEPERF | 1 | Was the physical examination performed? | Physical Exam Performed | If physical examination was performed as planned then select Yes, otherwise, select No. | text | PESTAT | If PEPERF="N" the value of PESTAT="NOT DONE". If PEPERF="Y", PESTAT= NULL. | (NY) | If used for an entire CRF or other set of multiple tests with PECAT and PESCAT, PETESTCD=PEALL. | Yes; No; | ||||
PECAT | 2 | What was category of the physical examination? | [PE Category]; NULL | Record the physical examination category, if not pre-printed on the CRF. | text | PECAT | N/A | Sponsor Defined | Y | |||||
PEDAT | 4 | What was the date of the physical examination? | Exam Date | Record complete date of examination using this format (DD-MON-YYYY). | text | PEDTC | N/A | prompt | ||||||
PESPID | 6 | [Sponsor defined question] | [Sponsor defined] | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | text | PESPID | N/A | |||||||
PETEST | 7 | What was the body system examined? | [Body System] | Per protocol, perform physical examinations of specified body systems. | text | PETEST; PETESTCD |
N/A |
|||||||
PERES | 8 | Were the results normal, abnormal or not done? | (Result) | Indicate the overall assessment for each exam category/body system listed. | text | PEORRES | If PERES="Normal", populate PEORRES with the value of PERES. If PERES="Abnormal", populate PEORRES with the value of PEDESC. | N/A | NORMAL; ABNORMAL; NOT DONE | |||||
PEDESC | 9 | If the result was abnormal, what were the findings? | Abnormal Findings | Record all abnormal findings for the given body system in the space provided. | text | PEORRES | If PERES="Normal", populate PEORRES with the value of PERES. If PERES="Abnormal", populate PEORRES with the value of PEDESC. | N/A |
| |||||
PECLSIG | 10 | Was the physical examination result clinically significant? | Clinically Significant | Was the physical examination result clinically significant? If Yes select Yes, otherwise, select No. | text | SUPPPE.QVAL | SUPPPE.QVAL where SUPPPE.QNAM ="PECLSIG" and SUPPPE.QLABEL="Clinically Significant". | (NY) | YES; NO | |||||
PEEVAL | 11 | Who was the evaluator? | [Evaluator/Reporter] | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | text | PEEVAL | (EVAL) | |||||||
PEREASND | 12 | What is the reason that data was not collected? | Reason Not Done | Provide the reason the assessment was not done. | text | PEREASND | N/A | |||||||
PEBODSYS | 13 | What was the body system/ organ system? | [Body System/Organ System] | N/A | text | PEBODSYS | N/A | |||||||
PELOC | 15 | What was the anatomical location of the body system examined or the finding? | Anatomical Location | Indicate the anatomical location of the abnormal finding. | text | PELOC | (LOC) | |||||||
PELAT | 16 | What was the side of the anatomical location of the body system examined or the finding? | Side | Record the side of the anatomical location of the abnormal finding. | text | PELAT | (LAT) | RIGHT; LEFT; BILATERAL | ||||||
PEDIR | 17 | What was the directionality of the anatomical location of the of the body system examined or the finding? | Directionality | Record the directionality of the anatomical location of the abnormal finding. | text | PEDIR | (DIR) | UPPER; LOWER | ||||||
PEMETHOD | 19 | What was the method used for the test or examination? | Method | Record the method of test or examination. | text | PEMETHOD | (METHOD) | CLINICAL EVALUATION; PATHOLOGICAL EVALUATION |
|
8.3.12 QRS - Questionnaires, Ratings, and Scales
Description/Overview for the CDASHIG QRS - Questionnaires, Ratings, and Scales Domain
Questionnaires, ratings, and scales (QRS) are standardized and often validated instruments, and the data collected using them are represented in SDTMIG domains including Questionnaires (QS), Disease Response and Clin Classification (RS), and Functional Tests (FT). Refer to the SDTMIG or the QRS web page (https://www.cdisc.org/foundational/qrs) for complete information on these domains. CDISC publishes supplemental specifications called QRS Supplements, including example annotated CRFs (aCRFs) for many of these instruments. CDISC Operational Procedure 017 (https://www.cdisc.org/system/files/) describes the development methodology for new QRS terminology. Because the nature of QRS precludes implementers from modifying the published data collection structure, the CDASHIG Metadata Table does not include specifications for QRS. Instead, implementers should refer to instrument-specific QRS Supplements on the QRS web page for example aCRFs, instrument-specific assumptions, and data examples.
For definitions and descriptions of the different types of questionnaires, ratings, and scales, visit the QRS web page.
The released QRS documentation is maintained on the CDISC QRS web page.
Specification for the CDASHIG QRS - Questionnaires, Ratings, and Scales Domain
Reference the QRS Supplements posted on the QRS web page (https://www.cdisc.org/foundational/qrs) and the specifications for specific domains (QS, RS, and FT) in the SDTMIG.
Assumptions for the CDASHIG QRS - Questionnaires, Ratings, and Scales Domain
1. CDISC standards for QRS include controlled terminology for test codes (--TESTCD), test names (--TEST), standard timing values, standard results for database values, and an aCRF with SDTMIG domain variable names. These standards can be used to create an electronic data collection (EDC) structure following the same conventions that would be used for any Findings class domain. The SDTMIG QS, RS, and FT domains utilize a normalized data structure; that is, 1 variable (--TEST) is used to capture the test name and another variable (--ORRES) is used to capture the result. Even though these domain variables are presented as a normalized structure in the CDASHIG Metadata Table, implementers using a denormalized structure (1 variable for each test) should create variable names that mirror the values in QRS controlled terminology (e.g., QSTESTCD, RSTESTCD, FTTESTCD).
2. Electronic representations of QRS instruments should reflect the title, subheadings, and exact numbering and wording of questions as they appear in original versions.
3. Electronic response fields should allow either the original response (--ORRES) or coded value (--STRESC) to be input—but usually not both, to avoid discrepancies.
4. Checkboxes that appear on validated QRS instruments should remain checkboxes in the CRF/eCRF.
5. Copyrighted instruments may include the copyright notice on the eCRF/CRF. For more copyright Information about QRS instruments, see the QRS web page (https://www.cdisc.org/foundational/qrs).
6. Instrument-specific assumptions are included in the QRS Supplements posted on the QRS web page. http://www.cdisc.org/qrs
Example CRF for the CDASHIG QRS - Questionnaires, Ratings, and Scales Domain
Example 1
Title: European Quality of Life Five Dimension Five Level Scale Questionnaire (EQ-5D-5L)
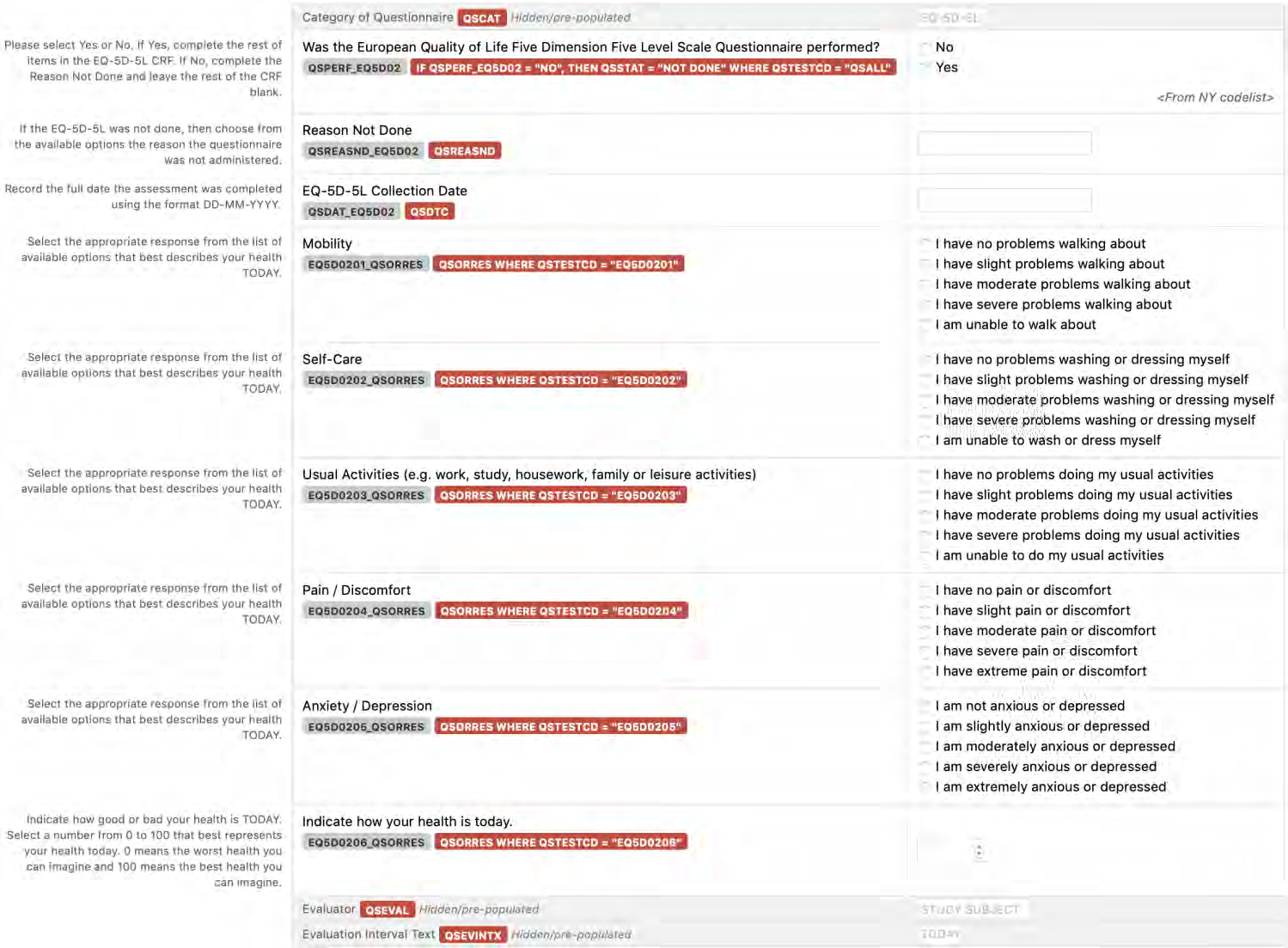
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTM Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
QSCAT | 1 | What is the category of the questionnaire? | Category of Questionnaire | Record the questionnaire category, if not pre-printed on the CRF. | Text | QSCAT |
| EQ-5D-5L | Yes | ||||
QSPERF_EQ5D02 | 2 | Was the European Quality of Life Five Dimension Five Level Scale Questionnaire performed? | EQ-5D-5L Performed | Please select Yes or No. If Yes, complete the rest of items in the EQ-5D-5L CRF. If No, complete the Reason Not Done and leave the rest of the CRF blank. | Text | N/A | IF QSPERF_EQ5D02 = "NO", THEN QSSTAT = "NOT DONE" WHERE QSTESTCD = "QSALL" | (NY) | No;Yes | radio | |||
QSREASND_EQ5D02 | 3 | What was the reason that the EQ- 5D-5L was not done? | Reason Not Done | If the EQ-5D-5L was not done, then choose from the available options the reason the questionnaire was not administered. | Text | QSREASND |
|
| Prompt | ||||
QSDAT_EQ5D02 | 4 | What was the date the EQ-5D-5L was collected? | EQ-5D-5L Collection Date | Record the full date the assessment was completed using the format DD-MM-YYYY. | Date | QSDTC |
|
| Prompt | ||||
EQ5D0201_QSORRES | 6 | Mobility | EQ5D02-Mobility | Select the appropriate response from the list of available options that best describes your health TODAY. | Text | QSORRES | QSORRES WHERE QSTESTCD = "EQ5D0201" | I have no problems walking about; | radio | ||||
EQ5D0202_QSORRES | 7 | Self-Care | EQ5D02-Self-Care | Select the appropriate response from the list of available options that best describes your health TODAY. | Text | QSORRES | QSORRES WHERE QSTESTCD = "EQ5D0202" | I have no problems washing or dressing myself; I have slight problems washing or dressing myself; I have moderate problems washing or dressing myself; I have severe problems washing or dressing myself; I am unable to wash or dress myself | radio | ||||
EQ5D0203_QSORRES | 8 | Usual Activities (e.g. work, study, housework, family or leisure activities) | EQ5D02-Usual Activities | Select the appropriate response from the list of available options that best describes your health TODAY. | Text | QSORRES | QSORRES WHERE QSTESTCD = "EQ5D0203" | I have no problems doing my usual activities; I have slight problems doing my usual activities; I have moderate problems doing my usual activities; I have severe problems doing my usual activities; I am unable to do my usual activities | radio | ||||
EQ5D0204_QSORRES | 9 | Pain / Discomfort | EQ5D02- Pain/Discomfort | Select the appropriate response from the list of available options that best describes your health TODAY. | Text | QSORRES | QSORRES WHERE QSTESTCD = "EQ5D0204" | I have no pain or discomfort; I have slight pain or discomfort; I have moderate pain or discomfort; I have severe pain or discomfort; I have extreme pain or discomfort | radio | ||||
EQ5D0205_QSORRES | 10 | Anxiety / Depression | EQ5D02- Anxiety/Depression | Select the appropriate response from the list of available options that best describes your health TODAY. | Text | QSORRES | QSORRES WHERE QSTESTCD = "EQ5D0205" | I am not anxious or depressed; I am slightly anxious or depressed; I am moderately anxious or depressed; I am severely anxious or depressed; I am extremely anxious or depressed | radio | ||||
EQ5D0206_QSORRES | 11 | Indicate how your health is today. | EQ5D02-EQ VAS Score | Indicate how good or bad your health is TODAY. Select a number from 0 to 100 that best represents your health today. 0 means the worst health you can imagine and 100 means the best health you can imagine. | integer | QSORRES | QSORRES WHERE QSTESTCD = "EQ5D0206" | ||||||
QSEVAL | 12 | Who was the evaluator? | Evaluator | Text | QSEVAL | STUDY SUBJECT | Yes | ||||||
QSEVINTX | 13 | Evaluation Interval Text | Evaluation Interval Text |
| Text | QSEVINTX |
| TODAY | Yes |
For additional examples, see the aCRFs that are part of the QRS Supplements on the QRS web page (https://www.cdisc.org/foundational/qrs).
Example Data for the CDASHIG QRS - Questionnaires, Ratings and Scales Domain
See the examples in the QRS Supplements posted on the QRS web page (https://www.cdisc.org/foundational/qrs).
8.3.13 RP - Reproductive System Findings
Description/Overview for the CDASHIG RP - Reproductive System Findings Domain
The RP domain is used to collect all reproductive detail information about a subject, such as reproductive ability, reproductive history (e.g., number of previous pregnancies, number of births), pregnancy during the study, and so on. All reproductive system findings for a subject are contained in the RP domain rather than other SDTMIG domains. Although sponsors previously may have reported this information in the Subject Characteristics (SC) domain, this information is now consolidated into the RP domain.
Specification for the CDASHIG RP - Reproductive System Findings Domain
Reproductive System Findings Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | RP | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM- based dataset creation before submission. |
Findings | RP | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | RP | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | RP | N/A | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | RP | N/A | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD- MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable RPDTC in ISO 8601 format. | N/A | N/A | The date the reproductive system findings were collected can be determined from the Visit Data variable (VISDAT) and applying that date to all of the reproductive system findings at that visit, or the collection date can be included on the RP CRF using the date (RPDAT) field. |
Findings | RP | N/A | N/A | 6 | RPCAT | Category for Repro System Findings | A grouping of topic- variable values based on user-defined characteristics. | What was the category of the reproductive system? | [Reproductive System Category]; NULL | Char | O | Record the reproductive system category, if not preprinted on the CRF. | RPCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | RP | N/A | N/A | 7 | RPSCAT | Subcategory for Repro System Findings | A sub-division of the RPCAT values based on user-defined characteristics. | What was the subcategory of the reproductive system? | Reproductive System Subcategory]; NULL | Char | O | Record the reproductive system subcategory, if not preprinted on the CRF. | RPSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. RPSCAT can only be used if there is an RPCAT and it must be a subcategorization of RPCAT. |
Findings | RP | N/A | N/A | 8 | RPPERF | Reproductive System Evaluation Performed | An indication whether or not a planned measurement, series of measurements, test, or observation was performed. | Was a reproductive system evaluation performed? | Reproductive System Evaluation Performed | Char | O | Indicate whether or not a planned reproductive system evaluation was done. | RPSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTMIG variable RPSTAT. If the CDASH field RPPERF="N", the value of RPSTAT will be "NOT DONE". If RPPERF="Y", RPSTAT should be null. A combination of SDTMIG variables (e.g., RPCAT and RPSCAT, RPTPT) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable RPTESTCD would be populated as RPALL and an appropriate test name (RPTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This may be implemented for a series of reproductive system evaluations, or a specific reproductive system test. This general prompt question is used as a data management tool to verify that missing results are confirmed missing. |
Findings | RP | N/A | N/A | 9 | RPREASND | RP Reason Not Performed | An explanation of why the data are not available. | What was the reason the reproductive system test was not collected? | Reason Not Done | Char | O | Provide the reason the measurement or test was not done. | RPREASND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | The reason the data are not available may be chosen from a sponsor-defined list (e.g., broken equipment, subject refused) or entered as free text. When PCREASND is used, the SDTMIG variable PCSTAT should also be populated in the SDTM-based dataset. |
Findings | RP | N/A | N/A | 10 | RPYN | Any Reproductive System Findings | General prompt question regarding whether or not there are any reproductive system findings available. | Were there any reproductive system findings? | Any Reproductive System Findings | Char | O | Indicate if the there are reproductive system findings. If yes, include the appropriate details where indicated on the CRF. | N/A | Does not map to an SDTMIG variable. The SDTM aCRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification that all other fields on the CRF were deliberately left blank. |
Findings | RP | N/A | N/A | 11 | RPSPID | RP Sponsor- Defined Identifier | A sponsor-defined identifier. In CDASH, This is typically used for preprinted or auto- generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor-defined question] | [Sponsor defined] | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | RPSPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Because SPID is a sponsor- defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile data. May be used to record preprinted number (e.g. line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Findings | RP | N/A | N/A | 12 | RPTEST | Reproductive System Findings Test Name | Descriptive name for Reproductive System Finding. | What is the reproductive finding name? | [Reproductive System Findings Test Name] | Char | HR | Record the name of the reproductive system finding if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | RPTEST; RPTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable RPTESTCD may be determined from the value collected in RPTEST. The SDTMIG variables RPTESTCD and RPTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | (RPTEST) | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | RP | N/A | N/A | 13 | RPORRES | RP Result or Finding in Original Units | Result of the finding defined in reproductive system finding, as originally received or collected. | What was the result for the reproductive system question? | (Result) | Char | HR | Record reproductive system finding. | RPORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Both quantitative results and interpretive findings or summaries may be recorded here. |
Findings | RP | N/A | N/A | 14 | RPORRESU | RP Original Units | The unit of the result as originally received or collected. | What was the unit of the result? | Unit | Char | R/C | Record or select the original unit in which these data were collected, if not preprinted on CRF. | RPORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | N/A | Should be preprinted on the CRF with the associated test when possible, rather than collected in a field that requires the site to enter text. |
Findings | RP | N/A | N/A | 15 | RPDAT | Reproductive System Finding Date | The date on which the reproductive system result or finding was collected, represented in an unambiguous date format (e.g., DD- MON-YYYY) . | What was the date the reproductive system question was collected? | Collection Date | Char | R/C | Record date of collection using this format (DD-MON- YYYY). | RPDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable RPDTC in ISO 8601 format. | N/A | N/A | This should be a complete date. The date of collection may be determined from the date of visit (VISDAT). |
Assumptions for the CDASHIG RP - Reproductive System Findings Domain
Any information on medications related to reproduction (e.g., contraceptives, fertility treatments) should not be collected in the RP domain; instead, they will need to be collected in the Concomitant/Prior Medications (CM) domain.
Example CRF for the CDASHIG RP - Reproductive System Findings Domain
Example 1
Title: Reproductive System Findings
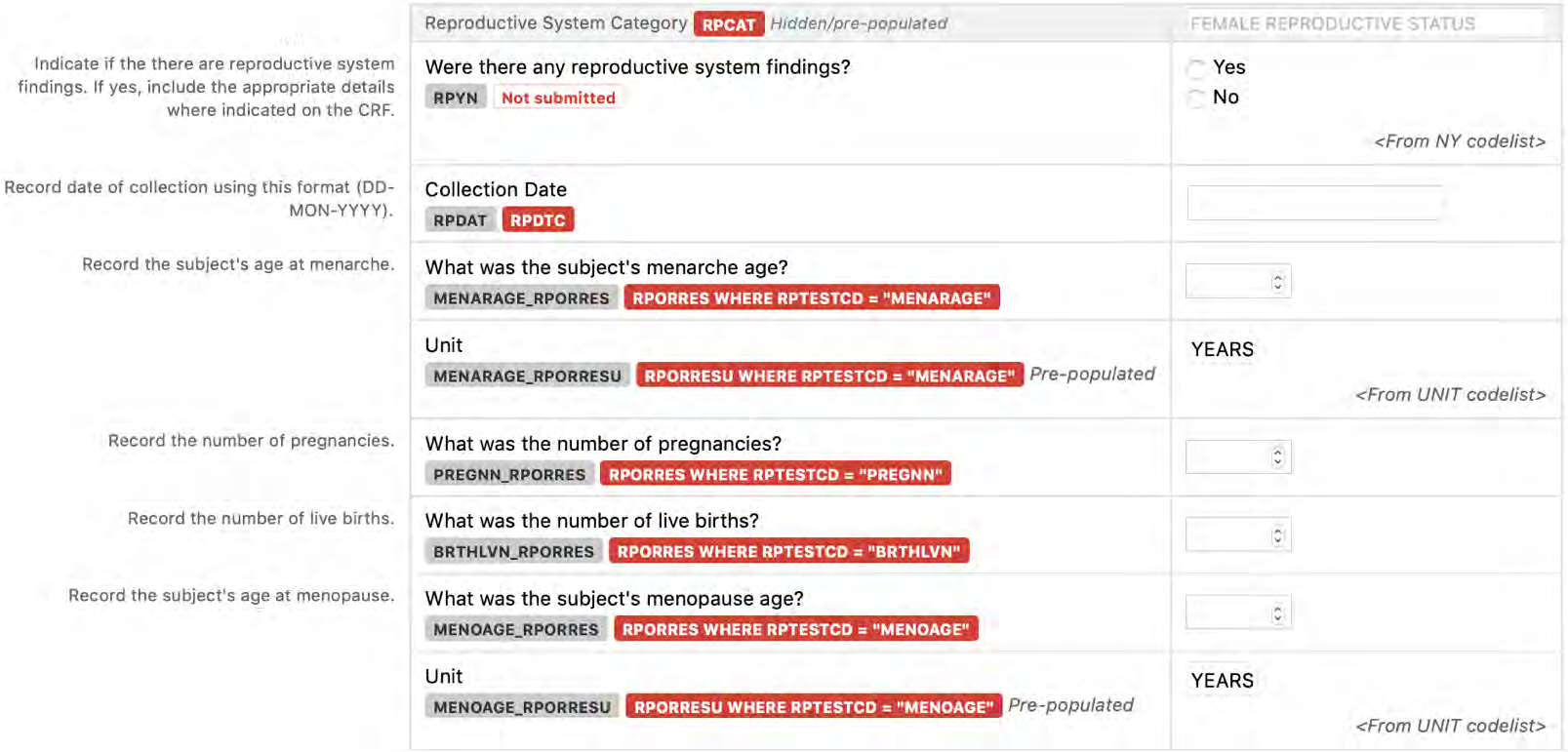
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre-Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
RPCAT | 1 | What was the category of the reproductive system? | Reproductive System Category | Record the reproductive system category, if not pre-printed on the CRF. | Text | RPCAT | FEMALE REPRODUCTIVE STATUS | Yes | |||||
RPYN | 2 | Were there any reproductive system findings? | Any Reproductive System Findings | Indicate if the there are reproductive system findings. If yes, include the appropriate details where indicated on the CRF. | Text | N/A | (NY) | Yes; No | radio | ||||
RPDAT | 4 | What was the date the reproductive system question was collected? | Collection Date | Record date of collection using this format (DD- MON-YYYY). | Date | RPDTC | Prompt | ||||||
MENARAGE_RPORRES | 5 | What was the subject's menarche age? | Menarche Age | Record the subject's age at menarche. | Integer | RPORRES | RPORRES WHERE RPTESTCD = "MENARAGE" |
| |||||
MENARAGE_RPORRESU | 6 | What was the unit? | Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | Text | RPORRESU | RPORRESU WHERE RPTESTCD = "MENARAGE" | (UNIT) | YEARS | Prompt | |||
PREGNN_RPORRES | 7 | What was the number of pregnancies? | Number of Pregnancies | Record the number of pregnancies. | Integer | RPORRES | RPORRES WHERE RPTESTCD = "PREGNN" |
| |||||
BRTHLVN_RPORRES | 8 | What was the number of live births? | Number of Live Births | Record the number of live births. | Integer | RPORRES | RPORRES WHERE RPTESTCD = "BRTHLVN" |
| |||||
MENOAGE_RPORRES | 9 | What was the subject's menopause age? | Menopause Age | Record the subject's age at menopause. | Integer | RPORRES | RPORRES WHERE RPTESTCD = "MENOAGE" |
| |||||
MENOAGE_RPORRESU | 10 | What was the unit? | Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | Text | RPORRESU | RPORRESU WHERE RPTESTCD = "MENOAGE" | (UNIT) |
| YEARS | Prompt |
8.3.14 RS - Disease Response and Clin Classification
Description/Overview for the CDASHIG RS - Disease Response and Clin Classification Domain
The CDASHIG RS domain describes assessment of disease response to treatment or clinical classifications, which are often based on published criteria. Clinical classifications may be based solely on objective data from clinical records, or they may involve a clinical judgment or interpretation of the directly observable signs, behaviors, or other physical manifestations related to a condition or subject status.
Specification for the CDASHIG RS - Disease Response and Clin Classification Domain
Disease Response and Clin Classification Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | RS | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | RS | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | RS | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | RS | N/A | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | RS | N/A | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD- MON-YYYY format. | N/A | This field is not an SDTM variable. If the date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM), concatenate the CDASH VISDAT/VISTIM components and populate the SDTMIG variable RSDTC in ISO 8601 format. | N/A | N/A | If the date the tests were collected can be determined from the Visit Date variable (VISDAT), apply that date to all of the tests at that visit, or the collection date can be collected on the CRF using the date (RSDAT). In this domain, it may not be appropriate to use the visit date as RSDTC. |
Findings | RS | N/A | N/A | 6 | RSCAT | Category for Response or Clin Class | A grouping of topic-variable values based on user-defined characteristics. | What is the [category/ criteria] for the [disease response/clinical classification] or What is the [response/clinical classification] criteria? | [Disease Response/Clinical Classification Category]; NULL | Char | R/C | N/A | RSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | (CCCAT);(ONCRSCAT) | This is most commonly either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response is typically a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. There are separate codelists used for categorizations of records about oncology response criteria (ONCRCAT) or other clinical classification (CRSCAT). Collect if multiple clinical classifications, or disease responses are active in a single study/database; otherwise, information should be distinguished somewhere on a form (e.g., table name, title, tab). |
Findings | RS | N/A | N/A | 7 | RSSCAT | Subcategory for Response or Clin Class | A sub-division of the RSCAT values based on user-defined characteristics. | What is the sub - category for the [disease response/clinical classification]? | [Disease Response/Clinical Classification Sub- Category]; NULL | Char | R/C | N/A | RSSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. RSSCAT can only be used if there is an RSCAT and it must be a subcategorization of RSCAT. |
Findings | RS | N/A | N/A | 8 | RSPERF | Response or Clin Class Performed | An indication whether or not a planned disease response or clinical classification assessment was performed. | Was the [(disease) response/clinical classification] assessment performed? | [Disease Response/Clinical Classification] Assessment | Char | O | Indicate whether or not the [disease response/clinical classification] assessment was performed. | RSSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTMIG variable RSSTAT. If RSPERF="N", the value of RSSTAT will be "NOT DONE". If RSPERF="Y", RSSTAT should be null. A combination of SDTMIG variables ( e.g., RSCAT and RSSCAT, RSTPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable RSTESTCD would be populated as RSALL and an appropriate test name (RSTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | A Not Done check box, which indicates the test was "NOT DONE". Typically, there would be one check box for each measurement. This field can be useful to confirm that a blank result field is meant to be blank. |
Findings | RS | N/A | N/A | 9 | RSREASND | Response or Clin Class Reason Not Done | An explanation of why the data are not available. | Why was the [disease response/clinical classification] assessment not performed? | Reason Response Assessment Not Performed | Char | O | If the response was not collected, indicate why. | RSREASND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | The reason the data are not available may be chosen from a sponsor defined list (e.g., Not Imaged, Patient Refusal, Site Error) or entered as free text. When RSREASND is used, the SDTMIG variable RSSTAT should also be populated in the SDTM-based dataset. |
Findings | RS | N/A | N/A | 10 | RSEVAL | Response or Clin Class Evaluator | The role of the person who provided the information. | What was the role of the person performing the [disease response/clinical classifiaction] assessment? | Evaluator | Char | R/C | Indicate who performed the assessment. | RSEVAL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EVAL) | N/A | Used only for results that are subjective (assigned by a person or a group). RSEVAL is expected for oncology response criteria. It can be null when the investigator provides all the data in a study. It should contain no null values when data from 1 of more evaluator are used in a study. |
Findings | RS | N/A | N/A | 11 | RSEVALID | Response or Clin Class Evaluator ID | Used to distinguish multiple evaluators with the same role. | What is the evaluator identifier? | Evaluator Identifier | Char | O | Identify the evaluator providing this evaluation. | RSEVALID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (MEDEVAL) | N/A | When multiple assessors play the role identified in RSEVAL, values of RSEVALID will attribute a row of data to a particular assessor. |
Findings | RS | N/A | N/A | 12 | RSLNKID | Response or Clin Class Link ID | An identifier used to link the disease response assessment to the related record in another domain which was used to determine the response result. | What was the [Disease Response or Clinical Classification ]Link ID Identifier? | [Disease Response or Clinical Classification ]Link ID | Char | O | If collected, record the unique [Disease Response or Clinical Classification ] Link ID Identifier. | RSLNKID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This variable is used to provide a unique code in order to link records across related CRFs (e.g., RS and TR) when appropriate. Sponsors develop their own conventions for populating RSLNKID. |
Findings | RS | N/A | N/A | 13 | RSLNKGRP | Response or Clin Class Link Group | A grouping identifier used to link the disease response assessment to a group of related record in another domain which was used to determine the response result. | What was the [Disease Response or Clinical Classification] Link Group Identifier? | [Disease Response or Clinical Classification ]Link Group | Char | O | If collected, record the unique [Disease Response or Clinical Classification ] Link Group Identifier. | RSLNKGRP | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This variable is used to provide a unique code in order to link a group of records across related CRFs (e.g., RS and TR) when appropriate. Sponsors develop their own conventions for populating RSLNKGRP. |
Findings | RS | N/A | N/A | 14 | RSTEST | Response or Clin Class Assessment Name | Descriptive name of the disease response or clinical classification used to obtain the measurement or finding. | What was the [disease response/clinical classification] test name? | [Disease Response / Clinical Classification Test Name] | Char | HR | Record the name of the RS test, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | RSTEST; RSTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable RSTESTCD may be determined from the value collected in RSTEST. The SDTMIG variables RSTESTCD and RSTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. | |
Findings | RS | N/A | N/A | 15 | RSORRES | Response or Clin Class Original Result | Result of the disease response or clinical classification as originally received, collected,or calculated. | What was the [disease response/clinical classification]? | (Result) | Char | HR | Indicate the response classification | RSORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Both quantitative results and interpretive findings or summaries may be recorded here. |
Findings | RS | N/A | N/A | 16 | RSORRESU | Response or Clin Class Original Units | The unit of the result as originally received or collected. | What was the [disease response/clinical classification] unit? | Unit | Char | HR | Record or select the original units in which these data were collected, if not preprinted on CRF | RSORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | N/A | Should be preprinted on the CRF with the associated test when possible, rather than collected in a field that requires the site to enter text. Should be included if applicable and not available elsewhere. |
Assumptions for the CDASHIG RS - Disease Response and Clin Classification Classifications
1. This domain is for clinical classifications, including oncology disease response criteria.
2. Whenever possible, all physical manifestations that are collected to determine the disease response or clinical classification should be represented in the topic-based domains (e.g., labs, vital signs, tumor results, clinical events) to which they pertain. For example, if a lab test value is collected and then scored for a response evaluation or clinical classification measure, the lab test value should be collected in the LB domain using the rules that apply to that domain.
3. The RS domain is applicable for representing responses to assessment criteria used in oncology studies.
4. When using the RS domain to represent response evaluation or clinical classification determined from data from other domains (i.e., relates back to another source domain), the appropriate identifier must be collected to allow the relevant RELRECs to be created. These may include --LNKID, --LNKGRP, – SPID, REFID, or the CDASH --NO variables.
5. RSCAT is used to group a set of assessments based on a disease response criterion (published or protocol- defined) or a clinical classification. There are two codelists for RSCAT:
a. ONCRSCAT contains controlled terminology terms for oncology disease response assessments.
b. CCCAT contains controlled terminology for other clinical classifications instruments.
6. Within CDISC, Clinical Classification instruments represented in the RS domain fall under the concept of Questionnaires, Ratings and Scales (QRS).
a. Oncology response criteria do not currently follow the processes for other clinical classifications instruments. Note that in oncology studies, RSSTRESC is subject to controlled terminology in the SDTM datasets.
b. For other clinical classification instruments, QRS Naming Rules apply to the codelists. CDISC publishes standard QRS Supplements to the SDTMIG along with controlled terminology.
i. All standard supplement development is coordinated with the CDISC SDTM QRS Subteam as the governing body. The process involves drafting the controlled terminology and defining measure-specific, standardized values for qualifier, timing, and result variables to populate the SDTM QS, FT, and RS domains. These supplements are developed based on user demand and therapeutic area standards development needs. Sponsors should always consult the CDISC website to review the terminology and supplements prior to modeling any QRS measure data in the RS domain.
ii. Sponsors may participate and/or request the development of additional supplements and terminology through the CDISC SDTM QRS Subteam and the Controlled Terminology QRS Subteam.
iii. Once generated, the Clinical Classifications Supplement is posted on the CDISC website (http://www.cdisc.org/qrs).
iv. Sponsors should always consult the published QRS Supplements for guidance on submitting derived information in SDTM-based domains.
7. When a clinical classification result is based on multiple procedures, scans, images, or physical exams performed on different dates, the individual procedure, scan, image, and physical exam dates should be collected. RSDTC data may then be derived from these individual dates as specified by the sponsor.
8. The RS domain is intended for collected data. This includes records derived by the investigator or with a data collection tool, but not sponsor-derived records. Sponsor-derived records and results should be provided in an analysis dataset. However, totals and subtotals in clinical classification measures are considered collected data if recorded by an assessor. If these totals are operationally derived through a data collection tool (e.g., eCRF or ePRO device), then RSDRVL should be "Y".
Example CRF for the CDASHIG RS - Disease Response and Clin Classification
Example 1
Title: Child-Pugh Classification
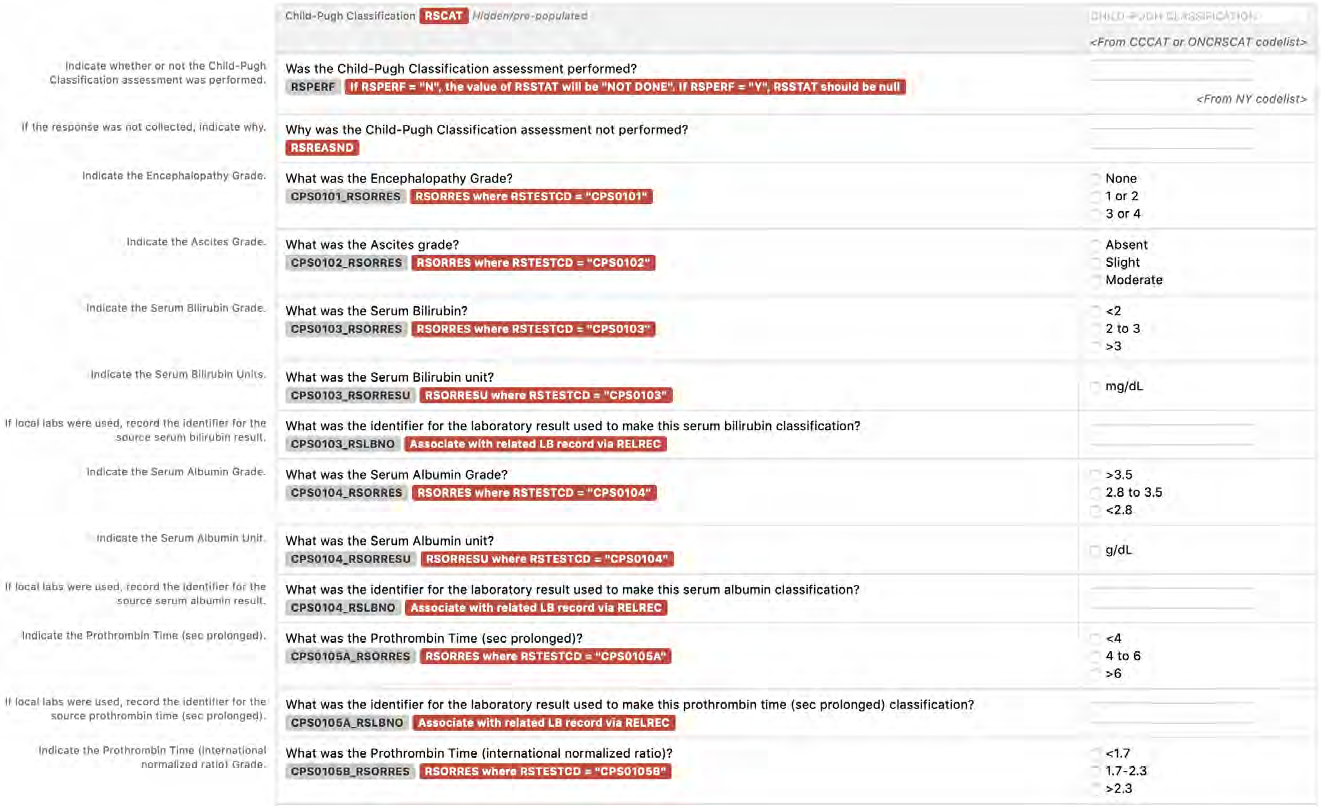
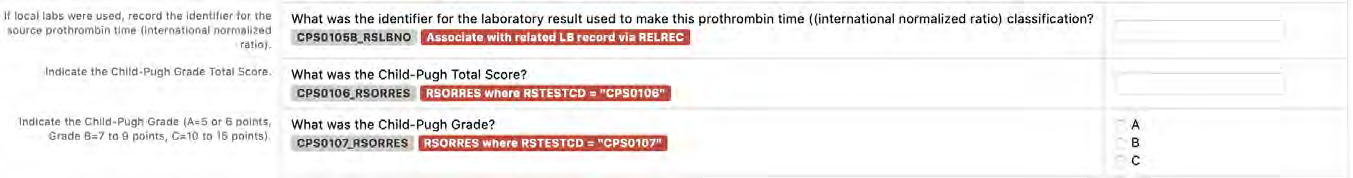
CRF Metadata
| Order Number | CDASH Variable | Question Text | Prompt | Data Type | Case Report Form Completion Instructions | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Codelist Name | Permissible Values | Pre-Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | RSCAT | What is the clinical classification criteria? | Child-Pugh Classification | text | N/A | RSCAT | N/A | (CCCAT);(ONCRSCAT) | CHILD-PUGH CLASSIFICATION | prompt | Y | ||
2 | RSPERF | Was the Child-Pugh Classification assessment performed? | Child-Pugh Classification Assessment Performed | text | Indicate whether or not the Child-Pugh Classification assessment was performed. | RSSTAT | If RSPERF = "N", the value of RSSTAT will be "NOT DONE". If RSPERF = "Y", RSSTAT should be null | (NY) | |||||
3 | RSREASND | Why was the Child-Pugh Classification assessment not performed? | Reason Child-Pugh Classification assessment Not Performed | text | If the response was not collected, indicate why. | RSREASND | N/A | N/A | |||||
4 | CPS0101_RSORRES | What was the Encephalopathy Grade? | Encephalopathy Grade | text | Indicate the Encephalopathy Grade. | RSORRES where RSTESTCD = "CPS0101" | N/A | None; 1 or 2;3 or 4; | |||||
5 | CPS0102_RSORRES | What was the Ascites grade? | Ascites Grade | text | Indicate the Ascites Grade. | RSORRES where RSTESTCD = "CPS0102" | N/A | Absent;Slight;Moderate | |||||
6 | CPS0103_RSORRES | What was the Serum Bilirubin? | Serum Bilirubin | text | Indicate the Serum Bilirubin Grade. | RSORRES where RSTESTCD = "CPS0103" | N/A | <2; 2 to 3;>3 | |||||
7 | CPS0103_RSORRESU | What was the Serum Bilirubin unit? | Serum Bilirubin Unit | text | Indicate the Serum Bilirubin Units. | RSORRESU where RSTESTCD = "CPS0103" | N/A | mg/dL | |||||
8 | CPS0103_RSLBNO | What was the identifier for the laboratory result used to make this serum bilirubin classification? | Related Laboratory Result Identifier | text | If local labs were used, record the identifier for the source serum bilirubin result. | N/A | Associate with related LB record via RELREC | N/A | |||||
9 | CPS0104_RSORRES | What was the Serum Albumin Grade? | Serum Albumin Grade | text | Indicate the Serum Albumin Grade. | RSORRES where RSTESTCD = "CPS0104" | N/A | >3.5;2.8 to 3.5 ; <2.8; | |||||
10 | CPS0104_RSORRESU | What was the Serum Albumin unit? | Serum Albumin Unit | text | Indicate the Serum Albumin Unit. | RSORRESU where RSTESTCD = "CPS0104" | N/A | g/dL | |||||
11 | CPS0104_RSLBNO | What was the identifier for the laboratory result used to make this serum albumin classification? | Related Laboratory Result Identifier | text | If local labs were used, record the identifier for the source serum albumin result. | N/A | Associate with related LB record via RELREC | N/A | |||||
12 | CPS0105A_RSORRES | What was the Prothrombin Time (sec prolonged)? | Prothrombin Time (sec prolonged) Result | text | Indicate the Prothrombin Time (sec prolonged). | RSORRES where RSTESTCD = "CPS0105A" | N/A | <4;4 to 6; >6 | |||||
13 | CPS0105A_RSLBNO | What was the identifier for the laboratory result used to make this prothrombin time (sec prolonged) classification? | Related Laboratory Result Identifier | text | If local labs were used, record the identifier for the sourceprothrombin time (sec prolonged). | N/A | Associate with related LB record via RELREC | N/A | |||||
14 | CPS0105B_RSORRES | What was the Prothrombin Time (international normalized ratio)? | Prothrombin Time (international normalized ratio Result | text | Indicate the Prothrombin Time (international normalized ratio) Grade. | RSORRES where RSTESTCD = "CPS0105B" | N/A | <1.7;1.7-2.3;>2.3; | |||||
15 | CPS0105B_RSLBNO | What was the identifier for the laboratory result used to make this prothrombin time ((international normalized ratio) classification? | Related Laboratory Result Identifier | text | If local labs were used, record the identifier for the source prothrombin time (international normalized ratio). | N/A | Associate with related LB record via RELREC | N/A | |||||
16 | CPS0106_RSORRES | What was the Child-Pugh Total Score? | Child-Pugh Total Score | text | Indicate the Child-Pugh Grade Total Score. | RSORRES where RSTESTCD = "CPS0106" | N/A |
| |||||
17 | CPS0107_RSORRES | What was the Child-Pugh Grade? | Child-Pugh Grade | text | Indicate the Child-Pugh Grade (A=5 or 6 points, Grade B=7 to 9 points, C=10 to 15 points). | RSORRES where RSTESTCD = "CPS0107" |
| N/A | A;B;C; |
8.3.15 SC - Subject Characteristics
Description/Overview for the CDASHIG SC - Subject Characteristics Domain
The SC domain describes protocol-specified characteristics of the study subjects and serves as an extension of the data contained in the Demographics (DM) domain. It is important to note that:
1. Data in this domain are collected only once per subject.
2. SC data that are collected once at the beginning of the trial and are not expected to change during the trial.
3. SC contains data such as additional information about education level, marital status, and national origin.
4. There is extensible CDASHIG controlled terminology for SCTEST. These data might be useful, for example, for risk-benefit or quality-of-life analyses, or for sub-setting a subject population.
5. The SDTMIG SC domain utilizes a normalized data structure; that is, 1 variable (SCTEST) is used to capture the test name and another variable (SCORRES) is used to capture the result. Subject Characteristics are presented as a normalized structure in the CDASHIG Metadata Table, but implementers using a denormalized structure (1 variable for each test) should create variable names that mirror the SCTESTCDs in controlled terminology.
Specification for the CDASHIG SC - Subject Characteristics Domain
Subject Characteristics Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | SC | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM- based dataset creation before submission. |
Findings | SC | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | SC | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | SC | N/A | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | SC | N/A | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable SCDTC in ISO 8601 format. | N/A | N/A | The date the subject characteristics were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the subject characteristics at that visit, or the collection date can be included on the SC CRF using the date (SCDAT) field. |
Findings | SC | N/A | N/A | 6 | SCCAT | Category for Subject Characteristic | A grouping of topic-variable values based on user-defined characteristics. | What was the category of the subject characteristics? | [Subject Characteristics Category ]; NULL | Char | O | Record the subject characteristics category, if not preprinted on the CRF. | SCCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | SC | N/A | N/A | 7 | SCSCAT | Subcategory for Subject Characteristic | A sub-division of the SCCAT values based on user-defined characteristics. | What was the subcategory of the subject characteristics? | [Subject Characteristics Subcategory]; NULL | Char | O | Record the subject characteristics subcategory, if not preprinted on the CRF. | SCSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. SCSCAT can only be used if there is an SCCAT and it must be a subcategorization of SCCAT. |
Findings | SC | N/A | N/A | 8 | SCPERF | SC Assessment Performed | An indication whether or not any subject characteristics were collected. | Were subject characteristics collected? | Subject Characteristics Collected | Char | O | Indicate if subject characteristics information was collected. If yes, record the appropriate details. | SCSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTM variable SCSTAT. If SCPERF="N", the value of SCSTAT will be "NOT DONE". If SCPERF="Y", SCSTAT should be null. A combination of SDTMIG variables (e.g., SCCAT and SCSCAT, SCTPT) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable SCTESTCD would be populated as SCALL and an appropriate test name (SCTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | General prompt question to be used as a data management tool to verify that missing results are confirmed missing. |
Findings | SC | N/A | N/A | 9 | SCSPID | SC Sponsor- Defined Identifier | A sponsor- defined identifier. In CDASH, This is typically used for preprinted or auto-generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor-defined question] | [Sponsor defined] | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | SCSPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Because SPID is a sponsor-defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile data. May be used to record preprinted number (e.g., line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Findings | SC | N/A | N/A | 10 | SCDAT | Subject Characteristic Collection Date | The date of collection represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the date the subject characteristic(s) was collected? | Date | Char | R/C | Record date the subject characteristic(s) was collected using the format (DD-MON- YYYY). | SCDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable SCDTC in ISO 8601 format. | N/A | N/A | The date of collection can be determined from a collected date of the visit (VISDAT) and in such cases a date field is not required. |
Findings | SC | N/A | N/A | 11 | SCTEST | Subject Characteristic | Descriptive name of the subject characteristic of interest. | What is the Subject Characteristics name? | [Subject Characteristic Test Name] | Char | HR | Record the name of the Subject Characteristics if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | SCTEST;SCTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable SCTESTCD may be determined from the value collected in SCTEST. The SDTMIG variables SCTESTCD and SCTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | (SCTEST) | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | SC | N/A | N/A | 12 | SCORRES | SC Result or Finding in Original Units | Result of the subject characteristic as originally received or collected. | What is the subject's characteristic? | (Result) | Char | HR | Record the subject characteristic. | SCORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | N/A |
Findings | SC | N/A | Horizontal- Generic | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM- based dataset creation before submission. |
Findings | SC | N/A | Horizontal- Generic | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | SC | N/A | Horizontal- Generic | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be provided to the site using a prepopulated list in the system. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM. |
Findings | SC | N/A | Horizontal- Generic | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | SC | N/A | Horizontal- Generic | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable SCDTC in ISO 8601 format. | N/A | N/A | The date the subject characteristics were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the subject characteristics at that visit, or the collection date can be included on the SC CRF using the date (SCDAT) field. |
Findings | SC | N/A | Horizontal- Generic | 6 | [SCTESTCD]_SCCAT | Category for Subject Characteristic | A grouping of topic-variable values based on user-defined characteristics. | What was the category of the subject characteristics?? | [Subject Characteristics Category]; NULL | Char | O | Record the subject characteristics category, if not preprinted on the CRF. | SCCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. This CDASH variable name is an example of what "variable name" can be used in a denormalized data structure. |
Findings | SC | N/A | Horizontal- Generic | 7 | [SCTESTCD]_SCSCAT | Subcategory for Subject Characteristic | A sub-division of the SCCAT values based on user-defined characteristics. | What was the subcategory of the subject characteristics? | [Subject Characteristics Subcategory]; NULL | Char | O | Record the subject characteristics subcategory, if not preprinted on the CRF. | SCSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. SCSCAT can only be used if there is an SCCAT and it must be a subcategorization of SCCAT. This CDASH variable name is an example of what "variable name" can be used in a denormalized data structure. |
Findings | SC | N/A | Horizontal- Generic | 8 | [SCTESTCD]_SCPERF | SC Assessment Performed | An indication whether or not any subject characteristics were collected. | Were subject characteristics collected for [SCTESTCD]? | [SCTEST] Collected | Char | O | Indicate if subject characteristics information was collected. If yes, include the appropriate details where indicated on the CRF. | SCSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTM variable SCSTAT. If [SCTESTCD]_SCPERF ="N", the value of SCSTAT will be "NOT DONE". If [SCTESTCD]_SCPERF ="Y", SCSTAT should be null. A combination of SDTMIG variables (e.g., SCCAT and SCSCAT, SCTPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable SCTESTCD would be assigned SCALL and an appropriate test name ( SCTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | General prompt question to be used as a data management tool to verify that missing results are confirmed missing. This may be implemented for all tests collected on the same horizontal record or for each specific test. When the SDTM-based datasets are created, the value of SCPERF would apply to all tests on the same record. Use the CDASH variable [SCTESTCD]_SCPERF when implemented on a specific test basis. This CDASH variable name is an example of what "variable name" can be used in a denormalized data structure. |
Findings | SC | N/A | Horizontal- Generic | 9 | SCGRPID | Subject Characteristics Group ID | A sponsor- defined identifier used to tie together a block of related records in a single domain. | What is the test group identifier? | Test Group ID | Char | O | Record unique group identifier. Sponsor may insert additional instructions to ensure each record has a unique group identifier. | SCGRPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | It can be beneficial to use an identifier in a data query to communicate clearly to the site the specific record in question. This group identifier ties together all the tests collected on this horizontal record. This field may be populated by the sponsor's data collection system. |
Findings | SC | N/A | Horizontal- Generic | 10 | [SCTESTCD]_SCORRES | SC Result or Finding in Original Units | Result of the Subject Characteristics as originally received or collected. | What is the subject's [SCTEST]? | [SCTEST] Result | Char | HR | Record the subject characteristic. | SCORRES;SCTEST;SCTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | N/A |
Assumptions for the CDASHIG SC - Subject Characteristics Domain
1. The subject characteristics that should be collected are not specified by CDASH; this is a medical and scientific decision that should be based on the needs of the protocol.
2. The SDTMIG variable SCDTC can be determined from a collected date of the visit (VISDAT); in such cases, a CDASH date of collection field is not required on the CRF.
Example CRF for the CDASHIG SC - Subject Characteristics Domain
Example 1
Title: Subject Characteristics
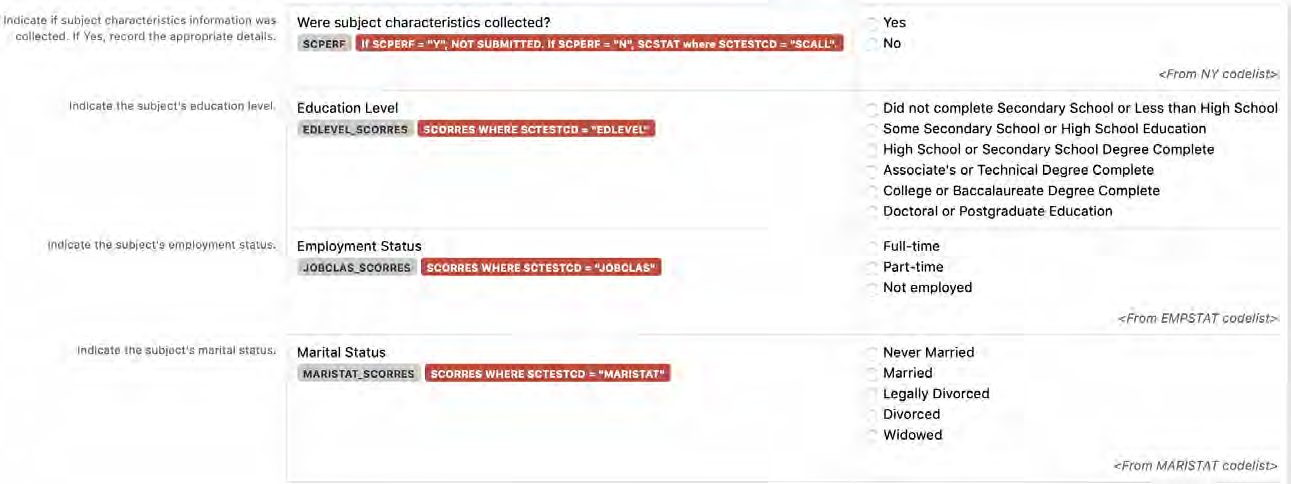
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTM Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
SCPERF | 1 | Were subject characteristics collected? | Subject Characteristics Collected | Indicate if subject characteristics information was collected. If Yes, record the appropriate details. | Text | SCSTAT | If SCPERF = "Y", NOT SUBMITTED. If SCPERF = "N", SCSTAT where SCTESTCD = "SCALL". | (NY) | Yes; No |
| |||
EDLEVEL_SCORRES | 2 | What is the subject's education level? | Education Level | Indicate the subject's education level. | Text | SCORRES;SCTEST;SCTESTCD | SCORRES WHERE SCTESTCD = "EDLEVEL" | Did not complete Secondary School or Less than High School; Some Secondary School or High School Education; High School or Secondary School Degree Complete; Associate’s or Technical Degree Complete; College or Baccalaureate Degree Complete; Doctoral or Postgraduate Education | prompt | radio | |||
JOBCLAS_SCORRES | 3 | What is the subject’s employment status? | Employment Status | Indicate the subject's employment status. | Text | SCORRES;SCTEST;SCTESTCD | SCORRES WHERE SCTESTCD = "JOBCLAS" | (EMPSTAT) | Full-time; Part-time; Not employed | prompt | radio | ||
MARISTAT_SCORRES | 4 | What is the subject's marital status? | Marital Status | Indicate the subject's marital status. | Text | SCORRES;SCTEST;SCTESTCD | SCORRES WHERE SCTESTCD = "MARISTAT" | (MARISTAT) | Never Married; Married; Legally Divorced; Divorced; Widowed |
| prompt | radio |
8.3.16 TU - Tumor/Lesion Identification Domain
Description/Overview for the CDASHIG TU - Tumor/Lesion Identification Domain
The TU domain represents data that uniquely identifies tumors/lesions (i.e., malignant tumors, culprit lesions, other sites of disease such as lymph nodes). Commonly, tumors/lesions are identified by an investigator and/or independent assessor and classified according to the disease assessment criteria. For example, for an oncology study using RECIST evaluation criteria, this equates to the identification of Target, Non-Target, or New tumors. When designing CRFs, it is common that a single CRF is designed to collect both the Tumor/Lesion Identification information and the results of any assessments on these identified tumors/lesions.
Specification for the CDASHIG TU - Tumor/Lesion Identification Domain
Tumor/Lesion Identification Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | TU | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | TU | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | TU | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | TU | N/A | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | TU | N/A | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. If the date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM), then concatenate the CDASH VISDAT/VISTIM components and populate the SDTMIG variable TUDTC in ISO 8601 format. | N/A | N/A | If the date the test was collected can be determined from the Visit Date variable (VISDAT), apply that date to all of the tests at that visit, or the collection date can be collected on the CRF using the date (TUDAT). In this domain, it may not be appropriate to use the visit date as TUDTC. |
Findings | TU | N/A | N/A | 6 | TUCAT | Category of Tumor/Lesion Identification | A grouping of topic- variable values based on user-defined characteristics. | What is the category of the [tumor/lesion] identification? | [Tumor/Lesion] Identification Category]; or NULL | Char | O | Record the Tumor Identification category, if not preprinted on the CRF. | TUCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This is most commonly either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | TU | N/A | N/A | 7 | TUSCAT | Subcategory Tumor/Lesion Identification | A sub-division of the TUCAT values based on user-defined characteristics. | What is the sub - category for the [tumor/lesion] identification? | [Tumor/Lesion] Identification Subcategory; or NULL | Char | O | Record the Tumor Identification subcategory, if not preprinted on the CRF. | N/A | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This is most commonly preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. TUSCAT can only be used if there is an TUCAT and it must be a subcategorization of TUCAT. |
Findings | TU | N/A | N/A | 8 | TUYN | Any Tumors/ Lesions Identification | An indication whether or not any tumors were identified. | Were any [target/non- target/new/sponsor- defined) [tumors/lesions] identified? | Any ([Target/Non- target/New/Sponsor- defined) [Tumors/Lesions] Identified | Char | O | Indicate if the there are [tumors/lesions] identified. If yes, include the appropriate details where indicated on the CRF. | N/A | Not Submitted. | (NY) | N/A | This is intended to be used as a data management tool to verify that missing tumor/lesions evaluations are confirmed missing. The sponsor may decide to map "No" responses using the appropriate SDTMIG variables. Typically, this would use the SDTMIG variable, TUOCCUR, with an appropriate TUTEST. |
Findings | TU | N/A | N/A | 9 | TUDAT | Tumor/Lesion Identification Date | The date of the [examination/procedure] used for tumor/lesion identification, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the date of the [examination/procedure] used for [tumor/lesion] identification? | [Tumor/Lesion] Identification Procedure Date | Char | R/C | Record the scan/image/physical exam date used to identify the tumor/lesion using this format (DD- MON-YYYY. | TUDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable TUDTC in ISO 8601 format. | N/A | N/A | This is the date of the scan/image/physical exam used to identified the tumor/lesion. It is not the date the MRI or scan was read. This is typically not the visit date. |
Findings | TU | N/A | N/A | 10 | TUEVAL | Tumor/Lesion Evaluator | The role of the person who provided the information. | Who provided the information?; or Who was the evaluator? | [Evaluator/Reporter] | Char | O | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | TUEVAL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EVAL) | N/A | Used only for results that are subjective (e.g., assigned by a person or a group). May be a preprinted, or collected. Sponsors may collect the data using a subset list of CT on the CRF. |
Findings | TU | N/A | N/A | 11 | TUEVALID | Tumor/Lesion Evaluator Identifier | Used to distinguish multiple evaluators with the same role. | What was the identifier of the evaluator? | [Evaluator/Reporter] Identifier | Char | O | Record the unique identifier assigned to the person making the evaluation. | TUEVALID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (MEDEVAL) | N/A | Collect if multiple evaluators are used in the study (may be omitted if multiple evaluators are not used); values should follow controlled terminology. |
Findings | TU | N/A | N/A | 12 | TULNKID | Tumor/Lesion Identification Link ID | An identifier used to link identified tumor/lesion to the assessment result. | What was the [Tumor/Lesion] (link) identifier? | [Tumor/ Lesion] ID | Char | HR | If collected, record the unique identifier for this tumor/lesion. | TULNKID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This variable is used to provide a unique code for each identified tumor in order to link records across related CRFs (TU and TR) when appropriate. Sponsors develop their own conventions for populating --LNKID. Typically, the lesion/tumor is assigned the --LNKID at baseline when the tumor/lesion is identified, and the this --LNKID is used at other visits to collect assessments on this tumor/lesion. |
Findings | TU | N/A | N/A | 13 | TUPRNO | Tumor/Lesion Related Procedure ID | The identifier for the procedure used to identify the tumor. | What was the identifier for the procedure used to identify this tumor? | Procedure Identifier | Char | O | Record the procedure [ID or Line Number] used to evaluate the tumor/lesion. | N/A | This does not map directly to an SDTMIG variable. For the SDTM submission datasets, may be used to create RELREC to link this record with a record in the PR domain. | N/A | N/A | Intent is to establish a link between the TU identified and the procedure undergone to identify this tumor/lesion. TUPRNO can be used to identify a relationship between records in the TU dataset and records in the PR dataset. See SDTMIG v3.2 Section 8.2 for information on RELREC. |
Findings | TU | N/A | N/A | 14 | TUMETHOD | Tumor/Lesion Method of Identification | Method of the test or examination. | What was the method used to [evaluate/identify] the tumor/lesion? | Method of [Evaluation/Identification] | Char | O | Record the method used to evaluate/identify the tumor/lesion. | TUMETHOD | Maps directly to the SDTM variable listed in the column with the heading "SDTM Target". | (METHOD) | N/A | The method used to identify the tumor/lesions. Sponsors may collect the method using a codelist which includes the most commonly known/generally recognized terms. The values will represent the method generically, not the product of the method (e.g., photograph) A sponsor may customize or restrict the list of values per response criteria or protocol needs. At a minimum, the primary method of identification should be entered and is expected to be consistent throughout the study; recording secondary methods is at the discretion of the sponsor. |
Findings | TU | N/A | N/A | 15 | TUREFID | Tumor/Lesion Identification Reference ID | An internal or external identifier such as image ID number (e.g., CT scan, MRI, ultrasound identifier). | What was the procedure [reference identifier/accession number]? | [Tumor/Lesion] Reference ID | Char | O | Record the internal or external identifier assigned to the procedure/method used to identify the tumor/lesion. | TUREFID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This variable may be used to collect a reference/accession number associated with the method used to identify the tumor/lesion. A sponsor may also decide to use the CDASH variable TUPRNO as an identifier for the procedure. |
Findings | TU | N/A | N/A | 16 | TUTEST | Tumor/Lesion Identification Test Name | Descriptive name of the measurement or finding. | What was the tumor/lesion Identification test name? | [Tumor or Lesion Identification Test Name] | Char | HR | Record the name of the TU test, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | TUTEST; TUTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable TUTESTCD may be determined from the value collected in TUTEST. The SDTMIG variables TUTESTCD and TUTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | (TUTEST) | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | TU | N/A | N/A | 17 | TUORRES | Tumor/Lesion Identification Result | Result of the tumor identification (e.g., classification or type of tumor). | What is the [type/classification] of [tumor/lesion] as defined by the criteria being employed? | (Result) | Char | HR | Record the Tumor Identification classification. This may be preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | TUORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Each test may be collected using the CDASH variable [TESTCD], for example, TUMERGE, TUSPLIT, TUMIDENT or [TESTCD]_TUORRES where TESTCD is the appropriate CT for the TU test code. This CDASH variable name is an example of what "variable name" can be used in a denormalized data structure. |
Findings | TU | N/A | N/A | 18 | TULOC | Location of the Tumor/Lesion | A description of the anatomical location of the identified tumor/lesion. | What was the anatomical location of the [tumor/lesion] identified? | Anatomical Location | Char | O | Record or select the anatomical location of the identified tumor/lesion. | TULOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. LAT, DIR, and PORTOT are used to further describe the anatomical location. A location detail text field (TULOCDTL) is conditional for entry (i.e., can be left blank) and allows the study site to specify the lesion in its own terms or can be used to distinguish tumors within the same location if other location qualifiers are not specific enough. |
Findings | TU | N/A | N/A | 19 | TULAT | Tumor/Lesion Identification Laterality | Qualifier for anatomical location further detailing the side of the body relevant for the event. | What was the laterality of the anatomical location? | [Tumor/Lesion Identification]Side | Char | O | Record the side of the body within the anatomical location of the identified [tumor/lesion]. | TULAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | TU | N/A | N/A | 20 | TUDIR | Tumor/Lesion Directionality | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What was the directionality of the anatomical location? | [Tumor/Lesion Identification] Directionality | Char | O | Record the directionality within the anatomical location of the identified [tumor/lesion]. | TUDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | TU | N/A | N/A | 21 | TULOCDTL | TU Identification Location Detail | A detail description of the location of the identified tumor/lesion. | What [were/are] additional details on the exact location of the tumor so that it can be distinguished from other tumors in the same anatomical location? | [Tumor/Lesion Identification] Location Detail | Char | O | Describe additional detail on the exact location of the tumor so that it can be distinguished from other tumors in the same anatomical location. | SUPPTU.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPTU dataset as the value of SUPPTU.QVAL where SUPPTU.QNAM="TULOCDTL" and SUPPTU.QLABEL= "TU Identification Location Detail". | N/A | N/A | Use if TULOC and TULAT and/or TUDIR values cannot provide uniqueness from other identified tumors. TULOCDTL is not meant to replace TULOC, TULAT, and/or TUDIR or serve as the free-text description field for TULOC (e.g., Location, Other). |
Findings | TU | N/A | N/A | 22 | TUNAM | Tumor/Lesion Identification Vendor Name | The name or identifier of the vendor (e.g., laboratory) that provided the test results. | What was the name of the vendor used? | [Tumor/Lesion Identification] Vendor Name | Char | O | Record the name of the vendor providing the evaluation. | TUNAM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Recommended to collect on the CRF if vendor names was not collected at the site/study level or if multiple vendors are used by a site. |
Assumptions for the CDASHIG TU - Tumor/Lesion Identification Domain
1. This domain only includes the information needed to identify the tumor/lesion. Results (e.g., size of a tumor/lesion) would be represented in the associated TR domain.
2. Typically in oncology studies, the initial identification of a tumor/lesion is done once, usually at baseline. New tumors/lesions identified subsequent to baseline or changes to tumors/lesions initially identified are also collected in this domain.
3. The identification information would typically include the location description.
4. The TULNKID variable is used to provide a unique code for each identified tumor/lesion. The unique code is not specified by CDASH; this is a decision that is based on the needs of the protocol. Suggestions for the unique TULNKID codes are provided in the oncology TAUGs and the SDTMIG.
5. TULNKID is used to relate an identification record in TU domain to assessment/result records in the TR domain. Therefore, the values must be identical. Suggestions for the unique TULNKID codes used for the identification of tumors/lesions in oncology trials are provided in the oncology TAUGs and the SDTMIG.
6. The SDTM identifiers, --GRPID, --REFID, and --SPID) must not be used to link the identified tumor/lesion with the results/assessments for this identified tumor. These identifiers are used for other purposes, as described in the SDTMIG.
7. TUEVAL and TUEVALID are used indicate who provided the data. TUEVALID provides more details when multiple evaluators are used. TUEVAL must be collected or defaulted when TUEVALID is collected.
Example CRFs for the CDASHIG TU - Tumor/Lesion Identification Domain
Example 1
Title: Tumor Identification/Evaluation
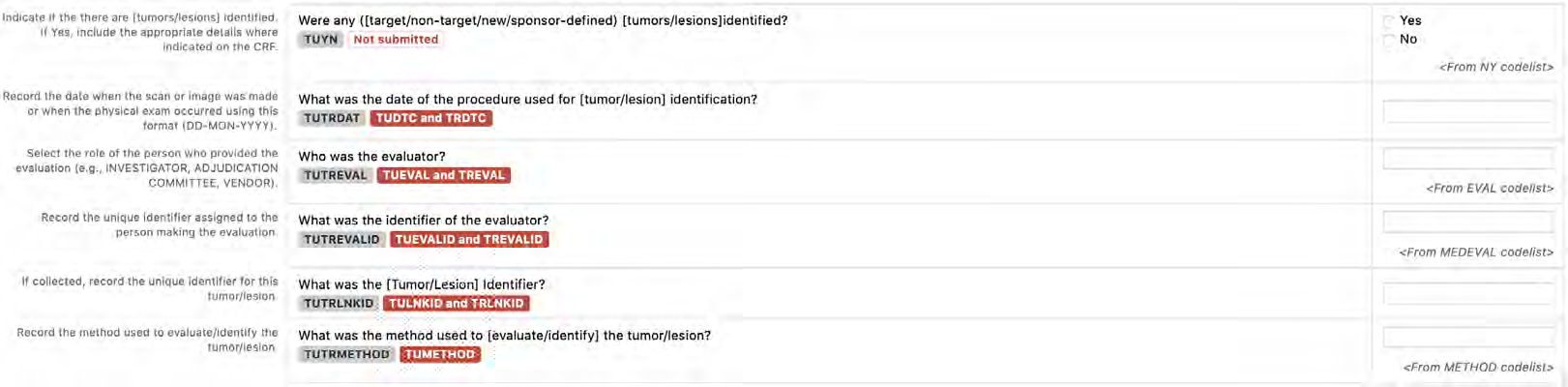
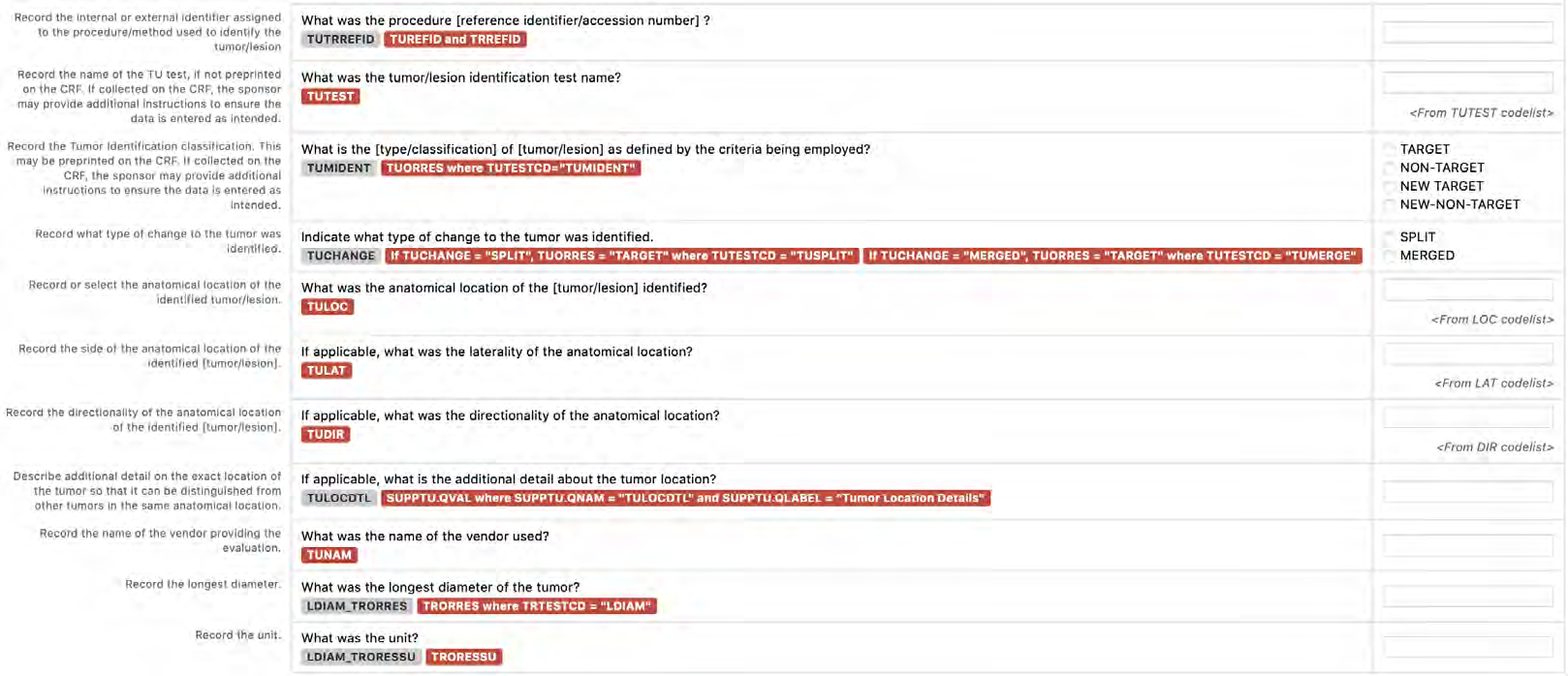
CRF Metadata
| Order Number | CDASHIG Variable | Question Text | Prompt | Type | Case Report Form Completion Instructions | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Codelist Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | TUYN | Were any ([target/non- target/new/sponsor-defined) [tumors/lesions]identified? | Any ([Target/Non- target/New/Sponsor-defined) Tumors/Lesions Identification | text | Indicate if the there are [tumors/lesions] identified. If Yes, include the appropriate details where indicated on the CRF. | N/A | (NY) | Yes; No | |||||
2 | TUTRDAT | What was the date of the procedure used for [tumor/lesion] identification? | Procedure Date | text | Record the date when the scan or image was made or when the physical exam occurred using this format (DD-MON-YYYY). | TUDTC;TRDTC | TUDTC and TRDTC | N/A | |||||
3 | TUTREVAL | Who was the evaluator? | Tumor Identification [Evaluator/Reporter] | text | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | TUEVAL;TREVAL | TUEVAL and TREVAL | (EVAL) | |||||
4 | TUTREVALID | What was the identifier of the evaluator? | Tumor Identification Evaluator Identifier | text | Record the unique identifier assigned to the person making the evaluation. | TUEVALID;TREVALID | TUEVALID and TREVALID | (MEDEVAL) | |||||
5 | TUTRLNKID | What was the [Tumor/Lesion] Identifier? | [Tumor/ Lesion] ID | text | If collected, record the unique identifier for this tumor/lesion. | TULNKID;TRLNKID | TULNKID and TRLNKID | N/A | |||||
6 | TUTRMETHOD | What was the method used to [evaluate/identify] the tumor/lesion? | Method of [Evaluation/Identification] | text | Record the method used to evaluate/identify the tumor/lesion. | TUMETHOD | (METHOD) | ||||||
7 | TUTRREFID | What was the procedure [reference identifier/accession number] ? | Reference ID | text | Record the internal or external identifier assigned to the procedure/method used to identify the tumor/lesion | TUREFID; | TUREFID and TRREFID | N/A | |||||
8 | TUTEST | What was the tumor/lesion identification test name? | Tumor/Lesion Identification Test Name | text | Record the name of the TU test, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | TUTEST | TUTEST | (TUTEST) | y | ||||
9 | TUMIDENT | What is the [type/classification] of [tumor/lesion] as defined by the criteria being employed? | Result | text | Record the Tumor Identification classification. This may be preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | TUORRES:TUEST:TUTESTCD: | TUORRES where TUTESTCD="TUMIDENT" | N/A | TARGET; NON- TARGET; NEW TARGET; NEW- NON-TARGET | ||||
10 | TUCHANGE | Indicate what type of change to the tumor was identified. | Changes to Tumor (Result) | text | Record what type of change to the tumor was identified. | TUORRES:TUEST:TUTESTCD | If TUCHANGE = "SPLIT", TUORRES = "TARGET" where TUTESTCD = "TUSPLIT" ; If TUCHANGE = "MERGED", TUORRES = "TARGET" where TUTESTCD = "TUMERGE" | N/A | SPLIT; MERGED | ||||
11 | TULOC | What was the anatomical location of the [tumor/lesion] identified? | Anatomical Location | text | Record or select the anatomical location of the identified tumor/lesion. | TULOC | (LOC) | ||||||
12 | TULAT | If applicable, what was the laterality of the anatomical location? | Side | text | Record the side of the anatomical location of the identified [tumor/lesion]. | TULAT | (LAT) | ||||||
13 | TUDIR | If applicable, what was the directionality of the anatomical location? | Directionality | text | Record the directionality of the anatomical location of the identified [tumor/lesion]. | TUDIR | (DIR) | ||||||
14 | TULOCDTL | If applicable, what is the additional detail about the tumor location? | Location Detail | text | Describe additional detail on the exact location of the tumor so that it can be distinguished from other tumors in the same anatomical location. | SUPPTU.QVAL | SUPPTU.QVAL where SUPPTU.QNAM = "TULOCDTL" and SUPPTU.QLABEL = "Tumor Location Details" | N/A | |||||
15 | TUNAM | What was the name of the vendor used? | Vendor Name | text | Record the name of the vendor providing the evaluation. | TUNAM | N/A | ||||||
16 | LDIAM_TRORRES | What was the longest diameter of the tumor? | Longest Diameter | text | Record the longest diameter. | TRORRES; TRTESTCD; TRTEST: | TRORRES where TRTESTCD = "LDIAM" | N/A | |||||
17 | LDIAM_TRORESSU | What was the unit? | Unit | text | Record the unit. | TRORESSU | N/A |
Example 2
This is an example of a single CRF collecting both acne lesion identification data (TU) and the lesion result data (TR). It collects the number of lesions of each type at each location. This is a normalized CRF. One record is collected for each anatomical location, and each type of lesion. The sponsor must determine how the TULNKID/TRLNKID are created and assigned. Other lesion types or location were not of interest.
At subsequent assessments, post-baseline, the CRF may be designed so that only the relevant questions are displayed (e.g., –EVAL, --LNKID, --DAT –REFID, TRORRES).
Title: Acne Vulgaris Lesion Evaluations
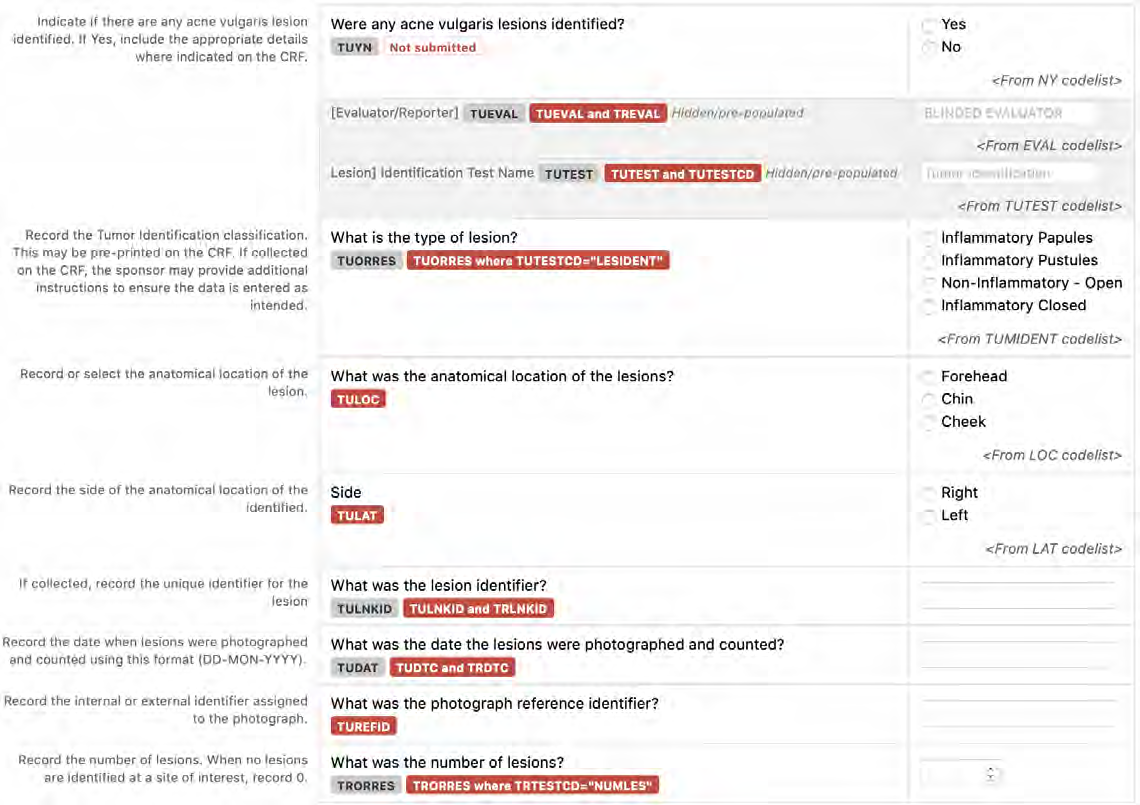
CRF Metadata
| Order Number | CDASH Variable | Question Text | Prompt | Data Type | Case Report Form Completion Instructions | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Codelist Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | TUYN | Were any acne vulgaris lesions identified? | Any Acne Vulgaris Lesions | text | Indicate if there are any acne vulgaris lesion identified. If Yes, include the appropriate details where indicated on the CRF. | N/A | (NY) | Yes;No | |||||
2 | TUEVAL | Who was the evaluator? | [Evaluator/Reporter] | text | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | TUEVAL; TREVAL | TUEVAL and TREVAL | (EVAL) | BLINDED EVALUATOR | checkbox | y | ||
3 | TUTEST | What was the lesion] Identification test name? | Lesion] Identification Test Name | text | Record the name of the TU test, if not pre- printed on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | TUTEST; TUTESTCD; | TUTEST and TUTESTCD | (TUTEST) | Tumor Identification | y | |||
4 | TUORRES | What is the type of lesion? | Result | text | Record the Tumor Identification classification. This may be pre-printed on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | TUORRES:TUTEST;TESTCD: | TUORRES where TUTESTCD="LESIDENT" | (TUMIDENT) | Inflammatory Papules; Inflammatory Pustules;Non- Inflammatory - Open;Inflammatory Closed | ||||
5 | TULOC | What was the anatomical location of the lesions? | Anatomical Location | text | Record or select the anatomical location of the lesion. | TULOC | (LOC) | Forehead; Chin; Cheek; | |||||
6 | TULAT | If applicable, what was the laterality of the anatomical location? | Side | text | Record the side of the anatomical location of the identified. | TULAT | (LAT) | Right; Left; | prompt | ||||
7 | TULNKID | What was the lesion identifier? | Lesion ID | text | If collected, record the unique identifier for the lesion | TULNKID; TRLNKID | TULNKID and TRLNKID | N/A | |||||
8 | TUDAT | What was the date the lesions were photographed and counted? | Date | text | Record the date when lesions were photographed and counted using this format (DD-MON-YYYY). | TUDTC; TRDTC; | TUDTC and TRDTC | N/A | |||||
9 | TUREFID | What was the photograph reference identifier? | Reference ID | text | Record the internal or external identifier assigned to the photograph. | TUREFID | N/A | ||||||
10 | TRORRES | What was the number of lesions? | Number of Lesions | integer | Record the number of lesions. When no lesions are identified at a site of interest, record 0. | TRORRES;TRTEST;TRTESTCD | TRORRES where TRTESTCD="NUMLES" |
8.3.17 TR - Tumor/Lesion Results
Description/Overview for the CDASHIG TR - Tumor/Lesion Results Domain
The TR domain represents measurements and/or assessments of the tumors/lesions identified in the Tumor/Lesion Identification (TU) domain. When the TR domain is used, there must be a corresponding TU domain present. This multiple-domain approach was developed largely to reduce the need to collect tumor identification information at each assessment (e.g., anatomical location). A unique tumor/lesion identification number (populated in TULNKID/TRLNKID) is used to link the tumor/lesion identification (TU) with the measurement/assessments (TR). When designing CRFs, it is common that a single CRF is designed to collect both the Tumor/Lesion Identification information and the results of any assessments on these identified tumors/lesions.
Specification for the CDASHIG TR - Tumor/Lesion Identification Domain
Tumor/Lesion Identification Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | TR | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | TR | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | TR | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | TR | N/A | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | TR | N/A | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. If the date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable TRDTC in ISO 8601 format. | N/A | N/A | If the date the test was collected can be determined from the Visit Date variable (VISDAT), apply that date to all of the tests at that visit, or the collection date can be collected on the CRF using the date (TRDAT). In this domain, it may not be appropriate to use the vist date as TRDTC. |
Findings | TR | N/A | N/A | 6 | TRLNKGRP | Tumor/Lesion Result Link Group | An identifier used to link related records across domains. | What was the [tumor/lesion] [link group] identifier? | [Tumor/Lesion] [Link Group] ID | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has the appropriate identifier. | TRLNKGRP | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Because TRLNKGRP is a sponsor-defined identifier, conformance to Question Text or Item Prompt is not applicable. This is typically used in oncology clincial trials. It is intended to group all the assessments at a evaluation time point represented in the TR domain with the associated response assessments represented in the RS domain. |
Findings | TR | N/A | N/A | 7 | TRCAT | Category of Tumor/Lesion Result | A grouping of topic-variable values based on user-defined characteristics. | What is the category of the [tumor/lesion] results? | [Tumor/Lesion Result Category]; or NULL | Char | O | Record the Tumor/Lesion Result category, if not preprinted on the CRF. | TRCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading or a preprinted category value on the CRF, and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | TR | N/A | N/A | 8 | TRSCAT | Subcategory of Tumor/Lesion Result | A subgrouping of topic-variable values based on user-defined characteristics. | What is the subcategory of the [tumor/lesion] results? | [Tumor/Lesion Result Subcategory]; or NULL | Char | O | Record the Tumor/Lesion Result subcategory, if not preprinted on the CRF. | TRSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be preprinted on the CRF or screen and prepopulated in the data management system. This is not typically a question to which the site would provide an answer. TRSCAT can only be used if there is an TRCAT and it must be a subcategorization of TRCAT. |
Findings | TR | N/A | N/A | 9 | TRSTAT | Tumor/Lesion Result Completion Status | The variable used to indicate that data are not available by having the site record the value as "Not Done". | Indicate if the [tumor/lesion] evaluation was not done. | Not Done | Char | O | Indicate if the [Tumor/Lesion] evaluation was not done. | TRSTAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (ND) | N/A | A Not Done check box, which indicates the test was NOT DONE. Typically, there would be one check box for each result. This field can be useful to confirm that a blank result field is meant to be blank. |
Findings | TR | N/A | N/A | 10 | TRREASND | Reason Tumor Measurement Not Performed | An explanation of why the data are not available. | What was the reason that the [tumor/lesion] was not [evaluated/assessed]? | Reason Not Done | Char | O | Provide the reason the result was not provided. | TRREASND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology may be used. The reason the data are not available may be chosen from a sponsor-defined codelist (e.g., broken equipment, subject refused) or entered as free text. When TRREASND is used, TRSTAT should also be populated in the SDTM-based dataset. |
Findings | TR | N/A | N/A | 11 | TREVAL | Tumor/Lesion Result Evaluator | The role of the person who provided the information. | Who provided the information? OR Who was the evaluator? | [Evaluator/Reporter] | Char | O | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | TREVAL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EVAL) | N/A | Used only for results that are subjective (e.g., assigned by a person or a group). May be a preprinted or collected. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | TR | N/A | N/A | 12 | TREVALID | Tumor/Lesion Result Evaluator Identifier | Used to distinguish multiple evaluators with the same role. | What was the identifier of the evaluator? | Evaluator Identifier | Char | O | Record the unique identifier assigned to the person making the evaluation. | TREVALID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (MEDEVAL) | N/A | Collect if multiple evaluators are used in the study (may be omitted if multiple evaluators are not used); values should follow controlled terminology. |
Findings | TR | N/A | N/A | 13 | TRDAT | Tumor/Lesion Result Date | The date of the procedure used for tumor/lesion assessment, represented in an unambiguous date format (e.g., DD- MON-YYYY). | What was the date of the procedure used for [tumor/lesion] assessment? | Date | Char | R/C | Record the date when the method used to assess the tumor/lesion occurred using this format (DD- MON-YYYY). | TRDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable TRDTC in ISO 8601 format. | N/A | N/A | This is the date of the scan/image/physical exam used to evaluate the tumor/lesion. It is not the date the MRI or scan was read. This is typically not the visit date. |
Findings | TR | N/A | N/A | 14 | TRLNKID | Tumor/Lesion Result Link ID | An identifier used to link identified tumor/lesion to the assessment result. | What was the [tumor/lesion] Identifier? | [Tumor/ Lesion] ID | Char | R/C | If collected, record the unique identifier for this tumor/lesion. | TRLNKID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This variable is used to provide a unique code for each identified tumor in order to link records across related CRFs (TU and TR) when appropriate. Sponsors develop their own conventions for populating -- LNKID. Typically, the lesion/tumor is assigned the -- LNKID at baseline when the tumor/lesion is identified, and this --LNKID is used at other visits to collect assessments on this tumor /lesion. Note: This variable may be collected using one CDASH variable name (e.g., TULNKID) and populated into TRLNKID when the submisson datasets are created. |
Findings | TR | N/A | N/A | 15 | TRTEST | Tumor/Lesion Assessment Test Name | Descriptive name of the measurement or finding. | What was the [tumor/ lesion] (assessment) test name? | [Tumor/Lesion] (Assessment) Test Name] | Char | HR | Record the name of the tumor lesion assessment, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | TRTEST; TRTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable TRTESTCD may be determined from the value collected in TRTEST. The SDTMIG variables TRTESTCD and TRTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | (TRTEST) | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | TR | N/A | N/A | 16 | TRORRES | TR Result or Finding in Original Units | Result of the tumor/lesion assessment. | What is the result for the [tumor/lesion assessment]? | (Result) | Char | HR | Record the Tumor/Lesion Assessment result. | TRORRES;TRTESTCD; TRTEST | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. In addition to the SDTMIG variable TRORRES, create TRTESTCD from the CDASH variable name and determine the value of TRTEST from TRTESTCD. The CDASH prompt may also contain the TRTEST. Use appropriate CDISC Controlled Terminology for the test and test code. | N/A | N/A | Result of the tumor/lesion assessment. Both quantitative and qualitatve results may be recorded here. |
Findings | TR | N/A | N/A | 17 | TRORRESU | TR Original Units | The unit of the result as originally received or collected. | What was the unit of the [result/measurement]? | Unit | Char | R/C | Record or select the original units in which these data were collected, if not preprinted on CRF. | TRORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | N/A | Usually the unit of the test is preprinted on the CRF. |
Findings | TR | N/A | N/A | 18 | TRNAM | Tumor/Lesion Result Vendor Name | The name or identifier of the vendor (e.g., laboratory) that provided the test results. | What was the name of the vendor used? | Vendor Name | Char | O | Record the name of the vendor providing the evaluation. | TRNAM | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Recommended to collect on the CRF if vendor names was not collected at the site/study level or if multiple vendors are used by a site. |
Assumptions for the CDASHIG TR - Tumor/Lesion Identification Domain
1. This domain only includes the information on the tumor/lesion assessment results (e.g., size of a tumor/lesion). This may be a single assessment or multiple assessments over time.
2. The identification information is provided in the TU domain. The TULNKID and TRLNKID variables contain the same unique code to link each identified tumor/lesion and each identified tumor result/assessment. The unique code is not specified by CDASH; this is a decision that is based on the needs of the protocol. Suggestions for the unique TULNKID/TRLNKID codes are provided in the oncology TAUGs and the SDTMIG.
3. The SDTM identifiers --GRPID, --REFID, and --SPID must not be used to link the identified tumor/lesion with the results/assessments for this identified tumor. These identifiers are used for other purposes, as described in the SDTMIG.
4. Link identifiers (e.g., TRLNKID, TRLNKGRP) may be used to link the assessments in the TR domain to the Disease Response and Clin Classification (RS) domain. The RS domain would typically provide the classification or response assessments associated with these TR results.
5. TREVAL and TREVALID are used indicate who provided the data. TREVALID provides more details when multiple evaluators are used. TREVAL must be collected or defaulted when TREVALID is collected.
Example CRFs for the CDASHIG TR - Tumor/Lesion Identification Domain
Example 1
Title: Tumor Identification/Evaluation
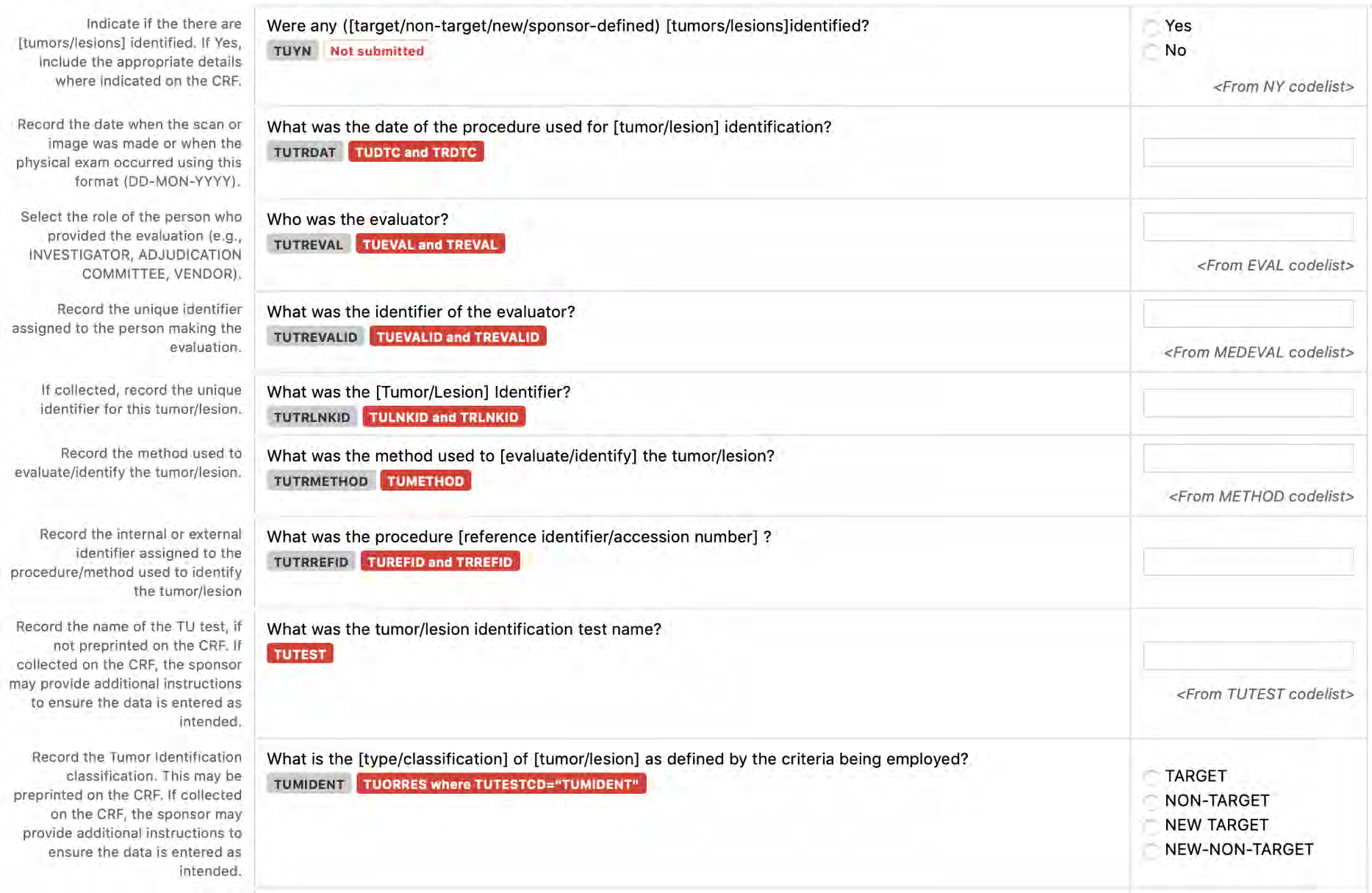
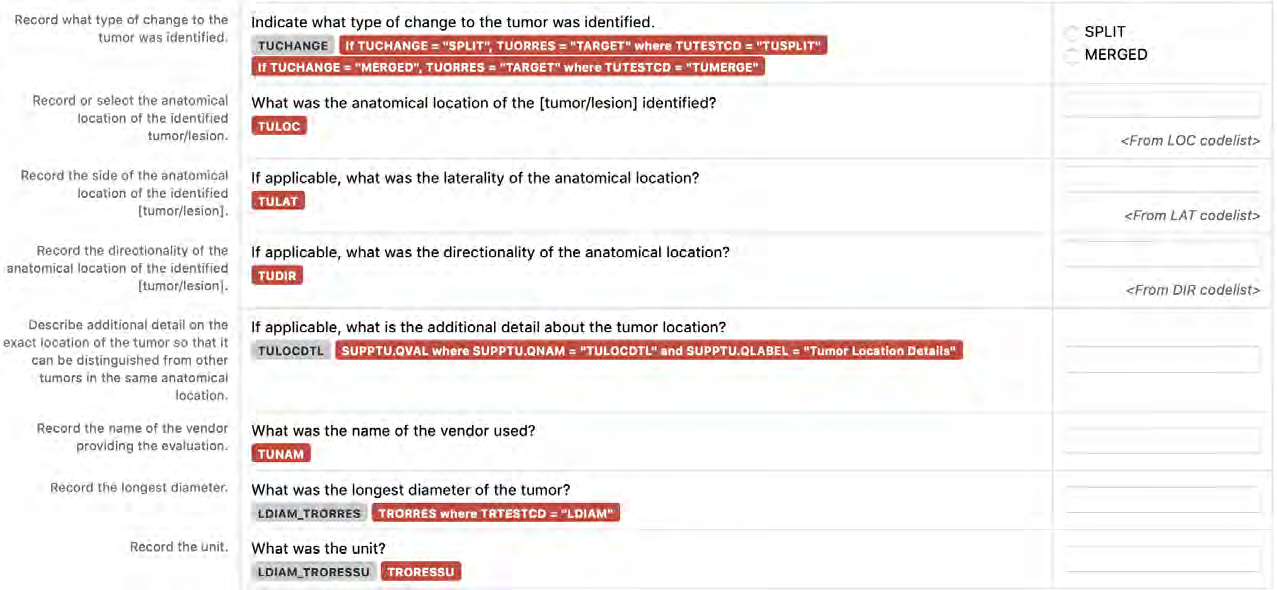
CRF Metadata
| Order Number | CDASHIG Variable | Question Text | Prompt | Type | Case Report Form Completion Instructions | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Codelist Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | TUYN | Were any ([target/non- target/new/sponsor-defined) [tumors/lesions]identified? | Any ([Target/Non- target/New/Sponsor-defined) Tumors/Lesions Identification | text | Indicate if the there are [tumors/lesions] identified. If Yes, include the appropriate details where indicated on the CRF. | N/A |
| (NY) | Yes; No | ||||
2 | TUTRDAT | What was the date of the procedure used for [tumor/lesion] identification? | Procedure Date | text | Record the date when the scan or image was made or when the physical exam occurred using this format (DD-MON-YYYY). | TUDTC;TRDTC | TUDTC and TRDTC | N/A | |||||
3 | TUTREVAL | Who was the evaluator? | Tumor Identification [Evaluator/Reporter] | text | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | TUEVAL;TREVAL | TUEVAL and TREVAL | (EVAL) | |||||
4 | TUTREVALID | What was the identifier of the evaluator? | Tumor Identification Evaluator Identifier | text | Record the unique identifier assigned to the person making the evaluation. | TUEVALID;TREVALID | TUEVALID and TREVALID | (MEDEVAL) | |||||
5 | TUTRLNKID | What was the [Tumor/Lesion] Identifier? | [Tumor/ Lesion] ID | text | If collected, record the unique identifier for this tumor/lesion. | TULNKID;TRLNKID | TULNKID and TRLNKID | N/A | |||||
6 | TUTRMETHOD | What was the method used to [evaluate/identify] the tumor/lesion? | Method of [Evaluation/Identification] | text | Record the method used to evaluate/identify the tumor/lesion. | TUMETHOD | (METHOD) | ||||||
7 | TUTRREFID | What was the procedure [reference identifier/accession number] ? | Reference ID | text | Record the internal or external identifier assigned to the procedure/method used to identify the tumor/lesion | TUREFID; | TUREFID and TRREFID | N/A | |||||
8 | TUTEST | What was the tumor/lesion identification test name? | Tumor/Lesion Identification Test Name | text | Record the name of the TU test, if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | TUTEST | TUTEST | (TUTEST) | y | ||||
9 | TUMIDENT | What is the [type/classification] of [tumor/lesion] as defined by the criteria being employed? | Result | text | Record the Tumor Identification classification. This may be preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | TUORRES:TUEST:TUTESTCD: | TUORRES where TUTESTCD="TUMIDENT" | N/A | TARGET; NON- TARGET; NEW TARGET; NEW- NON-TARGET | ||||
10 | TUCHANGE | Indicate what type of change to the tumor was identified. | Changes to Tumor (Result) | text | Record what type of change to the tumor was identified. | TUORRES:TUEST:TUTESTCD | If TUCHANGE = "SPLIT", TUORRES = "TARGET" where TUTESTCD = "TUSPLIT" ; If TUCHANGE = "MERGED", TUORRES = "TARGET" where TUTESTCD = "TUMERGE" | N/A | SPLIT; MERGED |
| |||
11 | TULOC | What was the anatomical location of the [tumor/lesion] identified? | Anatomical Location | text | Record or select the anatomical location of the identified tumor/lesion. | TULOC | (LOC) | ||||||
12 | TULAT | If applicable, what was the laterality of the anatomical location? | Side | text | Record the side of the anatomical location of the identified [tumor/lesion]. | TULAT | (LAT) | ||||||
13 | TUDIR | If applicable, what was the directionality of the anatomical location? | Directionality | text | Record the directionality of the anatomical location of the identified [tumor/lesion]. | TUDIR | (DIR) | ||||||
14 | TULOCDTL | If applicable, what is the additional detail about the tumor location? | Location Detail | text | Describe additional detail on the exact location of the tumor so that it can be distinguished from other tumors in the same anatomical location. | SUPPTU.QVAL | SUPPTU.QVAL where SUPPTU.QNAM = "TULOCDTL" and SUPPTU.QLABEL = "Tumor Location Details" | N/A | |||||
15 | TUNAM | What was the name of the vendor used? | Vendor Name | text | Record the name of the vendor providing the evaluation. | TUNAM | N/A | ||||||
16 | LDIAM_TRORRES | What was the longest diameter of the tumor? | Longest Diameter | text | Record the longest diameter. | TRORRES; TRTESTCD; TRTEST: | TRORRES where TRTESTCD = "LDIAM" | N/A | |||||
17 | LDIAM_TRORESSU | What was the unit? | Unit | text | Record the unit. | TRORESSU | N/A |
Example 2
This is an example of a single CRF collecting both acne lesion identification data (TU) and the lesion result data (TR). It collects the number of lesions of each type at each location. This is a normalized CRF. One record is collected for each anatomical location, and each type of lesion. The sponsor must determine how the TULNKID/TRLNKID are created and assigned. Other lesion types or location were not of interest.
At subsequent assessments, post-baseline, the CRF may be designed so that only the relevant questions are displayed (e.g., –EVAL, --LNKID, --DAT –REFID, TRORRES).
Title: Acne Vulgaris Lesion Evaluations
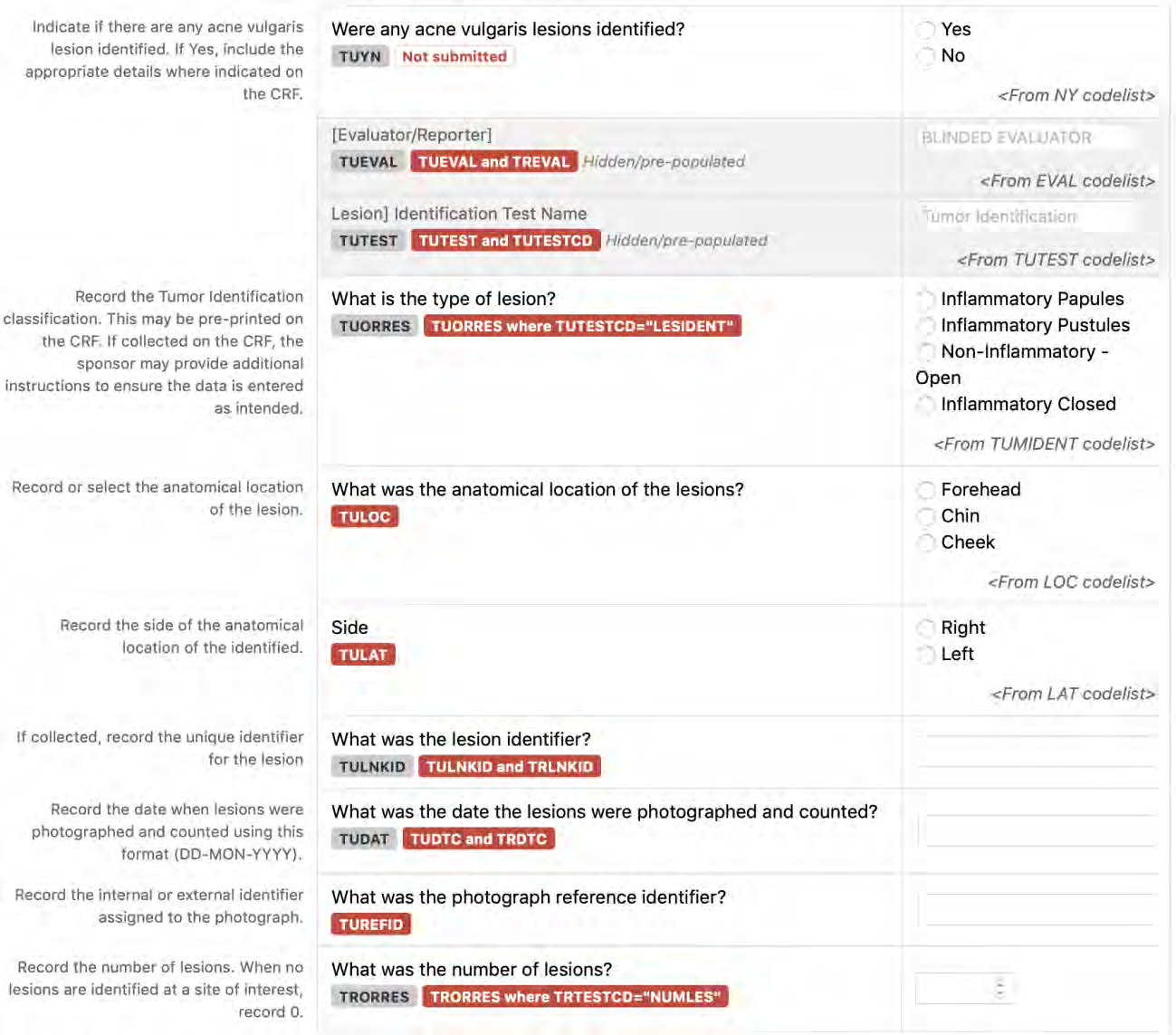
CRF Metadata
| Order Number | CDASH Variable | Question Text | Prompt | Data Type | Case Report Form Completion Instructions | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Codelist Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | TUYN | Were any acne vulgaris lesions identified? | Any Acne Vulgaris Lesions | text | Indicate if there are any acne vulgaris lesion identified. If Yes, include the appropriate details where indicated on the CRF. | N/A |
| (NY) | Yes;No | ||||
2 | TUEVAL | Who was the evaluator? | [Evaluator/Reporter] | text | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | TUEVAL; TREVAL | TUEVAL and TREVAL | (EVAL) | BLINDED EVALUATOR | checkbox | y | ||
3 | TUTEST | What was the lesion] Identification test name? | Lesion] Identification Test Name | text | Record the name of the TU test, if not pre- printed on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | TUTEST; TUTESTCD; | TUTEST and TUTESTCD | (TUTEST) | Tumor Identification | y | |||
4 | TUORRES | What is the type of lesion? | Result | text | Record the Tumor Identification classification. This may be pre-printed on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | TUORRES:TUTEST;TESTCD: | TUORRES where TUTESTCD="LESIDENT" | (TUMIDENT) | Inflammatory Papules; Inflammatory Pustules;Non- Inflammatory - Open;Inflammatory Closed | ||||
5 | TULOC | What was the anatomical location of the lesions? | Anatomical Location | text | Record or select the anatomical location of the lesion. | TULOC | (LOC) | Forehead; Chin; Cheek; | |||||
6 | TULAT | If applicable, what was the laterality of the anatomical location? | Side | text | Record the side of the anatomical location of the identified. | TULAT | (LAT) | Right; Left; | prompt | ||||
7 | TULNKID | What was the lesion identifier? | Lesion ID | text | If collected, record the unique identifier for the lesion | TULNKID; TRLNKID | TULNKID and TRLNKID | N/A | |||||
8 | TUDAT | What was the date the lesions were photographed and counted? | Date | text | Record the date when lesions were photographed and counted using this format (DD-MON-YYYY). | TUDTC; TRDTC; | TUDTC and TRDTC | N/A | |||||
9 | TUREFID | What was the photograph reference identifier? | Reference ID | text | Record the internal or external identifier assigned to the photograph. | TUREFID | N/A | ||||||
10 | TRORRES | What was the number of lesions? | Number of Lesions | integer | Record the number of lesions. When no lesions are identified at a site of interest, record 0. | TRORRES;TRTEST;TRTESTCD | TRORRES where TRTESTCD="NUMLES" |
|
8.3.18 VS - Vital Signs
Description/Overview for the CDASHIG VS - Vital Signs Domain
In CDASH, vital signs are measurements including but not limited to blood pressure, temperature, respiration, body surface area, body mass index (BMI), height, and weight.
Specification for the CDASHIG VS - Vital Signs Domain
Vital Signs Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | VS | N/A | Horizontal- Generic | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | VS | N/A | Horizontal- Generic | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | VS | N/A | Horizontal- Generic | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | VS | N/A | Horizontal- Generic | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | VS | N/A | Horizontal- Generic | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable VSDTC in ISO 8601 format. | N/A | N/A | The date the VS measurements were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the observations at that visit, or the collection date can be included on the VS CRF using the Vital Signs Date (VSDAT) field. |
Findings | VS | N/A | Horizontal- Generic | 6 | [VSTESTCD]_VSPERF | Vital Signs Performed | An indication whether or not a planned vital signs measurement, series of vital signs measurements, tests, or observations was performed. | Were [vital signs/[VSTEST] performed? | Vital Signs Performed ; [VSTEST] Performed | Char | O | Indicate if the vital signs were collected. If yes, include the appropriate details where indicated on the CRF. | VSSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTM variable VSSTAT. If VSPERF="N", the value of VSSTAT will be "NOT DONE". If VSPERF="Y", VSSTAT should be null. A combination of SDTMIG variables (e.g., VSCAT and VSSCAT, VSTPT) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable VSTESTCD would be populated as VSALL and an appropriate test name (VSTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This general prompt question is used as a data management tool to verify that missing results are confirmed missing. This may be implemented for all tests collected on the same horizontal record or for each specific test. When mapped to SDTM, the value of VSPERF would apply to all tests on the same record. Use the CDASH variable [VSTESTCD]_VSPERF when implemented on a specific test basis. |
Findings | VS | N/A | Horizontal- Generic | 7 | [VSTESTCD]_VSDAT | Vital Signs Date | The date of the vital signs measurement, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the date of the measurement(s)? | [VSTEST] Date | Char | R/C | Record date of measurements using this format (DD- MON-YYYY). | VSDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable VSDTC in ISO 8601 format. | N/A | N/A | A single date may be collected for all the vital sign measurements when they are performed on the same date. The date of each measurement can also be collected for each measurement using a CDASH variable [VSTESTCD]_VSDAT. The date of the measurements may be determined from a collected date of visit and in such cases a separate measurement date field is not required. |
Findings | VS | N/A | Horizontal- Generic | 8 | [VSTESTCD]_VSTIM | Vital Signs Time | The time of measurement, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the time of the measurement(s)? | [VSTEST] Time | Char | R/C | Record time of measurement (as complete as possible). | VSDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable VSDTC in ISO 8601 format. | N/A | N/A | A single collection time (e.g., VSTIM) may be collected for all the measurements when they are performed at the same time. The time of each measurement can also be collected using a CDASH variable [VSTESTCD]_VSTIM. |
Findings | VS | N/A | Horizontal- Generic | 9 | VSCAT | Category for Vital Signs | A grouping of topic-variable values based on user-defined characteristics. | What was the category of the vital signs? | [Vital Signs Category]; NULL | Char | O | Record the vital signs category, if not preprinted on the CRF. | VSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | VS | N/A | Horizontal- Generic | 10 | VSSCAT | Subcategory for Vital Signs | A sub-division of the VSCAT values based on user-defined characteristics. | What was the subcategory of the vital signs? | [Vital Signs Subcategory]; NULL | Char | O | Record the vital signs subcategory, if not preprinted on the CRF. | VSSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. VSSCAT can only be used if there is a VSCAT and it must be a subcategorization of VSCAT. |
Findings | VS | N/A | Horizontal- Generic | 11 | VSGRPID | Vital Signs Group ID | A sponsor- defined identifier used to tie a block of related records in a single domain. | What is the vital signs group identifier? | Test Group ID | Char | O | Record unique group identifier. Sponsor may insert additional instructions to ensure each record has a unique group identifier. | VSGRPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | It can be beneficial to use an identifier in a data query to communicate clearly to the site the specific record in question. This group identifier ties together all the tests collected on the same horizontal record. This field may be populated by the sponsor's data collection system. |
Findings | VS | N/A | Horizontal- Generic | 12 | [VSTESTCD]_VSTPT | Vital Signs Planned Time Point Name | A text description of planned time point when measurements should be taken as defined in the protocol. | What is the planned time point for this vital signs measurement? | [Planned Time Point Name] | Char | R/C | Record the planned time point labels for vital signs, if not preprinted on the CRF. | VSTPT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. See SDTMIG for additional information on representing time points. The SDTMIG time point anchors VSTPTREF (text description) and VSRFTDTC (date/time) may be needed, as well as SDTMIG variables VSTPTNUM, VSELTM. | N/A | N/A | Planned time points are needed to differentiate multiple sequential assessments. It is recommended that time points should be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Planned Time Point" can be included as the column header. The planned time point of each measurement can also be collected using a CDASH variable [VSTESTCD]_VSTPT. |
Findings | VS | N/A | Horizontal- Generic | 13 | [VSTESTCD]_VSSTAT | Vital Signs Completion Status | The variable used to indicate that data are not available by having the site recording the value as "Not Done". | Indicate if the [VSTEST] measurement was not done | Not Done | Char | O | Indicate if the vital signs measurement was not done. | VSSTAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (ND) | N/A | A single "Not Done" can be collected once for all the tests on the same horizontal record using VSSTAT. The value of VSSTAT applies to all measurements on that record when mapped to SDTM. If needed, for each test "NOT DONE" may be collected using the CDASH variable [VSTESTCD]_VSSTAT. |
Findings | VS | N/A | Horizontal- Generic | 14 | [VSTESTCD]_VSORRES | VS Result or Finding in Original Units | Result of the vital signs measurement as originally received or collected. | What was the result of the [VSTEST] measurement? | [VSTEST] (Result) | Char | HR | Record the vital sign results. | VSORRES; VSTEST; VSTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. In addition to the SDTMIG variable VSORRES, create VSTESTCD from the CDASH variable name and determine the value of VSTEST from VSTESTCD. The CDASH prompt may also contain the VSTEST. Use appropriate CDISC Controlled Terminology for the test and test code. | N/A | N/A | Each test may be collected using the CDASH variable [TESTCD] e.g., SYSBP or [TESTCD]_VSORRES where TESTCD is the appropriate CT for the VS test code e.g., SYSBP_VSORRES. This CDASH variable name is an example of what "variable name" can be used in a denormalized data structure. |
Findings | VS | N/A | Horizontal- Generic | 15 | [VSTESTCD]_VSORRESU | VS Original Units | The unit of the result as originally received or collected. | What was the unit of the [VSTEST] measurement? | [VSTEST] Unit | Char | R/C | Record or select the original unit in which these data were collected, if not preprinted on CRF. | VSORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (VSRESU) | N/A | A single Unit field can be collected once for all measurements collected on the same horizontal record using VSUNIT. The value of VSUNIT applies to all measurements on that record when mapped to SDTM. If needed for each measurement, unit may be collected using the CDASH variable [VSTESTCD]_VSSTAT. Should be preprinted on the CRF with the associated test when possible, rather than collected in a field that requires the site to enter text. |
Findings | VS | N/A | Horizontal- Generic | 16 | [VSTESTCD]_VSCLSIG | Vital Signs Clinical Significance | An indication whether the vital signs results were clinically significant. | Was the [VSTEST] result clinically significant? | [VSTEST] Clinically Significant | Char | O | Record whether vital sign result was clinically significant. | SUPPVS.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPVS dataset as the value of SUPPVS.QVAL where SUPPVS.QNAM = "VSCLSIG" and SUPPVS.QLABEL="Clinically Significant". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | In horizontal data collection, a CDASH variable [VSTESTCD]_VSCLSIG may be created for each VSTESTCD and added to the CRF if needed. |
Findings | VS | N/A | Horizontal- Generic | 17 | [VSTESTCD]_VSPOS | Vital Signs Position of Subject | The position of the subject during a measurement or examination. | What was the position of the subject during the [VSTEST] measurement? | [VSTEST] Position | Char | R/C | Record the position of subject at time of test (e.g. SITTING). | VSPOS | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (POSITION) | (VSPOS) | Results may be affected by whether conditions for vital signs as specified in the protocol were properly met. One common condition is the subject's position. If the protocol requires this type of information, then a CDASH variable [VSTESTCD]_VSPOS may be created for each VSTESTCD and added to the CRF, if needed. |
Findings | VS | N/A | Horizontal- Generic | 18 | [VSTESTCD]_VSLOC | Location of Vital Signs Measurement | A description of the anatomical location of the subject relevant to the collection of Vital Signs measurement. | What was the anatomical location where the [VSTEST] measurement was taken? | [VSTEST] Anatomical Location | Char | O | Record or select location on body where measurement was performed, if not preprinted on CRF. | VSLOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location (e.g., ARM for blood pressure). Sponsors may collect the data using a subset list of controlled terminology on the CRF. In horizontal data collection, a CDASH variable [VSTESTCD]_VSLOC may be created for each VSTESTCD and added to the CRF, if needed. LAT, DIR, and PORTOT are used to further describe the anatomical location. |
Findings | VS | N/A | Horizontal- Generic | 19 | [VSTESTCD]_VSLAT | Vital Signs Laterality | Qualifier for anatomical location further detailing the side of the body. | What was the side of the anatomical location of the [VSTEST] measurement? | Side | Char | O | Record the side of the anatomical location of the vital signs measurement. | VSLAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | VS | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | VS | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | VS | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What is the subject identifier? | Subject | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be provided to the site using a prepopulated list in the system. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM. |
Findings | VS | N/A | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is represented only in the SDTMIG DM domain. For more information, refer to SDTM Table 2.2.4. |
Findings | VS | N/A | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable VSDTC in ISO 8601 format. | N/A | N/A | The date the VS measurements were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the observations at that visit, or the collection date can be included on the VS CRF using the Vital Signs Date (VSDAT) field. |
Findings | VS | N/A | N/A | 6 | VSPERF | Vital Signs Performed | An indication whether or not a planned vital signs measurement, series of vital signs measurements, tests, or observations was performed. | Were vital signs performed? | Vital Signs Performed | Char | O | Indicate if the vital signs were collected. If yes, include the appropriate details where indicated on the CRF. | VSSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTM variable VSSTAT. If VSPERF="N", the value of VSSTAT will be "NOT DONE". If VSPERF="Y", VSSTAT should be null. A combination of SDTMIG variables (e.g., VSCAT and VSSCAT, VSTPT) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable VSTESTCD would be populated as VSALL and an appropriate test name VSTEST provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This general prompt question is used as a data management tool to verify that missing results are confirmed missing. |
Findings | VS | N/A | N/A | 7 | VSDAT | Vital Signs Date | The date of the vital signs measurement, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the date of the vital signs measurement? | Date | Char | R/C | Record date of measurements using this format (DD- MON-YYYY). | VSDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable VSDTC in ISO 8601 format. | N/A | N/A | The date of measurement can be determined from a collected date of visit (VISDAT) and in such cases a separate measurement date field is not required. |
Findings | VS | N/A | N/A | 8 | VSTIM | Vital Signs Time | The time of measurement, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the time of the vital signs measurement? | Time | Char | R/C | Record time of measurement (as complete as possible). | VSDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable VSDTC in ISO 8601 format. | N/A | N/A | Collect time if it is relevant for the analysis. |
Findings | VS | N/A | N/A | 9 | VSSPID | Vital Signs Sponsor- Defined Identifier | A sponsor- defined identifier. In CDASH, This is typically used for preprinted or auto-generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor-defined question] | [Sponsor defined] | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | VSSPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Because SPID is a sponsor- defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile data. May be used to record preprinted number (e.g. line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Findings | VS | N/A | N/A | 10 | VSTPT | Vital Signs Planned Time Point Name | A text description of planned time point when measurements should be taken as defined in the protocol. | What is the planned time point for this vital signs measurement? | [Planned Time Point Name] | Char | R/C | Record the planned time point labels for vital signs, if not preprinted on the CRF. | VSTPT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. See SDTMIG for additional information on representing time points. The SDTMIG time point anchors VSTPTREF (text description) and VSRFTDTC (date/time) may be needed, as well as SDTMIG variables VSTPTNUM, VSELTM. | N/A | N/A | Planned time points are needed to differentiate multiple sequential assessments. It is recommended that time points should be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Planned Time Point" can be included as the column header. |
Findings | VS | N/A | N/A | 11 | VSCAT | Category for Vital Signs | A grouping of topic-variable values based on user-defined characteristics. | What was the category of the vital signs? | [Vital Signs Category]; NULL | Char | O |
| Record the vital signs category, if not preprinted on the CRF. | VSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | VS | N/A | N/A | 12 | VSSCAT | Subcategory for Vital Signs A sub-division of the VSCAT values based on user-defined characteristics. | What was the subcategory of the vital signs? | [Vital Signs Subcategory]; NULL | Char | O | Record the vital signs subcategory, if not preprinted on the CRF. | VSSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. VSSCAT can only be used if there is a VSCAT and it must be a subcategorization of VSCAT. | |
Findings |
| VS | N/A | N/A | 13 | VSREPNUM | Vital Signs Repetition Number | The instance number of a test that is repeated within a given timeframe for the same test. The level of granularity can vary (e.g., within a time point, within a visit). | What was the repetition number within the time point for this measurement? | Repetition Number | Char | O | Record the repetition number of the measurement within the time point. | SUPPVS.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPVS dataset as the value of SUPPVS.QVAL where SUPPVS.QNAM= "VSREPNUM" and SUPPVS.QLABEL= "Repetition Number within time point". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | N/A | N/A The repetition number of the test/measurement within the time point may be preprinted on the CRF (e.g., multiple measurements of blood pressure, multiple analyses of a sample). |
Findings | VS | N/A | N/A | 14 | VSTEST | Vital Signs Test Name | Descriptive name of the test or examination used to obtain the measurement or finding. | What is the vital sign test name? | [Vital Signs Test Name] | Char | HR | Record the name of the vital sign test if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | VSTEST; VSTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. The SDTMIG variable VSTESTCD may be determined from the value collected in VSTEST. Both VSTESTCD and VSTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | (VSTEST) | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | VS | N/A | N/A | 15 | VSSTAT | Vital Signs Completion Status | The variable used to indicate that data are not available by having the site recording the value as "Not Done". | Indicate if the vital signs measurement was not done | Not Done | Char | O | Indicate if the vital sign measurement was not done. | VSSTAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (ND) | N/A | A Not Done check box, which indicates the test was NOT DONE. Typically, there would be one check box for each measurement. This field can be useful on individual vital signs tests to confirm that a blank result field is meant to be blank. |
Findings | VS | N/A | N/A | 16 | VSORRES | VS Result or Finding in Original Units | Result of the vital signs measurement as originally received or collected. | What was the result of the measurement? | (Result) | Char | HR | Record the vital sign result. | VSORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | N/A |
Findings | VS | N/A | N/A | 17 | VSORRESU | VS Original Units | The unit of the result as originally received or collected. | What was the unit of the measurement? | Unit | Char | R/C | Record or select the original unit in which these data were collected, if not preprinted on CRF. | VSORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (VSRESU) | N/A | Should be preprinted on the CRF with the associated test when possible, rather than collected in a field that requires the site to enter text. |
Findings | VS | N/A | N/A | 18 | VSCLSIG | Vital Signs Clinical Significance | An indication whether the vital sign result was clinically significant. | Was the result clinically significant? | Clinically Significant | Char | O | Record whether vital sign result was clinically significant. | SUPPVS.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPVS dataset as the value of SUPPVS.QVAL where SUPPVS.QNAM = "VSCLSIG" and SUPPVS.QLABEL="Clinically Significant". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | N/A |
Findings | VS | N/A | N/A | 19 | VSLOC | Location of Vital Signs Measurement | A description of the anatomical location of the subject relevant to the collection of Vital Signs measurement. | What was the anatomical location where the measurement was taken? | Anatomical Location | Char | O | Record or select location on body where measurement was performed, if not preprinted on CRF. | VSLOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location (e.g., ARM for blood pressure). Sponsors may collect the data using a subset list of controlled terminology on the CRF. LAT, DIR, and PORTOT are used to further describe the anatomical location. |
Findings | VS | N/A | N/A | 20 | VSPOS | Vital Signs Position of Subject | The position of the subject during a measurement or examination. | What was the position of the subject during the measurement? | Position | Char | R/C | Record the position of subject at time of test (e.g. SITTING). | VSPOS | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (POSITION) | (VSPOS) | Results may be affected by whether conditions for vital signs as specified in the protocol were properly met. One common condition is the subject's position. |
Findings | VS | N/A | N/A | 21 | VSDIR | Vital Signs Directionality | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What was the directionality of the anatomical location of the measurement? | Directionality | Char | O | Record the directionality. | VSDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | VS | N/A | N/A | 22 | VSLAT | Vital Signs Laterality | Qualifier for anatomical location further detailing the side of the body. | What was the side of the anatomical location of the vital signs measurement? | Side | Char | O | Record the side of the anatomical location of the vital signs measurement. | VSLAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Assumptions for the CDASHIG VS - Vital Signs Domain
1. Vital Signs may be collected using either a normalized or a denormalized horizontal data structure, depending on the functionality of the data management or data capture system being used.
2. In a denormalized structure, Vital Signs are collected using a unique variable name for each test, resulting in a wide, horizontal dataset with multiple test results in each record. The SDTMIG VS structure is normalized, using 1 variable (VSTEST) for the name of the test and 1 variable (VSORRES) for the collected results, resulting in a vertical data structure in which there is 1 record for each test/result. CDASH recommendations are intended to facilitate moving denormalized data to the SDTM normalized data structure, because standard transformation programming can be written when the variable naming syntax is consistent and uses CDISC root variables and Controlled Terminology.
3. The set of variables needed for a particular study may include only test result and result unit, or it may include other variables such as location, laterality, position, or method. The examples in this section show a couple of different ways that denormalized variable names can be created based on the general variable- naming rules of CDASH, using the root variables found in the CDASH Model in combination with the controlled terminology from the Vital Signs Test Code codelist (VSTESTCD) in a consistent syntax.
Example CRFs for the CDASHIG VS - Vital Signs Domain
Example 1
This example shows a vital signs data collection in a denormalized structure with variable names that could be 8 or more characters. The following syntax was used to create the denormalized variable names:
Title: Vital Signs
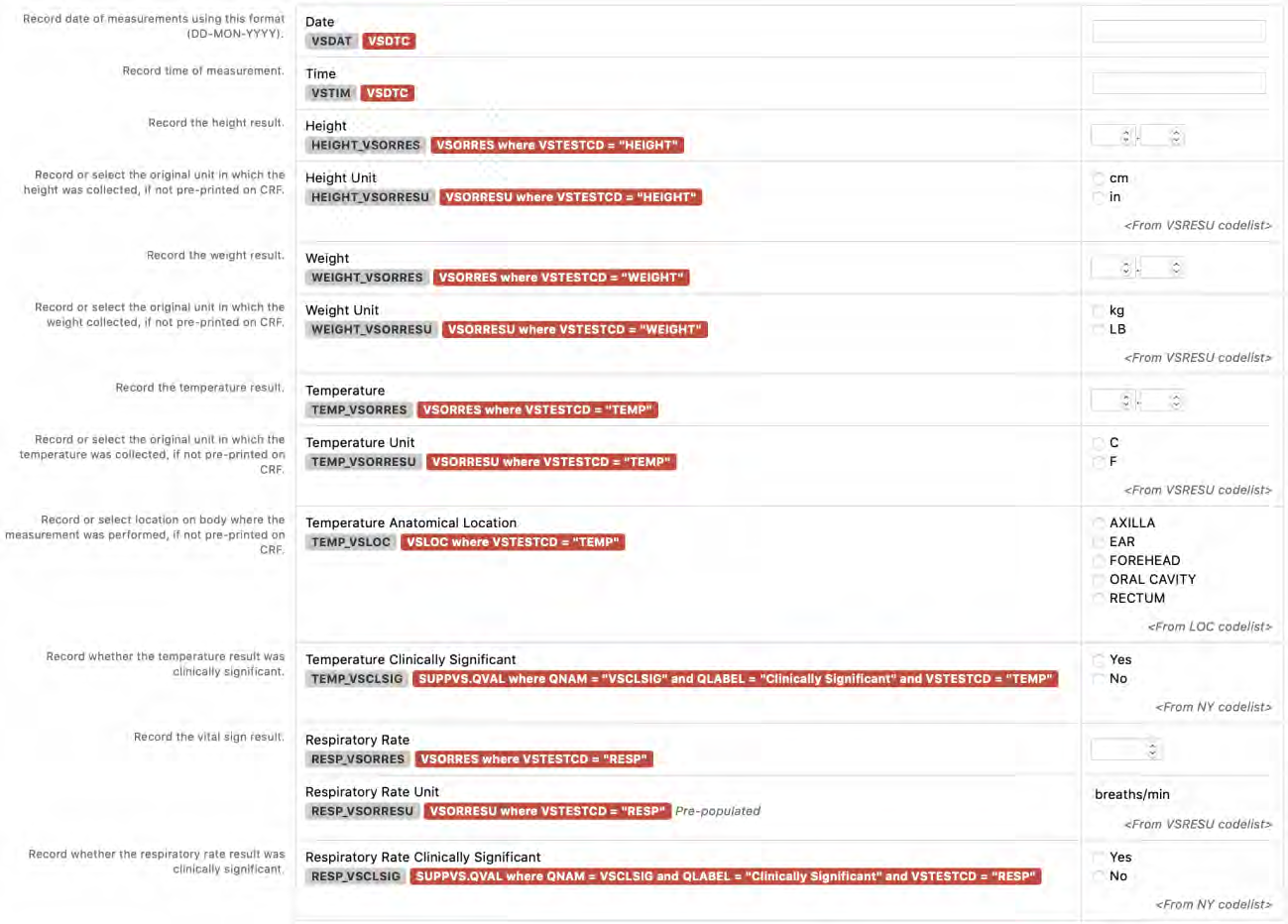
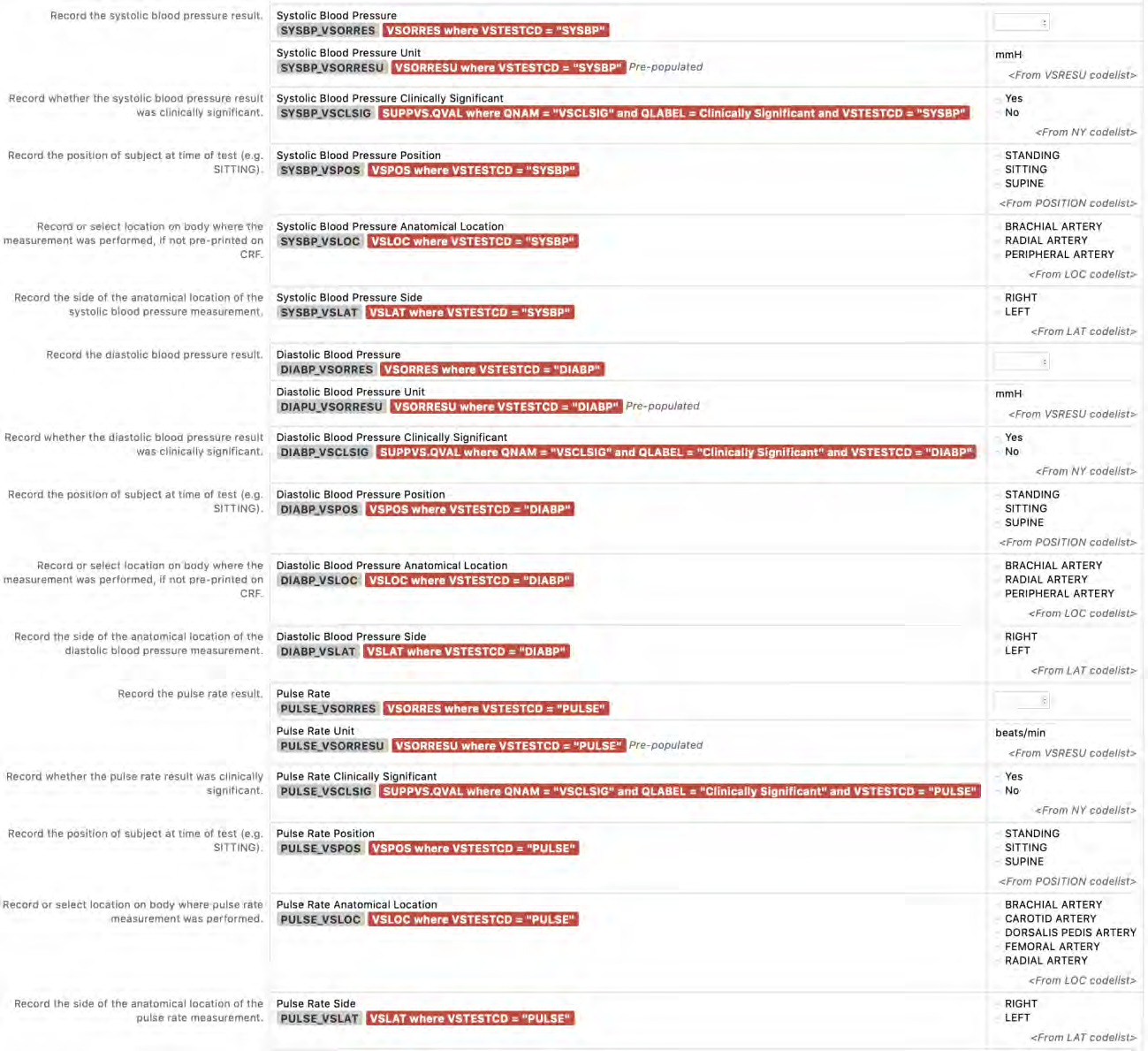
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
VSDAT | 1 | What was the date of the measurements? | Date | Record date of measurements using this format (DD-MON- YYYY). | Date | VSDTC | Prompt | ||||||
VSTIM | 2 | What was the time of the measurements? | Time | Record time of measurement. | Time | VSDTC | Prompt | ||||||
HEIGHT_VSORRES | 3 | What was the result of the height measurement? | Height | Record the height result. | Float | VSORRES; VSTEST; VSTESTCD | VSORRES where VSTESTCD = "HEIGHT" | Prompt | |||||
HEIGHT_VSORRESU | 4 | What was the unit of the height measurement? | Height Unit | Record or select the original unit in which the height was collected, if not pre-printed on CRF. | Text | VSORRESU | VSORRESU where VSTESTCD = "HEIGHT" | (VSRESU) | cm; in | Prompt | |||
WEIGHT_VSORRES | 5 | What was the result of the weight measurement? | Weight | Record the weight result. | Float | VSORRES; VSTEST; VSTESTCD | VSORRES where VSTESTCD = "WEIGHT" | Prompt | |||||
WEIGHT_VSORRESU | 6 | What was the unit of the weight measurement? | Weight Unit | Record or select the original unit in which the weight collected, if not pre-printed on CRF. | Text | VSORRESU | VSORRESU where VSTESTCD = "WEIGHT" | (VSRESU) | kg; LB | Prompt | |||
TEMP_VSORRES | 7 | What was the result of the temperature measurement? | Temperature | Record the temperature result. | Float | VSORRES; VSTEST; VSTESTCD | VSORRES where VSTESTCD = "TEMP" | Prompt | |||||
TEMP_VSORRESU | 8 | What was the unit of the temperature measurement? | Temperature Unit | Record or select the original unit in which the temperature was collected, if not pre-printed on CRF. | Text | VSORRESU | VSORRESU where VSTESTCD = "TEMP" | (VSRESU) | C; F | Prompt | |||
TEMP_VSLOC | 9 | What was the anatomical location where the temperature measurement was taken? | Temperature Anatomical Location | Record or select location on body where the measurement was performed, if not pre- printed on CRF. | Text | VSLOC | VSLOC where VSTESTCD = "TEMP" | (LOC) | AXILLA; EAR; FOREHEAD; ORAL CAVITY; RECTUM | Prompt | |||
TEMP_VSCLSIG | 10 | Was the temperature result clinically significant? | Temperature Clinically Significant | Record whether the temperature result was clinically significant. | Text | SUPPVS.QVAL | SUPPVS.QVAL where QNAM = "VSCLSIG" and QLABEL = "Clinically Significant" and VSTESTCD = "TEMP" | (NY) | Yes; No | Prompt | |||
RESP_VSORRES | 11 | What was the result of the respiratory rate measurement? | Respiratory Rate | Record the vital sign result. | Integer | VSORRES; VSTEST; VSTESTCD | VSORRES where VSTESTCD = "RESP" | Prompt | |||||
RESP_VSORRESU | 12 | What was the unit of the respiratory rate measurement? | Respiratory Rate Unit | Record or select the original unit in which the respiratory rate was collected, if not pre-printed on CRF. | Text | VSORRESU | VSORRESU where VSTESTCD = "RESP" | (VSRESU) | breaths/min | Prompt | |||
RESP_VSCLSIG | 13 | Was the respiratory rate result clinically significant? | Respiratory Rate Clinically Significant | Record whether the respiratory rate result was clinically significant. | Text | SUPPVS.QVAL | SUPPVS.QVAL where QNAM = VSCLSIG and QLABEL = "Clinically Significant" and VSTESTCD = "RESP" | (NY) | Yes; No | Prompt | |||
SYSBP_VSORRES | 14 | What was the result of the systolic blood pressure measurement? | Systolic Blood Pressure | Record the systolic blood pressureresult. | Integer | VSORRES; VSTEST; VSTESTCD | VSORRES where VSTESTCD = "SYSBP" | Prompt | |||||
SYSBP_VSORRESU | 15 | What was the unit of the systolic blood pressure measurement? | Systolic Blood Pressure Unit | Record or select the original unit in which the systolic blood pressure was collected, if not pre-printed on CRF. | Text | VSORRESU | VSORRESU where VSTESTCD = "SYSBP" | (VSRESU) | mmHg | Prompt | |||
SYSBP_VSCLSIG | 16 | Was the systolic blood pressure result clinically significant? | Systolic Blood Pressure Clinically Significant | Record whether the systolic blood pressure result was clinically significant. | Text | SUPPVS.QVAL | SUPPVS.QVAL where QNAM = "VSCLSIG" and QLABEL = Clinically Significant and VSTESTCD = "SYSBP" | (NY) | Yes; No | Prompt | |||
SYSBP_VSPOS | 17 | What was the position of the subject during the systolic blood pressure measurement? | Systolic Blood Pressure Position | Record the position of subject at time of test (e.g. SITTING). | Text | VSPOS | VSPOS where VSTESTCD = "SYSBP" | (POSITION) | STANDING; SITTING; SUPINE | Prompt | |||
SYSBP_VSLOC | 18 | What was the anatomical location where the systolic blood pressure measurement was taken? | Systolic Blood Pressure Anatomical Location | Record or select location on body where the measurement was performed, if not pre- printed on CRF. | Text | VSLOC | VSLOC where VSTESTCD = "SYSBP" | (LOC) | BRACHIAL ARTERY; RADIAL ARTERY; PERIPHERAL ARTERY | Prompt | |||
SYSBP_VSLAT | 19 | What was the side of the anatomical location of the systolic blood pressure measurement? | Systolic Blood Pressure Side | Record the side of the anatomical location of the systolic blood pressure measurement. | Text | VSLAT | VSLAT where VSTESTCD = "SYSBP" | (LAT) | RIGHT; LEFT | Prompt | |||
DIABP_VSORRES | 20 | What was the result of the diastolic blood pressure measurement? | Diastolic Blood Pressure | Record the diastolic blood pressure result. | Integer | VSORRES; VSTEST; VSTESTCD | VSORRES where VSTESTCD = "DIABP" | Prompt | |||||
DIAPU_VSORRESU | 21 | What was the unit of the diastolic blood pressure measurement? | Diastolic Blood Pressure Unit | Record or select the original unit in which the diastolic blood pressure was collected, if not pre-printed on CRF. | Text | VSORRESU | VSORRESU where VSTESTCD = "DIABP" | (VSRESU) | mmHg | Prompt | |||
DIABP_VSCLSIG | 22 | Was the diastolic blood pressure result clinically significant? | Diastolic Blood Pressure Clinically Significant | Record whether the diastolic blood pressure result was clinically significant. | Text | SUPPVS.QVAL | SUPPVS.QVAL where QNAM = "VSCLSIG" and QLABEL = "Clinically Significant" and VSTESTCD = "DIABP" | (NY) | Yes; No | Prompt | |||
DIABP_VSPOS | 23 | What was the position of the subject during the diastolic blood pressure measurement? | Diastolic Blood Pressure Position | Record the position of subject at time of test (e.g. SITTING). | Text | VSPOS | VSPOS where VSTESTCD = "DIABP" | (POSITION) | STANDING; SITTING; SUPINE | Prompt | |||
DIABP_VSLOC | 24 | What was the anatomical location where the diastolic blood pressure measurement was taken? | Diastolic Blood Pressure Anatomical Location | Record or select location on body where the measurement was performed, if not pre- printed on CRF. | Text | VSLOC | VSLOC where VSTESTCD = "DIABP" | (LOC) | BRACHIAL ARTERY; RADIAL ARTERY; PERIPHERAL ARTERY | Prompt | |||
DIABP_VSLAT | 25 | What was the side of the anatomical location of the diastolic blood pressure measurement? | Diastolic Blood Pressure Side | Record the side of the anatomical location of the diastolic blood pressure measurement. | Text | VSLAT | VSLAT where VSTESTCD = "DIABP" | (LAT) | RIGHT; LEFT | Prompt | |||
PULSE_VSORRES | 26 | What was the result of the pulse measurement? | Pulse Rate | Record the pulse rate result. | Integer | VSORRES; VSTEST; VSTESTCD | VSORRES where VSTESTCD = "PULSE" | Prompt | |||||
PULSE_VSORRESU | 27 | What was the unit of the pulse rate measurement? | Pulse Rate Unit | Record or select the original unit in which the pulse rate was collected, if not pre-printed on CRF. | Text | VSORRESU | VSORRESU where VSTESTCD = "PULSE" | (VSRESU) | beats/min | Prompt | |||
PULSE_VSCLSIG | 28 | Was the pulse rate result clinically significant? | Pulse Rate Clinically Significant | Record whether the pulse rate result was clinically significant. | Text | SUPPVS.QVAL | SUPPVS.QVAL where QNAM = "VSCLSIG" and QLABEL = "Clinically Significant" and VSTESTCD = "PULSE" | (NY) | Yes; No | Prompt | |||
PULSE_VSPOS | 29 | What was the position of the subject during the pulse rate measurement? | Pulse Rate Position | Record the position of subject at time of test (e.g. SITTING). | Text | VSPOS | VSPOS where VSTESTCD = "PULSE" | (POSITION) | STANDING; SITTING; SUPINE | Prompt | |||
PULSE_VSLOC | 30 | What was the anatomical location where the pulse rate measurement was taken? | Pulse Rate Anatomical Location | Record or select location on body where pulse rate measurement was performed. | Text | VSLOC | VSLOC where VSTESTCD = "PULSE" | (LOC) | BRACHIAL ARTERY; CAROTID ARTERY; DORSALIS PEDIS ARTERY; FEMORAL ARTERY; RADIAL ARTERY | Prompt | |||
PULSE_VSLAT | 31 | What was the side of the anatomical location of the pulse rate measurement? | Pulse Rate Side | Record the side of the anatomical location of the pulse rate measurement. | Text | VSLAT | VSLAT where VSTESTCD = "PULSE" | (LAT) | RIGHT; LEFT | Prompt |
Example 2
This example shows a vital signs data collection in a denormalized structure with variable names restricted to 8 characters or less. The VSTESTCD values were used to create the denormalized variable names.
Title: Vital Signs
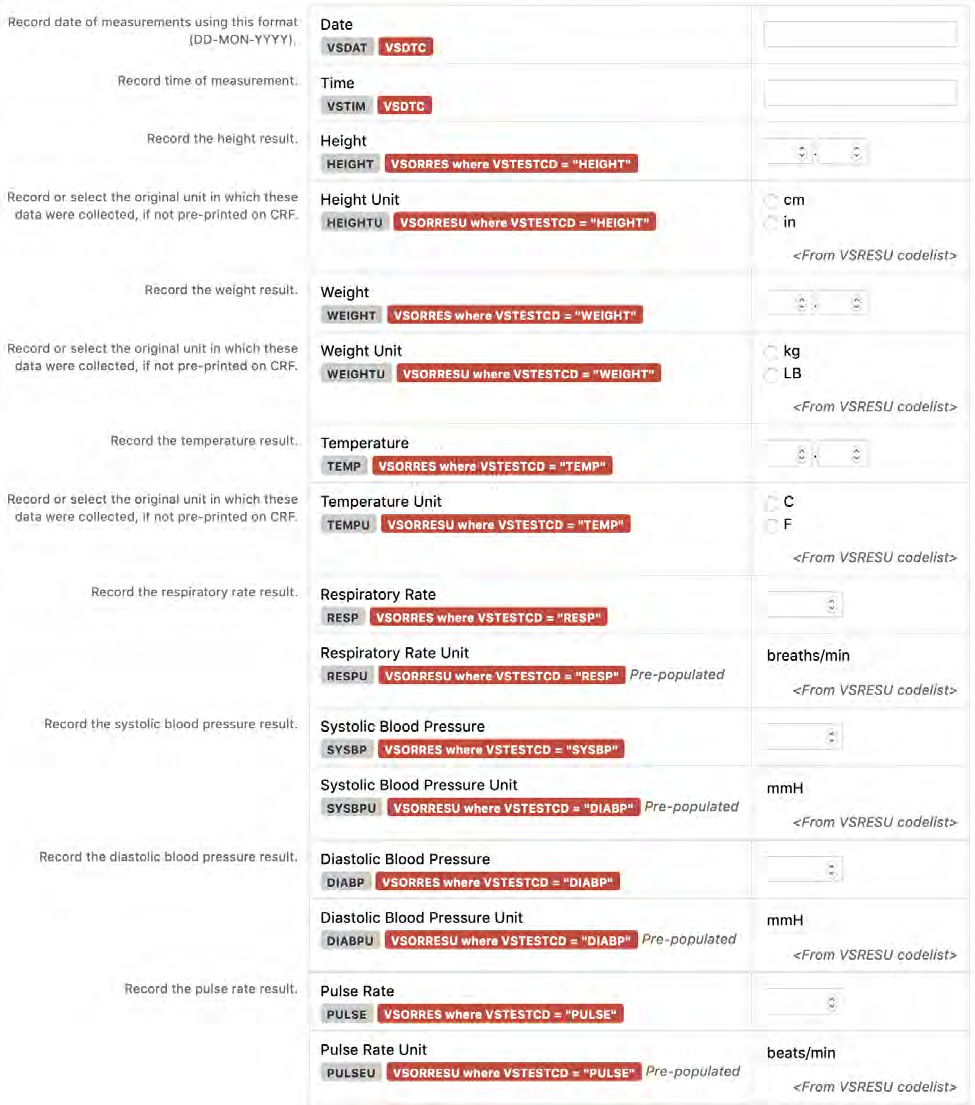
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre-Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
VSDAT | 1 | What was the date of the measurements? | Date | Record date of measurements using this format (DD-MON-YYYY). | Date | VSDTC | Prompt | ||||||
VSTIM | 2 | What was the time of the measurements? | Time | Record time of measurement. | Time | VSDTC | Prompt | ||||||
HEIGHT | 3 | What was the result of the height measurement? | Height | Record the height result. | Float | VSORRES; VSTEST; VSTESTCD | VSORRES where VSTESTCD = "HEIGHT" | Prompt | |||||
HEIGHTU | 4 | What was the unit of the height measurement? | Height Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | Text | VSORRESU | VSORRESU where VSTESTCD = "HEIGHT" | (VSRESU) | cm; in | Prompt | |||
WEIGHT | 5 | What was the result of the weight measurement? | Weight | Record the weight result. | Float | VSORRES; VSTEST; VSTESTCD | VSORRES where VSTESTCD = "WEIGHT" | Prompt | |||||
WEIGHTU | 6 | What was the unit of the weight measurement? | Weight Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | Text | VSORRESU | VSORRESU where VSTESTCD = "WEIGHT" | (VSRESU) | kg; LB | Prompt | |||
TEMP | 7 | What was the result of the temperature measurement? | Temperature | Record the temperature result. | Float | VSORRES; VSTEST; VSTESTCD | VSORRES where VSTESTCD = "TEMP" | Prompt | |||||
TEMPU | 8 | What was the unit of the temperature measurement? | Temperature Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | Text | VSORRESU | VSORRESU where VSTESTCD = "TEMP" | (VSRESU) | C; F | Prompt | |||
RESP | 9 | What was the result of the respiratory rate measurement? | Respiratory Rate | Record the respiratory rate result. | Integer | VSORRES; VSTEST; VSTESTCD | VSORRES where VSTESTCD = "RESP" | Prompt | |||||
RESPU | 10 | What was the unit of the respiratory rate measurement? | Respiratory Rate Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | Text | VSORRESU | VSORRESU where VSTESTCD = "RESP" | (VSRESU) | breaths/min | Prompt | |||
SYSBP | 11 | What was the result of the systolic blood pressure measurement? | Systolic Blood Pressure | Record the systolic blood pressure result. | Integer | VSORRES; VSTEST; VSTESTCD | VSORRES where VSTESTCD = "SYSBP" | Prompt | |||||
SYSBPU | 12 | What was the unit of the systolic blood pressure measurement? | Systolic Blood Pressure Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | Text | VSORRESU | VSORRESU where VSTESTCD = "DIABP" | (VSRESU) | mmHg | Prompt | |||
DIABP | 13 | What was the result of the diastolic blood pressure measurement? | Diastolic Blood Pressure | Record the diastolic blood pressure result. | Integer | VSORRES; VSTEST; VSTESTCD | VSORRES where VSTESTCD = "DIABP" | Prompt | |||||
DIABPU | 14 | What was the unit of the diastolic blood pressure measurement? | Diastolic Blood Pressure Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | Text | VSORRESU | VSORRESU where VSTESTCD = "DIABP" | (VSRESU) | mmHg | Prompt | |||
PULSE | 15 | What was the result of the pulse measurement? | Pulse Rate | Record the pulse rate result. | Integer | VSORRES; VSTEST; VSTESTCD | VSORRES where VSTESTCD = "PULSE" | Prompt | |||||
PULSEU | 16 | What was the unit of the pulse rate measurement? | Pulse Rate Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | Text | VSORRESU | VSORRESU where VSTESTCD = "PULSE" | (VSRESU) | beats/min | Prompt |
8.3.19 FA - Findings About Events or Interventions
Description/Overview for the CDASHIG FA - Findings About Events or Interventions Domain
The Findings class also includes the subtype Findings About, which is used to record findings related to observations in the Interventions or Events class.
Specification for the CDASHIG FA - Findings About Events or Interventions Domain
Findings About Events or Interventions Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | FA | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | FA | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | FA | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? [Subject/Participant] (Identifier) | > | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | FA | N/A | N/A | 4 | FAOBJ | Findings About Object of the Observation | A description of the object or focal point of the findings observation that is represented by FATEST. | [Sponsored-defined phrase] | [Sponsored-defined phrase] | Char | HR | [Protocol specific] | FAOBJ | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | The FAOBJ will usually be preprinted or hidden, and not solicited as an actual question. These FA domains are usually created by the sponsor. |
Findings | FA | N/A | N/A | 5 | FAYN | Findings About Collected | An indication whether or not any data was collected for the finding topic. | Has the subject had any [Findings topic(s)] (after/ before [study specific time frame])?; [Was/ Were] (there) any [Findings topic(s)] (reported) (after/before [study specific time frame])?; Were all eligibility criteria met? | Any [Finding Topic] | Char | O | Indicate if the there are findings. If yes, include the appropriate details where indicated on the CRF. | N/A | Does not map to an SDTM variable. The SDTM aCRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | N/A | This is a field that can be used in any CRF to indicate whether or not there is data to record. Used primarily as a data cleaning field. This provides verification that all other fields on the CRF were deliberately left blank. FAPERF should be used to capture a response about whether planned measurements, tests, observations were done. |
Findings | FA | N/A | N/A | 6 | FAPERF | Findings About Performed | An indication of whether or not a planned measurement, series of measurements, test, observation or specimen was performed or collected. | [Were any/Was the] [FATEST/topic] ([measurement(s)/test(s)/examin ation(s)/ specimen(s)/sample(s))] [performed/collected]? | ([FATEST/ topic] ([Measurement (s)/Test(s)/Examination(s)/Specimen(s)/ Sample(s)]) [Performed/Collected]? | Char | O | Indicate if the [FATESTs] was/were collected. If yes, include the appropriate details where indicated on the CRF. | FASTAT | This field does not map directly to an SDTM variable. May be used to populate a value into the SDTM variable FASTAT. If the CDASH variable FAPERF="N", the value of the STDM variable FASTAT is "OT DONE". If FAPERF="Y", FASTAT is null. A combination of SDTM variables (e.g., FACAT and FASCAT, FATPT ) is used to indicate that multiple tests were not done. In this situation, the SDTM variable FATESTCD would be populated with FAALL and an appropriate test name (FATEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | This field is used to capture a response to whether or not a planned measurement, test or observation was performed. A negative response can be collected as "N" and mapped to the FASTAT variable in SDTM as "NOT DONE". |
Findings | FA | N/A | N/A | 7 | FATEST | Findings About Test Name | Descriptive name for the test being performed. | What [is/was] the name (of the [measurement/test/examination]) ? | [Measurement/Test/Examination/] (Name) | Char | HR | Record the name of the FATEST if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | FATEST;FATE STCD | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. The SDTM variable FATESTCD may be determined from the value collected in FATEST. The SDTMIG variables FATESTCD and FATEST are required in SDTM. | N/A | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | FA | N/A | N/A | 8 | FATSTDTL | Findings About Test Detail | A further description of FATESTCD and FATEST. | What [is/was] the [measurement/test/ examination] detail name? | [Measurement/Test/Examination] Detail (Name) | Char | O | Record the detail of the [FATEST], if not preprinted on the CRF. | FATSTDTL | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | N/A | N/A | It is recommended that the test detail name be preprinted on the CRF. If the form is laid out as a grid, then words such as "Test," "Test Name" can be included as the column header. |
Findings | FA | N/A | N/A | 9 | FACAT | Category for Findings About | A grouping of topic- variable values based on user- defined characteristics. | What [is/was] the [type/category/name] (of the [measurement/test/examination/ specimen/sample])? | [Category/Category Value]; NULL | Char | O | Record the FA category, if not preprinted on the CRF. | FACAT | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | FA | N/A | N/A | 10 | FASCAT | Subcategory for Findings About | A sub-division of the FACAT values based on user-defined characteristics. | What [is/was] the [type/subcategory/name] (of the [measurement/test/examination/ specimen/sample])? | [FA Subcategory/FA Subcategory Value]; NULL | Char | O | Record the FA subcategory, if not preprinted on the CRF. | FASCAT | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor-defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. FASCAT can only be used if there is a FACAT and it must be a subcategorization of FACAT. |
Findings | FA | N/A | N/A | 11 | FAPOS | Findings About Position of Subject | The position of the subject during a measurement or examination. | In what position was the subject during the [measurement/ test/examination/specimen collection/sample collection]?; What was the position of the subject (during the [measurement/test/examination/s pecimen collection/sample collection])? | Position | Char | O | Record the position of the subject during the Findings About Test | FAPOS | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | (POSITION) | N/A | N/A |
Findings | FA | N/A | N/A | 12 | FAORRES | FA Result or Finding in Original Units | Result of the measurement or finding as originally received or collected. | What [is/was] the [result/amount/(subject's) characteristic] (of the [measurement/test/examination/q uestion/ assessment])? | ([Result/Amount] of) [value from FATEST] | Char | HR | Record the FATEST result. | FAORRES | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | N/A | N/A | In most cases, the Question Text and Item Prompt for the FAORRES are specific to the FATEST. The value of FATEST is most useful as the PROMPT on the field in which the RESULT for that test is collected. If the form is laid out as a grid, then words such as "Result" can be included as the column header. |
Findings | FA | N/A | N/A | 13 | FAORRESU | FA Original Units | The unit of the result as originally received or collected. | What [is/was] the unit (of the [measurement/test/examination]) ? | Unit | Char | R/C | Record or select the unit of measure associated with the test, if not preprinted on the CRF. | FAORRESU | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | (UNIT) | N/A | The Question Text and Item Prompt for the FAORRESU may be specific to the FATEST. Should be preprinted on the CRF with the associated test when possible, rather than collected in a field that requires the site to enter text (e.g., IN, LB, kg/L). |
Findings | FA | N/A | N/A | 14 | FAORNRLO | FA Normal Range Lower Limit- Orig Unit | The lower end of normal range or reference range for continuous results stored in --ORRES. | What [is/was] the lower limit of the reference range (for the [measurement/test/ examination])? | Normal Range Lower Limit | Char | O | Record the lower limit of the reference range of the Findings About test. | FAORNRLO | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | N/A | N/A | --ORNRLO should be populated only for continuous findings. The SDTM variable -- STNRC should be populated only for non-continuous results. These data may be obtained from the lab or the electronic equipment. These data could be derived from a site- or lab- specific set of normal ranges stored in a look-up table. |
Findings | FA | N/A | N/A | 15 | FAORNRHI | FA Normal Range Upper Limit- Orig Unit | The upper end of normal range or reference range for continuous results stored in --ORRES. | What [is/was] the upper limit of the reference range (for the [measurement/test/ examination])? | Normal Range Upper Limit | Char | O | Record the upper limit of the reference range of the Findings About test. | FAORNRHI | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | N/A | N/A | --ORNRHI should be populated only for continuous findings. The SDTM variable --STNRC should be populated only for non-continuous results. These data may be obtained from the lab or the electronic equipment. These data could be derived from a site- or lab-specific set of normal ranges stored in a look-up table. |
Findings | FA | N/A | N/A | 16 | FANRIND | Findings About Reference Range Indicator | An indication or description about how the value compares to the normal range or reference range. | How [did/do] the reported values compare within the [reference/normal/expected] range? | Comparison to [Reference/Expected/ Normal] Range | Char | O | Record where the test results were categorized within the respective reference range (e.g. HIGH, LOW, ABNORMAL). | FANRIND | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | (NRIND) | N/A | Reference ranges may be defined by FAORNRLO, FAORNRHI, FASTNRC or other objective criteria. Reference Range Indicator may be included if not derived or determined programmatically after data collection. Should not be used to indicate clinical significance. |
Findings | FA | N/A | N/A | 17 | FASTAT | Findings About Completion Status | The variable used to indicate that data are not available by having the site recording the value as "Not Done". | Was the [--TEST ] not [completed/answered/done/asses sed/ evaluated ]?; Indicate if the([--TEST] was) not [answered/assessed/done/evalua ted/ performed]. | Not Done | Char | O | Indicate if the [FATEST] measurement was not done. | FASTAT | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. If collected, the Origin (a column in the Define-XML) ="CRF", if populated from other sources such as a free text or sponsor-defined listing for FAREASND, the Origin ="DERIVED". | (ND) | N/A | Used only when the response value is collected as NOT DONE or NULL in lieu of or in addition to the CDASH FAPERF field. Typically a check box which indicates the test was NOT DONE. This field can be useful when multiple questions are asked to confirm that a blank result field is meant to be blank. |
Findings | FA | N/A | N/A | 18 | FAREASND | Findings About Reason Not Performed | An explanation of why the data are not available. | Was the [is/was] the reason that the [Findings topic/data/information/sponsor- defined phrase] was not [collected/answered/done/assess ed/ evaluated]? | Reason Not [Answered/Collected/Done/Evaluated/ Assessed/Available] | Char | O | Provide the reason why a Finding About test was not collected. | FAREASND | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology may be used. The reason the data are not available may be chosen from a sponsor-defined codelist (e.g., broken equipment, subject refused, etc.) or entered as free text. When --REASND is used, --STAT should also be populated in the SDTM-based dataset. |
Findings | FA | N/A | N/A | 19 | FASPEC | Findings About Specimen Type | The type of specimen used for a measurement. | What [is/was] the specimen type? Specimen Type | Char | O | Record the specimen material type. | FASPEC | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | (SPECTYPE) | N/A | The type of specimen used for a measure. Should be collected if not available elsewhere, or if required to differentiate multiple specimens. | |
Findings | FA | N/A | N/A | 20 | FASPCCND | Findings About Specimen Condition | Description of the condition of the specimen. | What [is/was] the condition of the specimen? | Specimen Condition | Char | O | Record condition of specimen. | FASPCCND | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | (SPECCOND) | N/A | May be collected using free or standardized text. Results may be affected by whether conditions for specimen were properly met. When Local Processing is used, sponsors may not routinely collect specimen condition. |
Findings | FA | N/A | N/A | 21 | FALOC | Location of the Finding About | The anatomical location of the subject relevant to the collection of the measurement. | What [is/was] the anatomical location (of the [measurement/test/examination]) or What [is/was] the anatomical location where the [measurement/specimen] was taken/collected)? | Anatomical Location | Char | O | Record or select location on body where measurement was performed, if not preprinted on CRF. | FALOC | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location. Example: ARM for blood pressure. Sponsors may collect the data using a subset list of controlled terminology on the CRF. LAT, DIR, and PORTOT are used to further describe the anatomical location. |
Findings | FA | N/A | N/A | 22 | FALAT | Laterality of Location of Finding About | Qualifier for anatomical location further detailing the side of the body. | What [is/was] the side (of the anatomical location of the [measurement/test/examination]) ? | Side | Char | O | Record the side of the anatomical location of the [FATEST] measurement. | FALAT | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | FA | N/A | N/A | 23 | FADIR | Findings About Directionality | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What [is/was] the directionality (of the anatomical location of the [measurement/test/ examination])? | Directionality | Char | O | Record the directionality. | FADIR | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | FA | N/A | N/A | 24 | FAPORTOT | FA Location Portion or Totality | Qualifier for anatomical location further detailing the distribution, which means arrangement of, apportioning of. | What [is/was] the portion or totality (of the anatomical location of the [measurement/test/examination]) ? | Portion or Totality | Char | O | Indicate the portion or totality anatomical location of the Findings About. | FAPORTOT | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | (PORTOT) | N/A | Collected when the sponsor needs to identify the specific portionality for the anatomical locations of the location of the FATEST. Sponsors may collect the data using a subset list of Controlled Terminology on the CRF. |
Findings | FA | N/A | N/A | 25 | FAMETHOD | Findings About Method | Method of the test or examination. | What was the method (used for the [measurement/test/examination]) ? | Method | Char | O | Record the method used to measure Findings About test. | FAMETHOD | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | (METHOD) | N/A | N/A |
Findings | FA | N/A | N/A | 26 | FALEAD | Findings About Lead | The lead or leads identified to capture the measurement for a test from an instrument. | What [is/was] the lead (used to measure [measurement/test/examination]) ? | Lead | Char | O | Record the lead used to measure Findings About test. | FALEAD | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | N/A | N/A | N/A |
Findings | FA | N/A | N/A | 27 | FAFAST | Findings About Fasting Status | An indication that the subject has abstained from food/water for the specified amount of time. | [Is/Was] the subject fasting (prior to the [test being performed/sample being collected])? | Fasting | Char | O | Record whether the subject was fasting prior to the test being performed. | FAFAST | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | (NY) | N/A | Results may be affected by whether the subject was fasting. This may not be relevant for all tests. |
Findings | FA | N/A | N/A | 28 | FAEVAL | Findings About Evaluator | The role of the person who provided the evaluation. | Who provided the (sponsor- defined phrase) information?; Who was the evaluator? | [Evaluator/Reporter] | Char | O | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | FAEVAL | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | (EVAL) | N/A | Used only for results that are subjective (e.g., assigned by a person or a group). May be a preprinted, or collected. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | FA | N/A | N/A | 29 | FAEVALID | Findings About Evaluator Identifier | An identifier used to distinguish multiple evaluators with the same role recorded in FAEVAL. | What [is/was] the identifier of the [evaluator name/reporter name] (providing the-sponsor-defined phrase-information)? | [Evaluator/Reporter] Identifier | Char | O | Record the unique identifier assigned to the person making the evaluation. | FAEVALID | Maps directly to the SDTM variable listed in the column with the heading SDTMIG Target. | (MEDEVAL) | N/A | This variable is used in conjunction with FAEVAL to provide an additional level of detail. |
Findings | FA | N/A | N/A | 30 | FACLSIG | Findings About Clinical Significance | An indication whether the test results were clinically significant | [Is/Was] the ([measurement/test/examination]) result clinically significant? | ([Measurement/Test/Examination/])/Clinically Significant | Char | O | Record whether [FATEST] result was clinically significant. | SUPPFA.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPFA dataset as the value of SUPPFA.QVAL when SUPPFA.QNAM = "CLSIG" and SUPPFA.QLABEL = "Clinical Significance". Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. | (NY) | N/A | N/A |
Assumptions for the CDASHIG FA - Findings About Events or Interventions Domain
1. The Findings About Events or Intervention domains use the same root variables as the Findings domain with the addition of the --OBJ variable
Example CRFs for the CDASHIG FA - Findings About Events or Interventions Domain
Example 1
In this sample CRF, data on symptoms associated with the clinical event of interest, migraine headaches, are collected.
Title: Migraine Symptoms
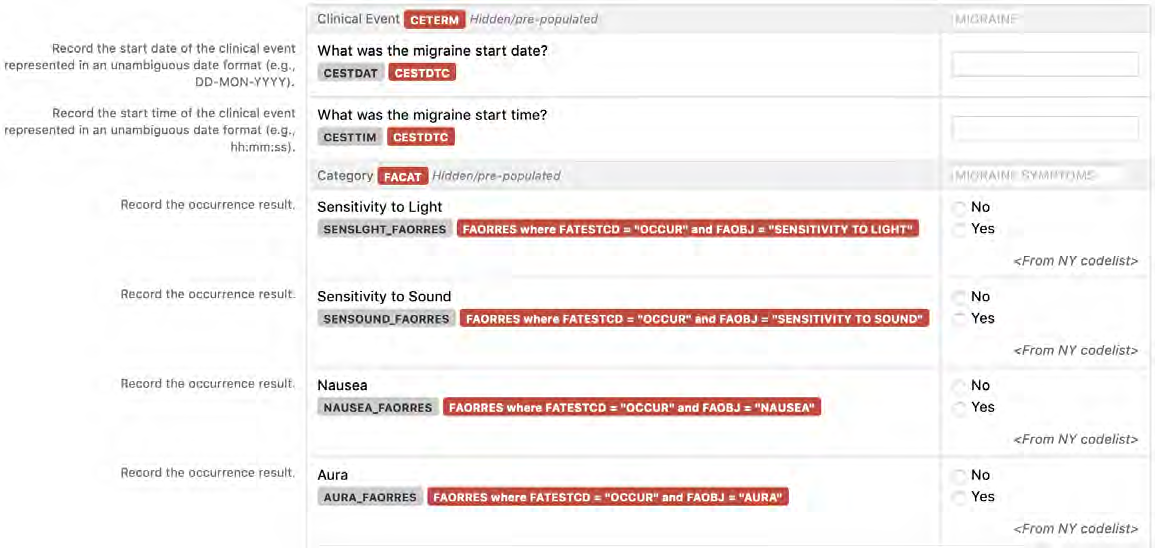
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target | SDTM Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CETERM | 1 | What is the clinical event term? | Clinical Event | Record the clinical event or [insert text corresponding to the specific clinical event]. | Text | CETERM | MIGRAINE | Prompt | Yes | ||||
CESTDAT | 2 | What was the migraine start date? | Start Date | Record the start date of the clinical event represented in an unambiguous date format (e.g., DD-MON-YYYY). | Date | CESTDTC | Qtext | ||||||
CESTTIM | 3 | What was the migraine start time? | Start Time | Record the start time of the clinical event represented in an unambiguous date format (e.g., hh:mm:ss). | Time | CESTDTC | Qtext | ||||||
FACAT | 4 | What was the category? | Category | Record the FA category, if not pre-printed on the CRF. | Text | FACAT | MIGRAINE SYMPTOMS | Prompt | Yes | ||||
SENSLGHT_FAORRES | 6 | What was the occurrence of sensitivity to light with the migraine? | Sensitivity to Light | Record the occurrence result. | Text | FAORRES | FAORRES where FATESTCD = "OCCUR" and FAOBJ = "SENSITIVITY TO LIGHT" | NY | No; Yes | Prompt | Radio | ||
SENSOUND_FAORRES | 7 | What was the occurrence of sensitivity to sound with the migraine? | Sensitivity to Sound | Record the occurrence result. | Text | FAORRES | FAORRES where FATESTCD = "OCCUR" and FAOBJ = "SENSITIVITY TO SOUND" | NY | No; Yes | Prompt | Radio | ||
NAUSEA_FAORRES | 8 | What was the occurrence of nausea with the migraine? | Nausea | Record the occurrence result. | Text | FAORRES | FAORRES where FATESTCD = "OCCUR" and FAOBJ = "NAUSEA" | NY | No; Yes | Prompt | Radio | ||
AURA_FAORRES | 9 | What was the occurrence of aura with the migraine? | Aura | Record the occurrence result. | Text | FAORRES | FAORRES where FATESTCD = "OCCUR" and FAOBJ = "AURA" | NY | No; Yes | Prompt | Radio |
Example
In this sample CRF, data about pre-specified symptoms of the disease under study are collected on a daily basis. The date of the assessment is captured, but start and end timing of the pre-populated symptoms are not.
Title: Findings About GERD Symptoms
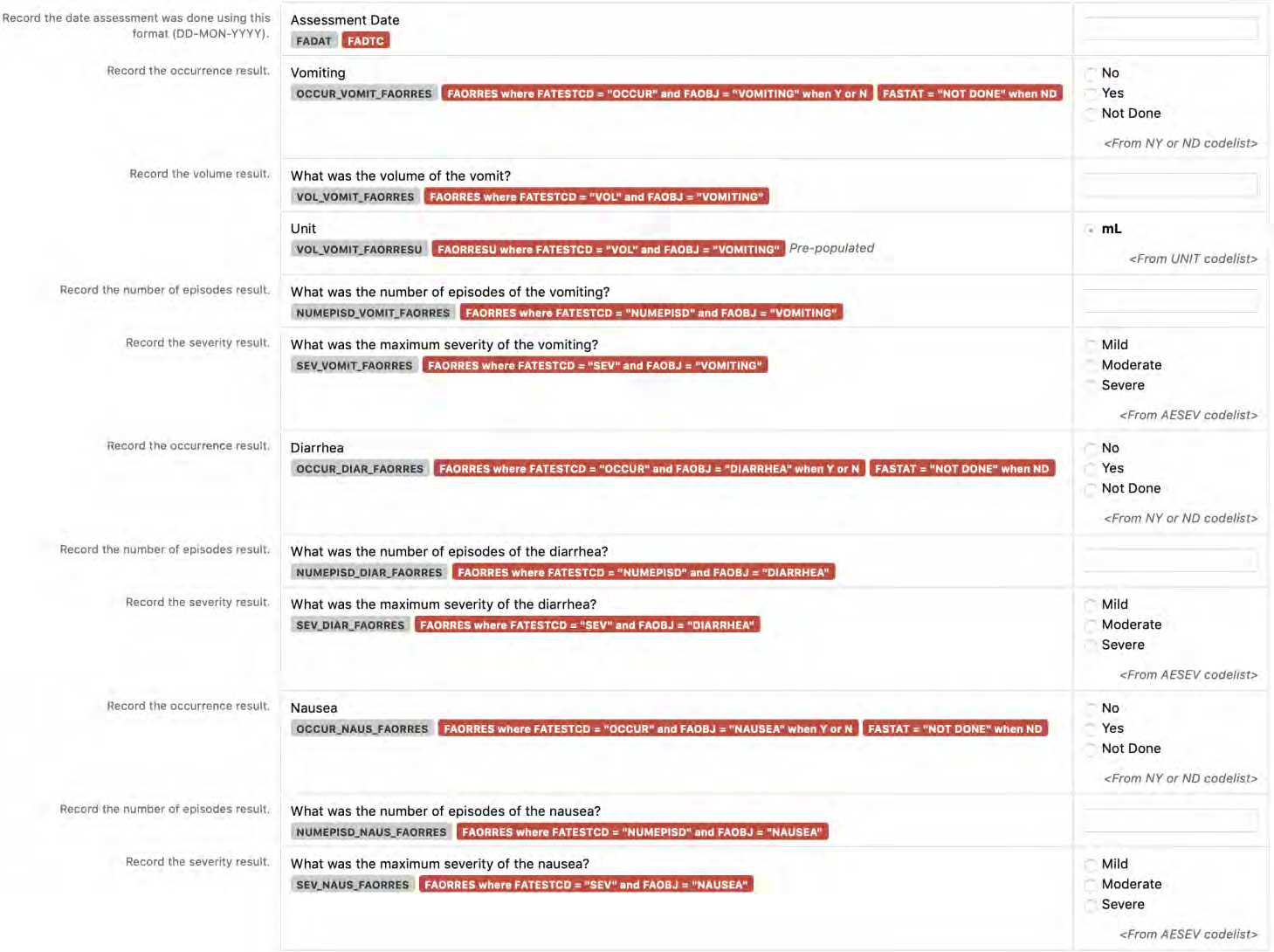
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target | SDTM Variable Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
FADAT | 1 | What is the date of the daily assessment? | Assessment Date | Record the date assessment was done using this format (DD-MON-YYYY). | Date | FADTC |
| Prompt | |||||
OCCUR_VOMIT_FAORRES | 2 | What was the occurrence of vomiting? | Vomiting | Record the occurrence result. | Text | FAORRES | FAORRES where FATESTCD = "OCCUR" and FAOBJ = "VOMITING" when Y or N; FASTAT = "NOT DONE" when ND | (NY); (ND) | No; Yes; Not Done | Prompt | Radio | ||
VOL_VOMIT_FAORRES | 3 | What was the volume of the vomit? | Volume of Vomit | Record the volume result. | Text | FAORRES | FAORRES where FATESTCD = "VOL" and FAOBJ = "VOMITING" |
| |||||
VOL_VOMIT_FAORRESU | 4 | What was the unit of the volume? | Unit | Record or select the unit of measure associated with the test, if not pre- printed on the CRF. | Text | FAORRESU | FAORRESU where FATESTCD = "VOL" and FAOBJ = "VOMITING" | (UNIT) | mL | mL | Prompt | Radio | |
NUMEPISD_VOMIT_FAORRES | 5 | What was the number of episodes of the vomiting? | Number of Episodes of Vomiting | Record the number of episodes result. | Text | FAORRES | FAORRES where FATESTCD = "NUMEPISD" and FAOBJ = "VOMITING" |
| |||||
SEV_VOMIT_FAORRES | 6 | What was the maximum severity of the vomiting? | Maximum Severity of Vomiting | Record the severity result. | Text | FAORRES | FAORRES where FATESTCD = "SEV" and FAOBJ = "VOMITING" | (AESEV) | Mild; Moderate; Severe |
| Radio | ||
OCCUR_DIAR_FAORRES | 7 | What was the occurrence of diarrhea? | Diarrhea | Record the occurrence result. | Text | FAORRES | FAORRES where FATESTCD = "OCCUR" and FAOBJ = "DIARRHEA" when Y or N; FASTAT = "NOT DONE" when ND | (NY); (ND) | No; Yes; Not Done | Prompt | Radio | ||
NUMEPISD_DIAR_FAORRES | 8 | What was the number of episodes of the diarrhea? | Number of Episodes of Diarrhea | Record the number of episodes result. | Text | FAORRES | FAORRES where FATESTCD = "NUMEPISD" and FAOBJ = "DIARRHEA" | ||||||
SEV_DIAR_FAORRES | 9 | What was the maximum severity of the diarrhea? | Maximum Severity of Diarrhea | Record the severity result. | Text | FAORRES | FAORRES where FATESTCD = "SEV" and FAOBJ = "DIARRHEA" | (AESEV) | Mild; Moderate; Severe |
| Radio | ||
OCCUR_NAUS_FAORRES | 10 | What was the occurrence of nausea? | Nausea | Record the occurrence result. | Text | FAORRES | FAORRES where FATESTCD = "OCCUR" and FAOBJ = "NAUSEA" when Y or N; FASTAT = "NOT DONE" when ND | (NY); (ND) | No; Yes; Not Done | Prompt | Radio | ||
NUMEPISD_NAUS_FAORRES | 11 | What was the number of episodes of the nausea? | Number of Episodes of Nausea | Record the number of episodes result. | Text | FAORRES | FAORRES where FATESTCD = "NUMEPISD" and FAOBJ = "NAUSEA" | ||||||
SEV_NAUS_FAORRES | 12 | What was the maximum severity of the nausea? | Maximum Severity of Nausea | Record the severity result. | Text | FAORRES | FAORRES where FATESTCD = "SEV" and FAOBJ = "NAUSEA" | (AESEV) | Mild; Moderate; Severe | Radio |
8.3.19.1 SR - Skin Response
Description/Overview for the CDASHIG SR - Skin Response Domain
The SR domain is a Findings About domain used to collect dermal responses to antigens.
Specification for the CDASHIG SR - Skin Response Domain
Skin Response Metadata Table
| Observation Class | Domain | Data Collection Scenario | Implementation Options | Order Number | CDASHIG Variable | CDASHIG Variable Label | DRAFT CDASHIG Definition | Question Text | Prompt | Data Type | CDASHIG Core | Case Report Form Completion Instructions | SDTMIG Target | Mapping Instructions | Controlled Terminology Codelist Name | Subset Controlled Terminology/ CDASH Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findings | SR | N/A | N/A | 1 | STUDYID | Study Identifier | A unique identifier for a study. | What is the study identifier? | [Protocol/Study] | Char | HR | N/A | STUDYID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | While this field is not typically captured on a CRF, it should be displayed clearly on the CRF and/or the EDC system. This field can be included into the database or populated during SDTM-based dataset creation before submission. |
Findings | SR | N/A | N/A | 2 | SITEID | Study Site Identifier | A unique identifier for a site within a study. | What is the site identifier? | Site (Identifier) | Char | HR | N/A | DM.SITEID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically preprinted in the header of each CRF page for single site studies. For studies with multiple sites, this field may be left blank so that the number can be recorded by the site, or it may be preprinted on the CRFs that are shipped to each site. EDC: This should be prepopulated. |
Findings | SR | N/A | N/A | 3 | SUBJID | Subject Identifier for the Study | A unique subject identifier within a site and a study. | What [is/was] the (study) [subject/participant] identifier? | [Subject/Participant] (Identifier) | Char | HR | Record the identifier for the subject. | DM.SUBJID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Paper: This is typically recorded in the header of each CRF page. EDC: The subject identifiers may be system generated. This CDASH variable is typically collected in all CDASH domains. However, this CDASH variable is populated only in the SDTMIG DM domain. For more information, refer to the SDTM Implementation Guide. |
Findings | SR | N/A | N/A | 4 | VISIT | Visit Name | The name of a clinical encounter that encompasses planned and unplanned trial interventions, procedures, and assessments that may be performed on a subject. | What is the visit name? | [Visit] | Char | R/C | N/A | VISIT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | The name of the visit is typically preprinted on the CRF, and should match the name of the visit in the protocol. May be used to derive the SDTM variable VISITNUM. Note: Sponsors may have CDASH visit numbering or visit naming conventions to handle special circumstances (e.g., unscheduled visits). In these cases, the appropriate visit numbers and visit names, may need to be populated when the SDTM submission datasets are created. |
Findings | SR | N/A | N/A | 5 | VISDAT | Visit Date | Date the clinical encounter occurred (or started). | What [is/was] the date of the visit? | (Visit) Date | Char | R/C | Record the [date/start date] of the visit using DD-MON-YYYY format. | N/A | This field is not an SDTM variable. The date of a measurement, test, observation can be determined from the date/time of visit (VISDAT/VISTIM) and then concatenating the CDASH VISDAT/VISTIM components and populating the SDTMIG variable SRDTC in ISO 8601 format. | N/A | N/A | The date the skin response measurements were collected can be determined from the Visit Date variable (VISDAT) and applying that date to all of the skin response measurements at that visit, or the collection date can be included on the Skin Response CRF using the date (SRDAT) field. |
Findings | SR | N/A | N/A | 6 | SRPERF | Skin Response Test Performed | An indication whether or not a planned skin response measurement, series of skin response measurements, tests, or observations was performed. | Was a skin response test performed? | Skin Response Test Performed | Char | O | Indicate if a skin response test as performed. | SRSTAT | This does not map directly to an SDTMIG variable. May be used to derive a value into the SDTM variable SRSTAT. If SRPERF="N", the value of SRSTAT will be "NOT DONE". If SRPERF="Y", SRSTAT should be null. A combination of SDTMIG variables (e.g., SRCAT and SRSCAT, SRTPT ) is used to indicate that multiple tests were not done. In this situation, the SDTMIG variable SRTESTCD would be populated as SRALL and an appropriate test name (SRTEST) provided. See SDTMIG v3.2 Section 4.1.5.1.2. | (NY) | N/A | General prompt question to be used as a data management tool to verify that missing results are confirmed missing. |
Findings | SR | N/A | N/A | 7 | SRREASND | Skin Response Reason Not Done | An explanation of why the data are not available. | What was the reason the test was not done? | Reason Not Done | Char | O | Provide the reason why the test or examination was not done. | SRREASND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | The reason the data are not available may be chosen from a sponsor- defined list (e.g., broken equipment, subject refused) or entered as free text. When SRREASND is used, the SDTMIG variable SRSTAT should also be populated in the SDTM- based dataset. |
Findings | SR | N/A | N/A | 8 | SRCAT | Skin Response Category for Test | A grouping of topic-variable values based on user-defined characteristics. | What was the category of the skin response? | [Skin Response Category]; NULL | Char | O | Record the skin response category, if not preprinted on the CRF. | SRCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Category" can be included as the column header. |
Findings | SR | N/A | N/A | 9 | SRSCAT | Skin Response Subcategory for Test | A sub-division of the SRCAT values based on user-defined characteristics. | What was the subcategory of the skin response? | [Skin Response Subcategory]; NULL | Char | O | Record the skin response subcategory, if not preprinted on the CRF. | SRSCAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | Sponsor-defined controlled terminology. This would most commonly be either a heading, or a preprinted category value on the CRF and not a question to which the site would provide an answer. If a question is asked, the response would typically be a sponsor- defined codelist. If the form is laid out as a grid, then words such as "Subcategory" can be included as the column header. SRSCAT can only be used if there is an SRCAT and it must be a subcategorization of SRCAT. |
Findings | SR | N/A | N/A | 10 | SRSPID | Skin Response Sponsor- Defined Identifier | A sponsor- defined identifier. In CDASH, This is typically used for preprinted or auto-generated numbers on the CRF, or any other type of identifier that does not already have a defined CDASH identifier field. | [Sponsor-defined question] | [Sponsor defined] | Char | O | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | SRSPID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. May be used to create RELREC to link this record with a record in another domain. | N/A | N/A | Because SPID is a sponsor-defined identifier, conformance to Question Text or Item Prompt is not applicable. Typically used as an identifier in a data query to communicate clearly to the site the specific record in question or to reconcile data. May be used to record preprinted number (e.g., line number, record number) on the CRF. This field may be populated by the sponsor's data collection system. |
Findings | SR | N/A | N/A | 11 | SROBJ | Skin Response Object of the Observation | A description of the object or focal point of the findings observation that is represented by SRTEST. | What intervention was performed to elicit the skin response? | [Intervention] Performed | Char | HR | Record the name of the antigen administered to the skin to elicit the skin response. | SROBJ | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | N/A |
Findings | SR | N/A | N/A | 12 | SRRFTDAT | SR Date of Reference Time Point | The date of the reference time point, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the date of the [intervention] performed to elicit the skin response? | [Intervention] Administration Date | Char | R/C | Record date of the test material administration using this format (DD-MON- YYYY). | SRRFTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable SRRFTDTC in ISO 8601 format. | N/A | N/A | A complete date is expected. If the date of administration is collected on a separate CRF (e.g., VISDAT), then it should not be collected on the SR CRF. |
Findings | SR | N/A | N/A | 13 | SRRFTTIM | SR Time of Reference Time Point | The time of the reference time point, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the time of the [intervention] performed to elicit the skin response? | [Intervention] Administration Time | Char | R/C | Record time of the test material administration using this format (hh:mm:ss). | SRRFTDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable SRRFTDTC in ISO 8601 format. | N/A | N/A | Collect time if it is relevant for the analysis of the skin response (e.g., multiple [intervention] administrations.) |
Findings | SR | N/A | N/A | 14 | SRLOC | SR Location Used for Measurement | A description of the anatomical location of the subject relevant to the collection of skin response test. | What was the anatomical location of the skin response measurement? | Anatomical Location | Char | O | Record or select location on body where measurement was performed, if not preprinted on CRF. | SRLOC | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LOC) | N/A | Collected or preprinted when the sponsor needs to identify the specific anatomical location (e.g., ARM for skin response). Sponsors may collect the data using a subset list of controlled terminology on the CRF. LAT, DIR, and PORTOT are used to further describe the anatomical location. |
Findings | SR | N/A | N/A | 15 | SRLAT | Skin Response Laterality | Qualifier for anatomical location further detailing the side of the body. | What was the side of the anatomical location of the skin response measurement? | Side | Char | O | Record the side of the anatomical location where the test was performed. | SRLAT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (LAT) | N/A | May be preprinted or collected when the sponsor needs to identify the specific side of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | SR | N/A | N/A | 16 | SRTEST | Skin Response Test or Examination Name | Descriptive name of the test or examination used to obtain the measurement or finding. | What was the skin response test name? | [Skin Response Test Name] | Char | HR | Record the name of the skin response test if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | SRTEST;SRTESTCD | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. SRTESTCD may be determined from the value collected in SRTEST. The SDTMIG variables SRTESTCD and SRTEST are required in the SDTM submission datasets. Use appropriate CDISC Controlled Terminology for the test and test code. | (SRTEST) | N/A | Required to identify which test the result is for. It is recommended that the test names be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Test" can be included as the column header. |
Findings | SR | N/A | N/A | 17 | SRTPT | Skin Response Planned Time Point Name | A text description of planned time point when measurements should be taken as defined in the protocol. | What was the planned time point for skin response measurement? | [Planned Time Point Name] | Char | R/C | Record the planned time point labels for skin response, if not preprinted on the CRF. | SRTPT | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. See SDTMIG for additional information on representing time points. Time point anchors SRTPTREF (text description) and SRRFTDTC (date/time) may be needed, as well as SDTMIG variables SRTPTNUM, SRELTM. | N/A | N/A | Planned time point are needed to differentiate multiple sequential assessments. It is recommended that time points should be preprinted on the CRF rather than collected in a field that requires the site to enter text. If the form is laid out as a grid, then words such as "Planned Time Point" can be included as the column header. |
Findings | SR | N/A | N/A | 18 | SRDAT | Skin Response Observation Date | The date of the measurements, represented in an unambiguous date format (e.g., DD-MON- YYYY). | What was the date of the skin response measurement? | Date | Char | R/C | Record date of measurements using this format (DD-MON- YYYY). | SRDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable SRDTC in ISO 8601 format. | N/A | N/A | The date of measurement can be determined from a collected date of the visit (VISDAT) and in such cases a separate measurement date field is not required. |
Findings | SR | N/A | N/A | 19 | SRTIM | Skin Response Observation Time | The time of measurement, represented in an unambiguous time format (e.g., hh:mm:ss). | What was the time of the skin response measurement? | Time | Char | R/C | Record time of measurement (as complete as possible). | SRDTC | This does not map directly to an SDTMIG variable. For the SDTM submission dataset, concatenate all collected CDASH DATE and TIME components and populate the SDTMIG variable SRDTC in ISO 8601 format. | N/A | N/A | The time of measurement (if required) can be determined from a collected time of the visit (VISTIM) and in such cases a separate measurement date field is not required. |
Findings | SR | N/A | N/A | 20 | SRDIR | Skin Response Directionality | Qualifier further detailing the position of the anatomical location relative to the center of the body, organ, or specimen. | What was the directionality of the anatomical location of the skin response measurement? | Directionality | Char | O | Record the directionality of the anatomical location where the test was performed. | SRDIR | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (DIR) | N/A | May be preprinted or collected when the sponsor needs to identify the directionality of the anatomical location. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | SR | N/A | N/A | 21 | SREVAL | Skin Response Evaluator | The role of the person who provided the evaluation. | Who was the evaluator? | [Evaluator/Reporter] | Char | O | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | SREVAL | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (EVAL) | N/A | Used only for results that are subjective (e.g., assigned by a person or a group). May be a preprinted or collected. Sponsors may collect the data using a subset list of controlled terminology on the CRF. |
Findings | SR | N/A | N/A | 22 | SREVALID | Skin Response Evaluator Identifier | Used to distinguish multiple evaluators with the same role recorded in SREVAL. | What was the identifier of the evaluator providing the skin response information? | [Evaluator/Reporter] Identifier | Char | O | Record the unique identifier assigned to the person making the evaluation. | SREVALID | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (MEDEVAL) | N/A | If EVALID needs to be collected for each test on the horizontal record the CDASH variable [SRTESTCD]_ EVALID can be used. |
Findings | SR | N/A | N/A | 23 | SRORRES | SR Results or Findings in Original Units | Result of the skin response test as originally received or collected. | What was the result of the skin response measurement? | (Result) | Char | HR | Record the skin response test result. | SRORRES | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | N/A | N/A | N/A |
Findings | SR | N/A | N/A | 24 | SRORRESU | SR Original Units | The unit of the result as originally received or collected. | What was the unit of the skin response measurement? | Unit | Char | R/C | Record or select the unit of measure associated with the test, if not preprinted on the CRF. | SRORRESU | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (UNIT) | N/A | Should be preprinted on the CRF with the associated test when possible, rather than collected in a field that requires the site to enter text. |
Findings | SR | N/A | N/A | 25 | SRNRIND | Skin Response Reference Range Indicator | An indication or description about how the value compares to the normal range or reference range. | How [did/do] the reported values compare within the [reference/normal/expected] range? | Comparison to [Reference/Expected/Normal] Range | Char | O | Record where the test results were categorized within the respective reference range (e.g. HIGH, LOW, ABNORMAL). | SRNRIND | Maps directly to the SDTMIG variable listed in the column with the heading SDTMIG Target. | (NRIND) | N/A | The category of the value within the respective reference range. Ranges may be defined by SRORNRLO and SRORNRHI or other objective criteria. Reference Range Indicator may be included if not derived or determined programmatically after data collection. Should not be used to indicate clinical significance. |
Findings | SR | N/A | N/A | 26 | SRCLSIG | Skin Response Clinical Significance | An indication whether the skin response result was clinically significant. | Was the result clinically significant? | Clinically Significant | Char | O | Record whether the skin response result was clinically significant. | SUPPSR.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPSR dataset as the value of SUPPSR.QVAL where SUPPSR.QNAM ="SRCLSIG" and SUPPSR.QLABEL="Clinically Significant". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A | If this level of information is needed, it may be added to the CRF. |
Assumptions for the CDASHIG SR - Skin Response Domain
1. The SR domain is to be used when the skin response is the primary response that is being assessed (e.g., allergy test kits, tuberculosis skin test), rather than it being a secondary response to the actual injection.
2. The method of assessment is typically a skin-prick test.
Example CRFs for the CDASHIG SR - Skin Response Domain
Example 1
Title: Skin Test
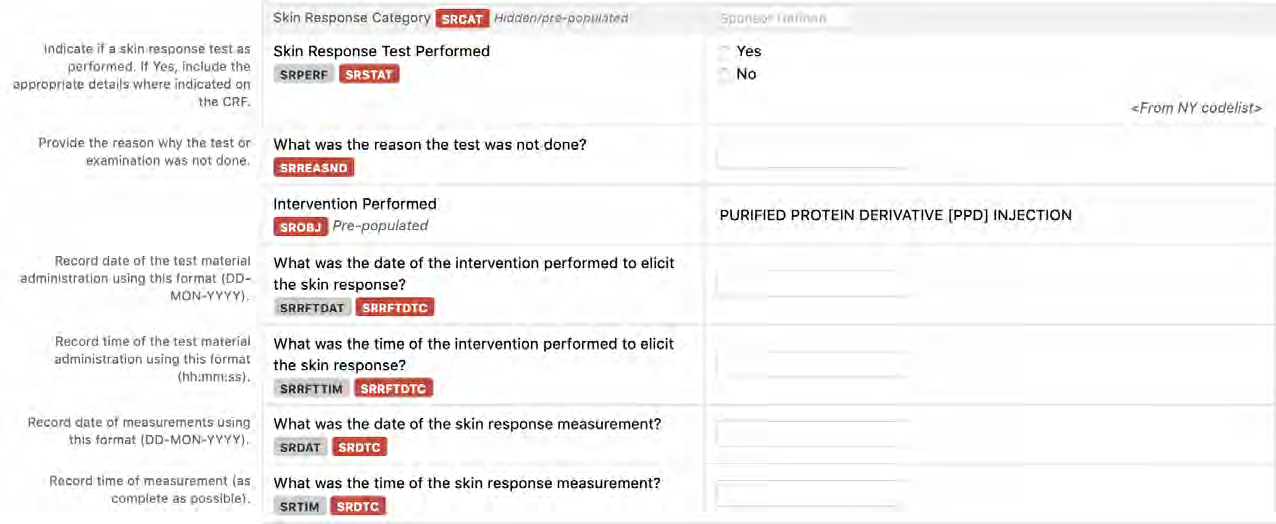
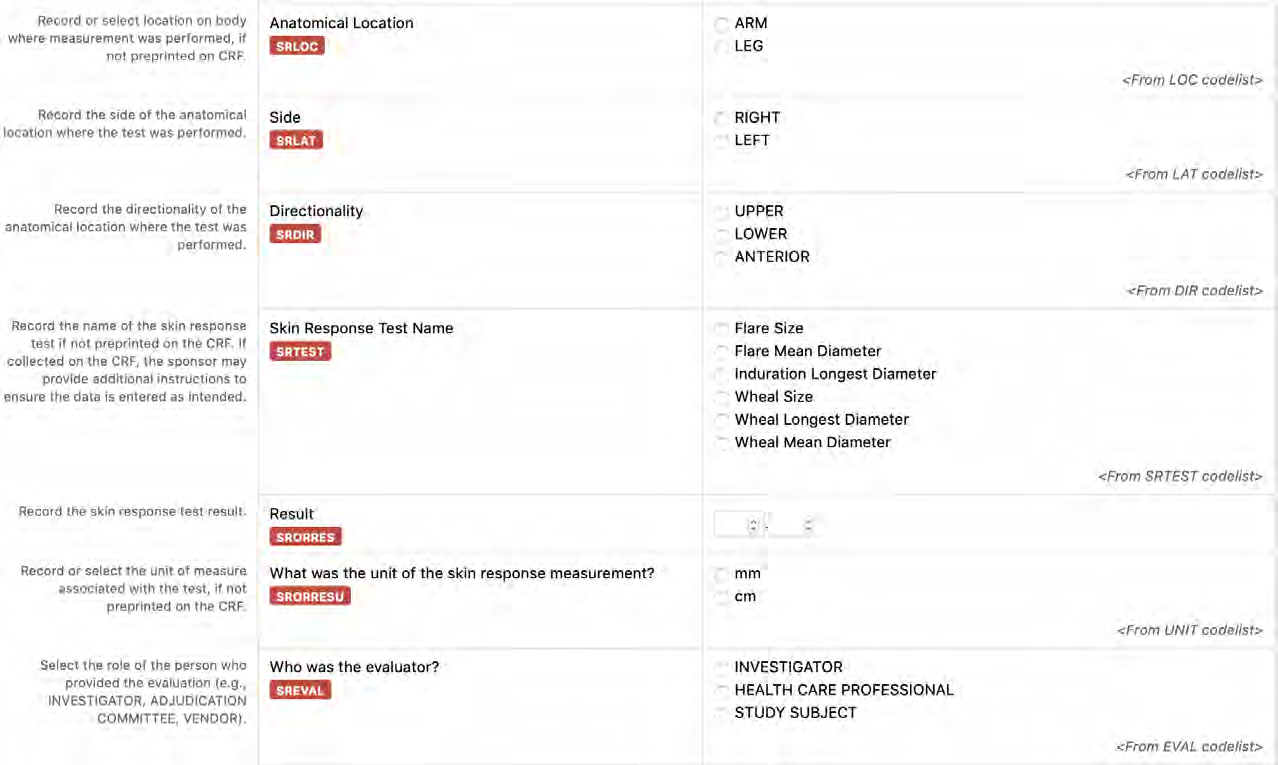
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTM Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre-Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
SRCAT | 1 | What was the category of the skin response? | Skin Response Category | Record the skin response category, if not pre-printed on the CRF. | Text | SRCAT | Sponsor Defined | Yes | |||||
SRPERF | 2 | Was a skin response test performed? | Skin Response Test Performed | Indicate if a skin response test as performed. If Yes, include the appropriate details where indicated on the CRF. | Text | SRSTAT | (NY) | Yes; No | prompt | ||||
SRREASND | 3 | What was the reason the test was not done? | Reason Not Done | Provide the reason why the test or examination was not done. | Text | SRREASND | |||||||
SROBJ | 4 | What intervention was performed to elicit the skin response? | Intervention Performed | Record the name of the antigen administered to the skin to elicit the skin response. | Text | SROBJ | PURIFIED PROTEIN DERIVATIVE [PPD] INJECTION | ||||||
SRRFTDAT | 5 | What was the date of the intervention performed to elicit the skin response? | Administration Date | Record date of the test material administration using this format (DD-MON-YYYY). | Date | SRRFTDTC | qtext | ||||||
SRRFTTIM | 6 | What was the time of the intervention performed to elicit the skin response? | Administration Time | Record time of the test material administration using this format (hh:mm:ss). | Time | SRRFTDTC | qtext | ||||||
SRDAT | 7 | What was the date of the skin response measurement? | Date | Record date of measurements using this format (DD- MON-YYYY). | Date | SRDTC | qtext | ||||||
SRTIM | 8 | What was the time of the skin response measurement? | Time | Record time of measurement (as complete as possible). | Time | SRDTC | qtext | ||||||
SRLOC | 10 | What was the anatomical location of the skin response measurement? | Anatomical Location | Record or select location on body where measurement was performed, if not preprinted on CRF. | Text | SRLOC | (LOC) | ARM; LEG | prompt | ||||
SRLAT | 11 | What was the side of the anatomical location of the skin response measurement? | Side | Record the side of the anatomical location where the test was performed. | Text | SRLAT | (LAT) | RIGHT; LEFT | prompt | ||||
SRDIR | 12 | What was the directionality of the anatomical location of the skin response measurement? | Directionality | Record the directionality of the anatomical location where the test was performed. | Text | SRDIR | (DIR) | UPPER; LOWER; ANTERIOR | prompt | ||||
SRTEST | 13 | What was the skin response test name? | Skin Response Test Name | Record the name of the skin response test if not preprinted on the CRF. If collected on the CRF, the sponsor may provide additional instructions to ensure the data is entered as intended. | Text | SRTEST | (SRTEST) | Flare Size; Flare Mean Diameter; Induration Longest Diameter; Wheal Size; Wheal Longest Diameter; Wheal Mean Diameter | prompt | ||||
SRORRES | 14 | What was the result of the skin response measurement? | Result | Record the skin response test result. | Float | SRORRES | prompt | ||||||
SRORRESU | 15 | What was the unit of the skin response measurement? | Unit | Record or select the unit of measure associated with the test, if not preprinted on the CRF. | Text | SRORRESU | (UNIT) | mm; cm | |||||
SREVAL | 16 | Who was the evaluator? | Evaluator | Select the role of the person who provided the evaluation (e.g., INVESTIGATOR, ADJUDICATION COMMITTEE, VENDOR). | Text | SREVAL | (EVAL) | INVESTIGATOR; HEALTH CARE PROFESSIONAL; STUDY SUBJECT |
Example 2
Title: Skin Response
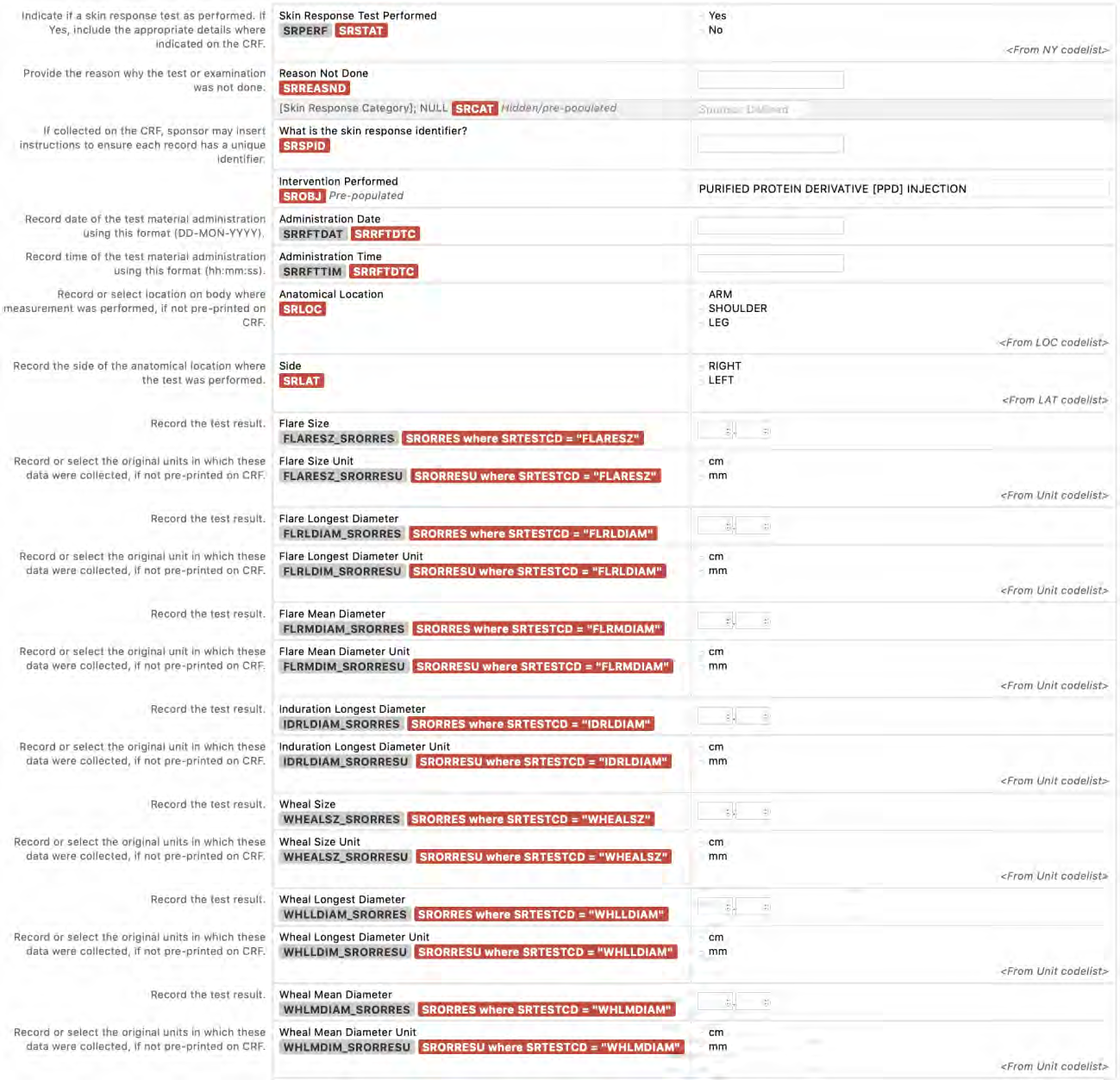
CRF Metadata
| Order | Question Text | Prompt | CRF Completion Instructions | Type | CDASH Variable | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology CodeList Name | Permissible Values | Pre-Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
2 | Was a skin response test performed? | Skin Response Test Performed | Indicate if a skin response test as performed. If Yes, include the appropriate details where indicated on the CRF. | Text | SRPERF | SRSTAT | NY | Yes; No | prompt | ||||
3 | What was the reason the test was not done? | Reason Not Done | Provide the reason why the test or examination was not done. | Text | SRREASND | SRREASND | prompt | ||||||
4 | What was the category of the skin response? | [Skin Response Category]; NULL | Record the skin response category, if not pre-printed on the CRF. | Text | SRCAT | SRCAT | Sponsor Defined | Yes | |||||
5 | What is the skin response identifier? | [Line Number/SR Number] | If collected on the CRF, sponsor may insert instructions to ensure each record has a unique identifier. | Text | SRSPID | SRSPID | |||||||
6 | What intervention was performed to elicit the skin response? | Intervention Performed | Record the name of the antigen administered to the skin to elicit the skin response. | Text | SROBJ | SROBJ | PURIFIED PROTEIN DERIVATIVE [PPD] INJECTION | ||||||
7 | What was the date of the [intervention] performed to elicit the skin response? | Administration Date | Record date of the test material administration using this format (DD- MON-YYYY). | Date | SRRFTDAT | SRRFTDTC | |||||||
8 | What was the time of the [intervention] performed to elicit the skin response? | Administration Time | Record time of the test material administration using this format (hh:mm:ss). | Time | SRRFTTIM | SRRFTDTC | |||||||
9 | What was the anatomical location of the skin response measurement? | Anatomical Location | Record or select location on body where measurement was performed, if not pre-printed on CRF. | Text | SRLOC | SRLOC | LOC | ARM; SHOULDER; LEG | prompt | ||||
10 | What was the side of the anatomical location of the skin response measurement? | Side | Record the side of the anatomical location where the test was performed. | Text | SRLAT | SRLAT | LAT | RIGHT; LEFT | prompt | ||||
11 | What was the result of the flare size measurement? | Flare Size | Record the test result. | float | FLARESZ_SRORRES | SRORRES;SRTEST;SRTESTCD | SRORRES where SRTESTCD = "FLARESZ" | prompt | |||||
12 | What was the unit of the flare size measurement? | Flare Size Unit | Record or select the original units in which these data were collected, if not pre-printed on CRF. | text | FLARESZ_SRORRESU | SRORRESU | SRORRESU where SRTESTCD = "FLARESZ" | Unit | cm; mm | prompt | |||
13 | What was the result of the flare longest diameter measurement? | Flare Longest Diameter | Record the test result. | float | FLRLDIAM_SRORRES | SRORRES; SRTEST; SRTESTCD | SRORRES where SRTESTCD = "FLRLDIAM" | prompt | |||||
14 | What was the unit of the flare longest diameter measurement? | Flare Longest Diameter Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | text | FLRLDIM_SRORRESU | SRORRESU | SRORRESU where SRTESTCD = "FLRLDIAM" | Unit | cm; mm | prompt | |||
15 | What was the result of the flare mean diameter measurement? | Flare Mean Diameter | Record the test result. | float | FLRMDIAM_SRORRES | SRORRES; SRTEST;SRTESTCD | SRORRES where SRTESTCD = "FLRMDIAM" | prompt | |||||
16 | What was the unit of the flare mean diameter measurement? | Flare Mean Diameter Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | text | FLRMDIM_SRORRESU | SRORRESU | SRORRESU where SRTESTCD = "FLRMDIAM" | Unit | cm; mm | prompt | |||
17 | What was the result of the induration longest diameter measurement? | Induration Longest Diameter | Record the test result. | float | IDRLDIAM_SRORRES | SRORRES;SRTEST; SRTESTCD | SRORRES where SRTESTCD = "IDRLDIAM" | prompt | |||||
18 | What was the unit of the induration longest diameter measurement? | Induration Longest Diameter Unit | Record or select the original unit in which these data were collected, if not pre-printed on CRF. | text | IDRLDIAM_SRORRESU | SRORRESU | SRORRESU where SRTESTCD = "IDRLDIAM" | Unit | cm; mm | prompt | |||
19 | What was the result of the wheal size measurement? | Wheal Size | Record the test result. | float | WHEALSZ_SRORRES | SRORRES; SRTEST; SRTESTCD | SRORRES where SRTESTCD = "WHEALSZ" | prompt | |||||
10 | What was the unit of the wheal size measurement? | Wheal Size Unit | Record or select the original units in which these data were collected, if not pre-printed on CRF. | text | WHEALSZ_SRORRESU | SRORRESU | SRORRESU where SRTESTCD = "WHEALSZ" | Unit | cm; mm | prompt | |||
11 | What was the result of the wheal longest diameter measurement? | Wheal Longest Diameter | Record the test result. | float | WHLLDIAM_SRORRES | SRORRES; SRTEST;SRTESTCD | SRORRES where SRTESTCD = "WHLLDIAM" | prompt | |||||
12 | What was the unit for the wheal longest diameter measurement? | Wheal Longest Diameter Unit | Record or select the original units in which these data were collected, if not pre-printed on CRF. | text | WHLLDIM_SRORRESU | SRORRESU | SRORRESU where SRTESTCD = "WHLLDIAM" | Unit | cm; mm | prompt | |||
13 | What was the result of the wheal mean diameter measurement? | Wheal Mean Diameter | Record the test result. | float | WHLMDIAM_SRORRES | SRORRES;SRTEST;SRTESTCD | SRORRES where SRTESTCD = "WHLMDIAM" | prompt | |||||
14 | What was the unit of the wheal mean diameter measurement? | Wheal Mean Diameter Unit | Record or select the original units in which these data were collected, if not pre-printed on CRF. | text | WHLMDIM_SRORRESU | SRORRESU | SRORRESU where SRTESTCD = "WHLMDIAM" | Unit | cm; mm | prompt |
8.4 Associated Persons Domains
Description/Overview for the CDASHIG AP Domains
Data may be collected about persons other than the subject under study. Appropriate consent may be needed when collecting data about caregivers, study partners, organ donors, and so on. (Consent would not be needed to collect data about a subject's family medical history.) These persons could be associated with the study itself, a particular study subject, or a device used in the study. The term associated persons is used to classify data collected about persons who are not subject participants in a clinical study. An associated person (AP) may be a family member or may have a non-familial relationship to a study subject (e.g., the associated person might be a caregiver or an organ or blood donor). An AP may also be unrelated to any study subject, such as a study technician who is inadvertently exposed to a study-related substance and has to be followed for adverse events.
AP domains parallel general observation class domains as well as the Demographics (DM) and potentially the Comments (CO) domains. The structure of AP domains is therefore the same as those for study-subject domains, with the exception of variables that are only applicable to study subjects (e.g., the identifiers USUBJID and SPDEVID). Unless otherwise stated, all other general assumptions about CDASHIG variables and domains will apply to AP data.
Refer to the SDTMIG supplement for Associated Persons (SDTMIG-AP) for further details about representing data collected about people other than study subjects in SDTM.
8.4.1 Assumptions for the CDASHIG AP Domains
1. The DOMAIN for every CDASHIG AP domain has a 4-character code, beginning with "AP" and followed by the respective domain abbreviation; for example, APCM (Associated Persons Concomitant Medication), APDM (Associated Persons Demographics), APMH (Associated Persons Medical History).
2. Required Variables for SDTM
a. AP SDTM datasets must include the required topic variable for its associated class (e.g., --TRT for an Interventions class domain) plus the following required variables specific to AP domains: an Associated Person Identifier (APID) and a description of the associated person's relationship to one or more study subjects (SREL), if any. The CRF for the AP data may collect both APID and SREL, or just SREL. When only SREL is collected, the APID variable is populated when the SDTM datasets are created.
b. The AP domain variable RSUBJID (Identifier for the related study subject) may be collected on the CRF. However, because there is typically just 1 related study subject, RSUBJID is not required to be collected and may be populated in the SDTMIG dataset from the study subject's identifier.
c. AP domains should include the commonly used variables for their respective class.
3. Prohibited Variables for SDTM
a. The same variables that are listed as not being allowed for use within a given class are also prohibited from use in an AP domain for that class. For example, when creating an AP Interventions Class domain, --ORRES, --ORRESU, --PERF, and --TERM are prohibited.
b. Careful consideration should be given if/when creating AP CRFs with CDASHIG fields that may map to multiple SDTMIG classes.
4. Special Considerations
a. The AP domain name is created by adding the prefix "AP" to the CDASHIG domain letters associated with the data to be reported (e.g., "APMH" where "MH" is the parent CDASHIG domain of interest).
b. For non-supplemental qualifiers (i.e., parent domain variables) individual CDASHIG variable names use the same names as CDASHIG items in the parent domain (e.g., MHTERM, MHSTDAT) variable. They do not have the prefix "AP" appended.
c. For supplemental qualifier variables, individual CDASH items are mapped to an AP domain-specific supplemental qualifier variable. Supplemental Qualifier variables for AP domains begin with SQAP
d. When any CRF includes the variables APID and/or SREL, the sponsor must ensure that the appropriate AP-- domain is used when the SDTM datasets are created. Sponsors may opt to include the CDASH variable "DOMAIN" which is prespecified to the applicable AP domain (e.g., APMH) to facilitate the creation of the SDTM datasets.
Specification for the CDASHIG AP Variables
Refer to the Identifier Variables at the end of the Domain Specific section of the CDASH Model for the CDASH attributes for variables APID, SREL, and RSUBJID.
Domain Specific
| Observation Class | Domain | Order Number | CDASH Variable | CDASH Variable Label | DRAFT CDASH Definition | Question Text | Prompt | Data Type | SDTM Target | Mapping Instructions | Controlled Terminology Codelist Name | Implementation Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Domain Specific | AE | 1 | AEACNOYN | Any Other Actions Taken | An indication whether any other action(s) were taken in response to the adverse event that are unrelated to study treatment dose changes or other non-study treatments given because of this adverse event. | Were any other actions taken in response to this adverse event? | Any Other Actions Taken | Char | N/A | Does not map to an SDTM variable. The SDTM Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification that the AEACNOTH field on the CRF was deliberately left blank. |
Domain Specific | AE | 2 | AERLNSYN | Related to Non- Study Treatment | An indication whether in the investigator's opinion the event may have been due to a treatment other than study treatment. | [Is/Was] this adverse event due to treatment other than study treatment? | Related to Non-Study Treatment | Char | N/A | Does not map to an SDTM variable. The SDTM Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | The intent/purpose of collecting this field is to help with data cleaning and monitoring. It provides verification that the CDASH AERELNST field on the CRF was deliberately left blank. |
Domain Specific | AE | 3 | AESCAN | Involves Cancer | An indication the serious event was associated with the development of cancer. | [Is/Was] the adverse event associated with the development of cancer? | Cancer | Char | AESCAN | Maps directly to the SDTM variable listed in the column with the heading "SDTM Target". | (NY) | Although deprecated, this variable is included for illustrative purposes if the sponsor is continuing to collect legacy data. If the details regarding a Serious AE are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Note for sponsors: The FDA Study Data Technical Conformance Guide v2.2 (June 12, 2015) Section 4.1.1.3 states "The entry of a "Y" for the serious adverse event variable, AESER, should have the assessment indicated, (e.g., as a death, hospitalization, or disability/permanent damage). Frequently, sponsors omit the assessment information, even when it has been collected on the CRF. The criteria that led to the determination should be provided. This information is critical during FDA review to support the characterization of serious AEs". |
Domain Specific | AE | 4 | AESCONG | Congenital Anomaly or Birth Defect | An indication the serious adverse event was associated with a congenital anomaly or birth defect. | [Is/Was] the adverse event associated with a congenital anomaly or birth defect? | Congenital Anomaly/Birth Defect | Char | AESCONG | Maps directly to the SDTM variable listed in the column with the heading "SDTM Target". | (NY) | If the details regarding a Serious AE are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Note for sponsors: The FDA Study Data Technical Conformance Guide v2.2 (June 12, 2015) Section 4.1.1.3 states "The entry of a "Y" for the serious adverse event variable, AESER, should have the assessment indicated, (e.g., as a death, hospitalization, or disability/permanent damage). Frequently, sponsors omit the assessment information, even when it has been collected on the CRF. The criteria that led to the determination should be provided. This information is critical during FDA review to support the characterization of serious AEs". |
Domain Specific | AE | 5 | AESDISAB | Persist or Signif Disability/Incapacity | An indication the serious adverse event was associated with a persistent or significant disability or incapacity. | Did the adverse event result in disability or permanent damage? | Disability or Permanent Damage | Char | AESDISAB | Maps directly to the SDTM variable listed in the column with the heading "SDTM Target". | (NY) | If the details regarding a Serious AE are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Note for sponsors: The FDA Study Data Technical Conformance Guide v2.2 (June 12, 2015) Section 4.1.1.3 states "The entry of a "Y" for the serious adverse event variable, AESER, should have the assessment indicated, (e.g., as a death, hospitalization, or disability/permanent damage). Frequently, sponsors omit the assessment information, even when it has been collected on the CRF. The criteria that led to the determination should be provided. his information is critical during FDA review to support the characterization of serious AEs". |
Domain Specific | AE | 6 | AESDTH | Results in Death | An indication the serious adverse event resulted in death. | Did the adverse event result in death? | Death | Char | AESDTH | Maps directly to the SDTM variable listed in the column with the heading "SDTM Target". | (NY) | If the details regarding a Serious AE are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Note for sponsors: The FDA Study Data Technical Conformance Guide v2.2 (June 12, 2015). Section 4.1.1.3 states "The entry of a "Y" for the serious adverse event variable, AESER, should have the assessment indicated, (e.g., as a death, hospitalization, or disability/permanent damage). Frequently, sponsors omit the assessment information, even when it has been collected on the CRF. The criteria that led to the determination should be provided. This information is critical during FDA review to support the characterization of serious AEs". |
Domain Specific | AE | 7 | AESHOSP | Requires or Prolongs Hospitalization | An indication the serious adverse event resulted in an initial or prolonged hospitalization. | Did the adverse event result in initial or prolonged hospitalization of the subject? | Hospitalization (Initial or Prolonged) | Char | AESHOSP | Maps directly to the SDTM variable listed in the column with the heading "SDTM Target". | (NY) | If the details regarding a Serious AE are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Note for sponsors: The FDA Study Data Technical Conformance Guide v2.2 (June 12, 2015) Section 4.1.1.3 states "The entry of a "Y" for the serious adverse event variable, AESER, should have the assessment indicated, (e.g., as a death, hospitalization, or disability/permanent damage). Frequently, sponsors omit the assessment information, even when it has been collected on the CRF. The criteria that led to the determination should be provided. This information is critical during FDA review to support the characterization of serious AEs". |
Domain Specific | AE | 8 | AESI | Adverse Event of Special Interest | An adverse event of special interest (serious or non-serious) is one of scientific and medical concern specific to the sponsor's product or program, for which ongoing monitoring and rapid communication by the investigator to the sponsor can be appropriate. Such an event might warrant further investigation in order to characterize and understand it. Depending on the nature of the event, rapid communication by the trial sponsor to other parties (e.g., regulators) might also be warranted. | [Is/Was] this event of special interest? | Adverse Event of Special Interest | Char | N/A | Does not map to an SDTM variable. The SDTM Annotated CRF is annotated to indicate that this field is NOT SUBMITTED. | (NY) | This CDASH field may be used just to trigger other CRF pages, or populate a value in AECAT or AESCAT. If submitted, this information could be submitted in a SUPP- -.QVAL dataset where SUPP--.QNAM= "AESI" and SUPP--.QLABEL = "Adverse Event of Special Interest". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. |
Domain Specific | AE | 9 | AESINTV | Needs Intervention to Prevent Impairment | An indication an adverse event required medical or surgical intervention to preclude permanent impairment of a body function, or prevent permanent damage to a body structure, due to the use of a medical product. | Did the adverse event require intervention to prevent permanent impairment or damage resulting from the use of a medical product? | Needs Intervention to Prevent Impairment | Char | SUPPAE.QVAL | This does not map directly to an SDTM variable. This information could be submitted in a SUPPAE dataset as the value of SUPPAE.QVAL where SUPPDS.QNAM = "AESINTV" and SUPPAE.QLABEL= "Needs Intervention to Prevent Impairment". Sponsors should see requirements for the reporting of adverse events involving medical devices. | (NY) | This criteria is used for serious adverse events associated with a medical product (e.g., device). SDTM does not contain a variable to report this criteria of seriousness. Sponsor could submit this in the SUPPAE dataset. Sponsors should see requirements for the reporting of adverse events involving medical devices to health authorities. |
Domain Specific | AE | 10 | AESLIFE | Is Life Threatening | An indication the serious adverse event was life threatening. | [Is/Was] the adverse event life threatening? | Life Threatening | Char | AESLIFE | Maps directly to the SDTM variable listed in the column with the heading "SDTM Target". | (NY) | If the details regarding a Serious AE are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Note for sponsors: The FDA Study Data Technical Conformance Guide v2.2 (June 12, 2015) Section 4.1.1.3 states "The entry of a "Y" for the serious adverse event variable, AESER, should have the assessment indicated, (e.g., as a death, hospitalization, or disability/permanent damage). Frequently, sponsors omit the assessment information, even when it has been collected on the CRF. The criteria that led to the determination should be provided. This information is critical during FDA review to support the characterization of serious AEs". |
Domain Specific | AE | 11 | AESMIE | Other Medically Important Serious Event | An indication additional categories for seriousness apply. | [Is/Was] the adverse event a medically important event not covered by other "serious" criteria? | Other Serious (Important Medical Events) | Char | AESMIE | Maps directly to the SDTM variable listed in the column with the heading "SDTM Target". | (NY) | If the details regarding a Serious AE are collected in the clinical database, then it is recommended that a separate Yes/No variable be defined for each Serious AE type. Note for sponsors: The FDA Study Data Technical Conformance Guide v2.2 (June 12, 2015) Section 4.1.1.3 states "The entry of a "Y" for the serious adverse event variable, AESER, should have the assessment indicated, (e.g., as a death, hospitalization, or disability/permanent damage). Frequently, sponsors omit the assessment information, even when it has been collected on the CRF. The criteria that led to the determination should be provided. This information is critical during FDA review to support the characterization of serious AEs". |
Domain Specific | AE | 12 | AESOD | Occurred with Overdose | An indication the serious event occurred with an overdose. | Did the adverse event occur with an overdose? | Overdose | Char | AESOD | Maps directly to the SDTM variable listed in the column with the heading "SDTM Target". | (NY) | Although deprecated, this variable is included for illustrative purposes if the sponsor is continuing to collect legacy data. If the details regarding a Serious AE are collected in the clinical database, then it is recommended that a separate Yes/No variable. Note for sponsors: The FDA Study Data Technical Conformance Guide v2.2 (June 12, 2015) Section 4.1.1.3 states "The entry of a "Y" for the serious adverse event variable, AESER, should have the assessment indicated, (e.g., as a death, hospitalization, or disability/permanent damage). Frequently, sponsors omit the assessment information, even when it has been collected on the CRF. The criteria that led to the determination should be provided. This information is critical during FDA review to support the characterization of serious AEs". |
Domain Specific | DM | 1 | RACEOTH | Race Other | A free-text field to be used when none of the pre-printed values for RACE are applicable or if another, unprinted selection should be added to those pre-printed values. | What was the other race? | Specify Other Race | Char | SUPPDM.QVAL | This does not map directly to an SDTMIG variable. This information could be submitted in a SUPPDM dataset as the value of SUPPDM.QVAL where SUPPDM.QNAM = "RACEOTH" and SUPP.QLABEL="RACE OTHER". See the SDTMIG Section 5-DM Domain. Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | N/A | When creating the Demographics form, it is suggested that you include the five standard race categories. If you choose, you might include another value of Specify, other with a free text field for extending the list of values. The RACEOTH variable contains the free text added by the site. The value(s) added in the optional variable might or might not "collapse up" into one of the five categories specified by the FDA Guidance. See SDTMIG V3.2 for examples of reporting this implementation. |
Domain Specific | DS | 1 | DSCONT | Subject Continue | The plan for subject continuation to the next phase of the study or another study at the time of completion of the CRF. | Will the subject continue? | Subject Continue | Char | SUPPDS.QVAL | This information could be submitted in a SUPPDS dataset as the value of SUPPDS.QVAL where SUPPDS.QNAM= "DSCONT" and SUPPDS.QLABEL="Subject Continue". Refer to the current SDTM and SDTMIG for instructions on placement of non-standard variables in SDTM domains. | (NY) | N/A |
Domain Specific | DS | 2 | DSNEXT | Next EPOCH | Identifies the study epoch or new study in which the subject will participate. | What [is/was] the next [Period/Epoch/Trial] the subject will [continue to/enter/enroll]? | Next [Period/Epoch/Trial] | Char | N/A | This variable does not map to an SDTM variable. The CRF is annotated to indicate that this field is NOT SUBMITTED. | N/A | Typically this is used as General prompt question to aid in monitoring, data cleaning and subject tracking. This information could be submitted in a SUPP--.QVAL dataset where SUPP--.QNAM = "--DSNEXT" and SUPP-- .QLABEL = "Next EPOCH". Refer to the current SDTM and SDTMIG for instructions on placement of non- standard variables in SDTM domains. |
Domain Specific | DS | 3 | DSUNBLND | Unblinded | An indication the subject's treatment information was revealed to any unauthorized site personnel during the trial. | [Is/Was] treatment (unblinded/unmasked) by the site? | Unblinded | Char | DSTERM | This does not map directly to an SDTM variable. If the CDASH field DSUNBLIND = "Y", then the SDTMIG variables DSDECOD and DSTERM = "TREATMENT UNBLINDED" and "DSCAT" = "OTHER EVENT". If DSUNBLIND = "N", then the CRF should be annotated to indicate that this value is NOT SUBMITTED. | (NY) | There may be multiple rows in the SDTM DS dataset to represent this information; each with the appropriate DSCAT values. One row could indicate the treatment was unblinded using DSCAT= "OTHER EVENT" and another row could indicate the status of the subject at the end of their participation in the trial using DSCAT = "DISPOSTION". If DSUNBLND is yes and information was collected about the reason for the unblinding, populate DSCAT with "OTHER EVENT" and the SDTMIG variables DSTERM with the free text and DSDECOD with the sponsor-defined standardized text (e.g., TREATMENT UNBLINDED). If DSUNBLND is yes, and the unblinding also resulted in the subject discontinuing the trial prematurely, populate DSCAT with "DISPOSTION" and use the SDTM IG variables DSTERM and DSDECOD to capture the applicable discontinuation details. If the unblinding occurred due to an Adverse Event, DSTERM contains the text of the Adverse Event, and in the AE domain the SDTMIG variable AEACNOTH ( "Were any other actions taken in response to this adverse event?" ) may include text of "Treatment Unblinded". DSUNBLND may also be used to document intentional unblinding at a protocol defined point in the trial. |
Domain Specific | MH | 1 | MHEVDTYP | Medical History Event Date Type | Specifies the aspect of the medical condition or event by which MHSTDTC and/or the MHENDTC is defined. | What was the medical history event date type? | Medical History Event Date Type | Char | SUPPMH.QVAL | This field does not map directly to an SDTM variable. This information could be submitted in a SUPPMH dataset as the value of SUPPMH.QVAL when SUPPMH.QNAM = "MHEVDTYP" and SUPPMH.QLABEL= "Medical History Event Date Type". | N/A | It is not related to the trials condition. It cannot be a value of PRIMARY DIAGNOSIS or SECONDARY DIAGNOSIS. The event date type may the date of DIAGNOSIS, SYMPTOMS, RELAPSE, INFECTION. |
Identifiers | AP-- | 1 | APID | Associated Persons Identifier | The identifier for a single associated person. | What is the associated person's identifier? | Associated Persons Identifier | Char | APID | Maps directly to the SDTM variable listed in the column with the heading "SDTM Target". | N/A | N/A |
Identifiers | AP-- | 2 | SREL | Subject, Device or Study Relationship | The relationship of the associated person to the study subject / participant. | What is the associated person's relationship to the (study) [subject/participant]? | Relationship to (Study) [Subject/Participant] | Char | SREL | Maps directly to the SDTM variable listed in the column with the heading "SDTM Target". | (RELSUB) | N/A |
Identifiers | AP-- | 3 | RSUBJID | Related Subject | The identifier for the study subject / participant. | What [is/was] the related (study) [subject/participant] identifier? | Related [Subject/Participant] (Identifier) | Char | RSUBJID | Maps directly to the SDTM variable listed in the column with the heading "SDTM Target". | N/A | This may be derived/populated from the study subject's identifier as recorded on the subject Demographics CRF. |
Example CRFs for the CDASHIG AP Domains
Example
This example CRF represents a use case where the study subject is an infant and the biological mother and biological father are associated persons.
Title: Associated Persons Demographics
CRF Metadata
| CDASH Variable | Order | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre- Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DOMAIN | 1 | Domain | Domain | N/A | Text | DOMAIN | (DOMAIN) | APDM | Yes | ||||
RSUBJID | 1 | What is the related study subject's identifier? | Related Subject Identifier | Record the identifier for the study subject/participant. This may be derived/populated from the study subject's identifier as recorded on the subject Demographics CRF. | Text | RSUBJID | Yes | ||||||
SREL | 1 | What is the associated person's relationship to the subject? | Relationship to Subject | Record the relationship of the associated person to the study subject. | Text | SREL | RELSUB | Mother, Biological; Father, Biological | |||||
APID | 2 | What is the associated persons identifier? | Associated Persons Identifier | Record the identifier for a single associated person. | Text | APID | Prompt | ||||||
BRTHDAT | 3 | What is the subject's date of birth? | Birth Date | Record the date of birth to the level of precision known (e.g., day/month/year, year, month/year) in this format (DD-MON-YYYY). | Date | BRTHDTC | Prompt | ||||||
SEX | 4 | What is the sex of the subject? | Sex | Record the appropriate sex. | Text | SEX | (SEX) | Male; Female | Prompt | radio | |||
ETHNIC | 5 | Do you consider yourself Hispanic/Latino or not Hispanic/Latino? | Ethnicity | Study participants should self-report ethnicity, with ethnicity being asked about before race. | Text | ETHNIC | (ETHNIC) | Hispanic or Latino; Not Hispanic or Latino; Not Reported |
| radio | |||
RACE | 6 | Which of the following five racial designations best describes you? (More than one choice is acceptable.) | Race | Study participants should self-report race, with race being asked about after ethnicity. | Text | RACE | (RACE) | American Indian or Alaska Native; Asian; Black or African American; Native Hawaiian or Other Pacific Islander; White | check |
Example 2
This example CRF represents a use case where the study subject is an infant and the biological mother is an associated person and data about her medications during breastfeeding are being collected.
Title: Concomitant Medications During Breastfeeding

CRF Metadata
| CDASH Variable | Order Number | Question Text | Prompt | CRF Completion Instructions | Type | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology Code List Name | Permissible Values | Pre-Populated Value | Query Display | List Style | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DOMAIN | 0 | Domain | Domain | N/A | Text | DOMAIN | (DOMAIN) | APCM | Yes | ||||
CMCAT | 1 | What is the category for the concomitant medication? | Concomitant Medication Category | Record the concomitant medication category, if not pre-printed on the CRF. | text | CMCAT | MEDICATION DURING BREASTFEEDING | Yes | |||||
SREL | 2 | What is the associated person's relationship to the subject? | Relationship to Subject | Record the relationship of the associated person to the study subject. | Text | SREL | (RELSUB) | MOTHER, BIOLOGICAL | Yes | ||||
RSUBJID | 3 | What is the related study subject's identifier? | Related Subject Identifier | Record the identifier for the study subject/participant. This may be derived/populated from the study subject's identifier as recorded on the subject Demographics CRF. | Text | RSUBJID | Yes | ||||||
APID | 4 | What is the associated persons identifier? | Associated Persons Identifier | Record the associated persons identifier for the mother, using the same value as noted on the Associated Persons Demographics CRF. | Text | APID | prompt | ||||||
CMYN | 5 | Were any concomitant medication(s) taken while breastfeeding? | Any Concomitant Medication(s) | Indicate if any concomitant medications were taken. If Yes, include the appropriate details where indicated on the CRF. | text | (NY) | Yes; No | prompt | Radio | ||||
CMTRT | 6 | What was the concomitant medication name? | Concomitant Medication | Record only 1 medication per line. Provide the full trade or proprietary name of the medication; otherwise, the generic name may be recorded. | text | CMTRT | |||||||
CMSCAT | 7 | What is the subcategory for the concomitant medication? | Concomitant Medication Subcategory | Record the concomitant medication subcategory. | text | CMSCAT | ANTIRETROVIRAL; GENERAL | ||||||
CMDOSE | 8 | What was the individual dose of the concomitant medication per administration? | Dose per administration | Record the dose of concomitant medication taken per administration (e.g., 200). | text | CMDOSE | |||||||
CMDOSU | 9 | What is the unit? | (Dose) Unit | Record the dose unit of the dose of concomitant medication taken (e.g., mg). | text | CMDOSU | (UNIT) | ||||||
CMDOSFRQ | 10 | What was the frequency of the concomitant medication? | Frequency | Record how often the concomitant medication was taken (e.g., BID, PRN). | text | CMDOSFRQ | (FREQ) | ||||||
CMROUTE | 11 | What was the route of administration of the concomitant medication? | Route | Provide the route of administration for the concomitant medication. | text | CMROUTE | (ROUTE) | ||||||
CMSTDAT | 12 | What was the concomitant medication start date? | Start Date | Record the date the concomitant medication was first taken, using this format: DD- MON-YYYY. If the subject has been taking the concomitant medication for a considerable amount of time prior to the start of the study, it is acceptable to have an incomplete date. Any concomitant medication taken during the study is expected to have a complete start date. Any prior concomitant medication that is exclusionary should have both a start and end date. | text | CMSTDTC | |||||||
CMONGO | 13 | Was the concomitant medication ongoing? | Concomitant Meds Ongoing | Record the concomitant medication/treatment as ongoing if the subject has not text stopped taking the concomitant medication/treatment at the time of data collection and the end date should be left blank. | text | CMENRF; CMENRTPT | CMENRF/CMENRTPT | (NY) | Yes | Checkbox | |||
CMENDAT | 14 | What was the concomitant medication end date? | End Date | Record the date the concomitant medication was stopped, using this format: DD- text MON-YYYY. If the subject has not stopped taking the concomitant medication, leave this field blank. | text | CMENDTC |
|
Example 3
This example CRF represents a use case where the study subject is a child and the biological parents are associated persons.
Title: Associated Persons STI Medical History and Risk Factors
CRF Metadata
| CDASH Variable | Order Number | Question Text | Prompt | Type | CRF Completion Instructions | SDTMIG Target Variable | SDTMIG Target Mapping | Controlled Terminology CodeList Name | Permissible Values | Pre- Populated Value | Displayed Query | List | Hidden |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DOMAIN | 0 | Domain | Domain | text | N/A | DOMAIN | (DOMAIN) | APMH | Yes | ||||
MHCAT | 1 | What was the category of the medical history? | Medical History Category | text | Indicate the category of the medical history event or condition. | MHCAT | RISK FACTORS | Y | |||||
MHSCAT | 2 | What was the subcategory of the medical history? | Medical History Subcategory | text | Indicate the subcategory of the medical history event or condition. | MHSCAT | HISTORY OF STI | Y | |||||
RSUBJID | 3 | What is the related study subject's identifier? | Related Subject Identifier | text | Record the identifier for the study subject/participant. This may be derived/populated from the study subject's identifier as recorded on the subject Demographics CRF. | RSUBJID | Y | ||||||
SREL | 4 | What is the associated person's relationship to the subject? | Relationship to Subject | text | Record the relationship of the associated person to the study subject. | SREL | RELSUB | Mother, Biological; Father, Biological | |||||
APID | 5 | What is the associated person's identifier? | Associated Persons Identifier | text | Record the identifier for the associated person using the same value as noted on the Associated Persons Demographics CRF. | APID | |||||||
HIV_MHTERM | 6 | N/A | Medical History Term | text | N/A | MHTERM | HIV | Y | |||||
HIV_MHOCCUR | 7 | Has the associated person been diagnosed with HIV? | HIV Occurrence | text | Indicate if the associated person has ever been diagnosed with HIV. | MHOCCUR | MHOCCUR where MHTERM = "HIV" | (NY) | Yes;No | Radio Buttons | |||
HIV_MHSTDAT | 8 | What was the medical condition or event diagnosis date? | Diagnosis Date | text | Record the diagnosis date of the medical event or condition. | MHSTDTC | N/A | Prompt | |||||
HIV_DIAG_MHEVDTYP | 9 | What was the medical history event date type? | Medical History Event Date Type | text | The instructions depend upon the format of the CRF. Sponsors may pre-print these values on the CRF or use them as defaulted or hidden text. | SQAPMH.QVAL | SQAPMH.QVAL where SQAPMH.QNAM = "MHEVDTYP" and SQAPMH.QLABEL = "Medical History Event Date Type" | DIAGNOSIS | Y | ||||
GONORRHEA_MHTERM | 10 | N/A | Medical History Term | text | N/A | MHTERM | GONORRHEA | Y | |||||
GONORRHEA_MHOCCUR | 11 | Has the associated person ever had gonorrhea? | Medical History Term | text | Indicate if the associated person has ever been diagnosed with gonorrhea. | MHOCCUR | MHOCCUR where MHTERM = "GONORRHEA" | (NY) | Yes;No | Radio Buttons | |||
GONORRHEA_MHSTDAT | 12 | What was the medical condition or event start date? | Start Date | text | Record the start date of the medical event or condition. | MHSTDTC | |||||||
GONORRHEA_MHONGO | 13 | Is the event ongoing at the time of collection of this history? | Ongoing | text | Indicate if the condition is ongoing at the time the MHENRF history is collected. | MHENRF | MHENRTPT where MHENTPT = Date of Collection | (NY) | Yes | checkbox | |||
GONORRHEA_MHENDAT | 14 | What was the medical condition or event end date? | End Date | text | Record the end date of the medical event or condition. | MHENDTC | |||||||
CHLAMYDIA_MHTERM | 15 | N/A | Medical History Term | text | N/A | MHTERM | CHLAMYDIA | Y | |||||
CHLAMYDIA_MHOCCUR | 16 | Has the associated person ever had chlamydia? | Medical History Term | text | Indicate if the associated person has ever been diagnosed with chlamydia. | MHOCCUR | MHOCCUR where MHTERM = "CHLAMYDIA" | (NY) | Yes;No | Radio Buttons | |||
CHLAMYDIA_MHSTDAT | 17 | What was the medical condition or event start date? | Start Date | text | Record the start date of the medical event or condition. | MHSTDTC | |||||||
CHLAMYDIA_MHONGO | 18 | Is the event ongoing at the time of collection of this history? | Ongoing | text | Indicate if the condition is ongoing. | MHENRTPT | MHENRTPT where MHENTPT = Date of Collection | (NY) | Yes; | checkbox | |||
CHLAMYDIA_MHENDAT | 19 | What was the medical condition or event end date? | End Date | text | Record the end date of the medical event or condition. | MHENDTC |
9 Appendices
Appendix A: CDASH Model and CDASHIG Team Contributors
The following table lists volunteers who actively contributed to the development of the CDASH Model 1.1 and CDASHIG 2.1:
| Name | Institution/Organization |
|---|---|
Melissa Binz | Pfizer |
Jorge Torres Borrero | Lung Biotechnology PBC |
Tammy Burns | EMD Serono |
Robert Butler | Gilead Sciences |
Dan Crawford | Veeva |
Carolyn Famatiga-Fay | CFSQUARED Solutions, LLC |
Nikki Flores | Gilead Sciences |
Erica Gonzales | PRA Health Sciences |
Kit Howard | CDISC |
Dawn Kaminski | eClinical Solutions |
Natalia Khelmer | Johnson & Johnson |
Joanna Kuzmicz | AstraZeneca |
Shannon Labout | Data Science Solutions LLC |
Kathleen Mellars | CDISC |
Valerie Paxton | Synteract |
Deborah Rittenhouse | CSL Behring |
Jerry Salyers | Data Standards Consulting |
Tisanna J. Shelton | Eli Lilly & Company |
Sheetal Sheth | KCT Data Inc. |
Benjamin C. Shim | Eli Lilly & Company |
Lauren Shinaberry | Abbvie Inc. |
Trisha Simpson | UCB |
Lorraine P. Spencer, Co-chair | Abbvie |
Alana St. Clair | CDISC |
Judy Tran | Merck |
Kim Truett | KCT Data Inc. |
Gary Walker | Gary G Walker, LLC |
Guang-Liang Wang | Otsuka |
Michael J. Ward, Co-chair | Eli Lilly & Company |
Appendix B: Glossary and Abbreviations
The following abbreviations and terms are used in this document. Additional definitions can be found in the CDISC Glossary (available at https://www.cdisc.org/standards/semantics/glossary).
ADaM | Analysis Data Model |
AE | Adverse event; also refers to the Adverse Events domain. |
ATC code | Anatomic Therapeutic Chemical code (from WHODrug) |
CDASH | Clinical Data Acquisition Standards Harmonization Project; the name for the project that delivers basic data collection fields. |
CDASHIG | Clinical Data Acquisition Standards Harmonization Implementation Guide for Human Clinical Trials; the associated implementation guide intended to be paired with CDASH Model. |
CDISC | Clinical Data Interchange Standards Consortium |
CDM | Clinical data management |
Clinical database | A repository of the study results data collected in a clinical trial. The format and structure of this repository may vary across sponsors and vendors. |
Collected | Within this document, collected refers to information that is recorded and/or transmitted to the sponsor. This includes data entered by the site on CRFs/eCRFs as well as vendor data such as core lab data. This term is a synonym for captured. |
CRF | Case report form (sometime case record form); a printed, optical, or electronic document designed to record all required information to be reported to the sponsor for each trial subject. |
CTCAE | Common Terminology Criteria for Adverse Events Dictionary |
Databased | To put (data) into a database. |
Dataset | A collection of structured data in a single file. |
Denormalized | The organization of data such that multiple observations (results) are presented in a single row of data. For example, the result values for PULSE, HEIGHT, WEIGHT would be presented in the same row of data with PULSE, HEIGHT, and WEIGHT as column headers. This is also called a horizontal data structure. |
Derived | Within this document, derived refers to information that is not directly entered into the specific data field by the investigator site or by a core lab. This category includes auto-encoded data, calculated data, and similar electronically generated data, but not pre-populated fields. |
Domain | A domain is a collection of data points related by a common topic, such as adverse events or demographics. |
eCOA | Electronic clinical outcome assessment |
eCRF | Electronic case report form |
ECG | Electrocardiogram |
EDC | Electronic data capture |
EHR | Electronic healthcare record |
Epoch | Interval of time in the planned conduct of a study. An epoch is associated with a purpose (e.g., screening, randomization, treatment, follow-up), which applies across all arms of a study. |
FDA | Food and Drug Administration; part of the US Department of Health and Human Services. The FDA is the regulatory authority for all pharmaceuticals (including biologics and vaccines) and medical devices in the US. |
GCDMP | Good Clinical Data Management Practices (GCDMP); SCDM publication on CDM processes |
HL7 | Health Level 7; standards for the exchange, integration, sharing, and retrieval of electronic health information |
ICD9 | International Classification of Diseases, Ninth Revision |
ICH | International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use |
ICH E2A | ICH guidelines on Clinical Safety Data Management: Definitions and Standards for Expedited Reporting |
ICH E2B | ICH guidelines on Clinical Safety Data Management: Data Elements For Transmission Of Individual Case Safety Reports |
ICH E2C | ICH guidelines on Clinical Safety Data Management: Periodic Safety Update Reports For Marketed Drugs |
ICH E3 | ICH guidelines on Structure and Content of Clinical Study Reports |
ICH E4 | ICH guidelines on Dose-response Information to Support Drug Registration |
ICH E5 | ICH guidelines on Ethnic Factors in the Acceptability of Foreign Clinical Data |
ICH E6 (R1) | ICH guideline for Good Clinical Practice |
ICH E9 | ICH guidelines on Statistical Principles for Clinical Trials |
ICH E11 | ICH guidelines on Clinical Investigation of Medicinal Products in the Pediatric Population |
ICH E14 | ICH guidelines on the Clinical Evaluation Of QT/QTc Interval |
ISO 8601 | International Organization for Standardization document of character representation of dates, date/times, intervals, and durations of time |
MedDRA | Medical Dictionary for Regulatory Activities; global standard medical terminology designed to supersede other terminologies (e.g., COSTART, ICD9) used in the medical product development process. |
MHLW | Japanese Ministry of Health, Labor and Welfare |
MRI | Magnetic resonance imaging |
N/A | Not applicable |
NCI | National Cancer Institute (NIH) |
NCI EVS | National Cancer Institute (NIH) Enterprise Vocabulary Services |
NIH | National Institutes of Health |
Normalized | The organization of data such that only 1 observation (result) is presented per row. For example, PULSE, HEIGHT, WEIGHT would be presented as values in individual rows (with the column header VSTESTCD) with each result presented in another column (same row) called VSORRES. This is also called a vertical data structure. |
PK | Pharmacokinetics; the study of the absorption, distribution, metabolism, and excretion of a drug. |
Preprinted (pre-printed) | Items that are part of the original printing on a paper CRF. For example, the unit required for a response, such as “years” for an age question. These data may or may not be stored in the database. |
Prepopulated (pre-populated) | Items that are part of the eCRF (or data collection device) that are not entered and cannot be modified. (Also see Preprinted). These data are stored in the study database. |
PRO | Patient-reported outcome |
Protocol deviation | A variation from processes or procedures defined in a protocol. Deviations usually do not preclude the overall evaluability of subject data for either efficacy or safety, and are often acknowledged and accepted in advance by the sponsor. Good clinical practice recommends that deviations be summarized by site and by category as part of the report of study results so that the possible importance of the deviations to the findings of the study can be assessed (cf Protocol violation; see also ICH E3). |
Protocol violation | A significant departure from processes or procedures that were required by the protocol. Violations often result in data that are not deemed evaluable for a per-protocol analysis, and may require that the subject(s) who violate the protocol be discontinued from the study (cf. Protocol deviation). |
SAP | Statistical analysis plan |
SCDM | Society for Clinical Data Management |
SDTM | Study Data Tabulation Model |
SDTMIG | Study Data Tabulation Model Implementation Guide |
SME | Subject matter expert |
SNOMED | Systematically organized computer processable collection of medical terms providing codes, terms, synonyms, and definitions used in clinical documentation and reporting. |
SOCs | System Organ Classes (from MedDRA) |
SOP | Standard Operating Procedure |
Study Treatment | The drug, device, therapy, or process under investigation in a clinical trial which has an effect on outcome of interest in a study (e.g., health-related quality of life, efficacy, safety, pharmacoeconomics; synonyms: intervention, therapeutic intervention, medical product) |
TAUG | Therapeutic area user guide |
Uncoded | Not coded; not having or showing a code. |
Variable naming fragment | A reusable pattern of characters in variable names that convey an equivalent meaning when applied to multiple variable names across classes and domains. |
WHO | World Health Organization |
WHO ART | World Health Organization Adverse Reaction Terminology has been developed over more than 30 years to serve as a basis for rational coding of adverse reaction terms. |
WHODrug (WHO-DD) | World Health Organization Drug Dictionary |
Appendix C: Revision History - Changes from CDASHIG v2.0 to CDASHIG v2.1
The release of the CDASHIG v2.1 and CDASH Model v1.1 entail changes from and supersedes prior versions of the CDASHIG v2.0 and CDASH Model v1.0, respectively. The most significant changes include:
• Inclusion of remaining SDTMIG v3.2 domains that were not published in CDASHIG v2.0 (i.e., MB, MS, TU, TR, RS, AP--)
• Conversion of all CRF examples to metadata table-defined aCRFs using the CRF Maker Tool
• Resolution of Jira issues submitted for enhancements and error corrections identified in CDASHIG v2.0 and CDASH Model v1.0
• Changed CDASHIG and CDASH Model Variable Labels, where needed, to more closely align with SDTMIG and SDTM Variable Labels, respectively
• Resolution of Jira issues submitted during Public Review
Appendix D: Representations and Warranties, Limitations of Liability, and Disclaimers
CDISC Patent Disclaimers
It is possible that implementation of and compliance with this standard may require use of subject matter covered by patent rights. By publication of this standard, no position is taken with respect to the existence or validity of any claim or of any patent rights in connection therewith. CDISC, including the CDISC Board of Directors, shall not be responsible for identifying patent claims for which a license may be required in order to implement this standard or for conducting inquiries into the legal validity or scope of those patents or patent claims that are brought to its attention.
Representations and Warranties
"CDISC grants open public use of this User Guide (or Final Standards) under CDISC's copyright."
Each Participant in the development of this standard shall be deemed to represent, warrant, and covenant, at the time of a Contribution by such Participant (or by its Representative), that to the best of its knowledge and ability: (a) it holds or has the right to grant all relevant licenses to any of its Contributions in all jurisdictions or territories in which it holds relevant intellectual property rights; (b) there are no limits to the Participant's ability to make the grants, acknowledgments, and agreements herein; and (c) the Contribution does not subject any Contribution, Draft Standard, Final Standard, or implementations thereof, in whole or in part, to licensing obligations with additional restrictions or requirements inconsistent with those set forth in this Policy, or that would require any such Contribution, Final Standard, or implementation, in whole or in part, to be either: (i) disclosed or distributed in source code form; (ii) licensed for the purpose of making derivative works (other than as set forth in Section 4.2 of the CDISC Intellectual Property Policy ("the Policy")); or (iii) distributed at no charge, except as set forth in Sections 3, 5.1, and 4.2 of the Policy. If a Participant has knowledge that a Contribution made by any Participant or any other party may subject any Contribution, Draft Standard, Final Standard, or implementation, in whole or in part, to one or more of the licensing obligations listed in Section 9.3, such Participant shall give prompt notice of the same to the CDISC President who shall promptly notify all Participants.
No Other Warranties/Disclaimers. ALL PARTICIPANTS ACKNOWLEDGE THAT, EXCEPT AS PROVIDED UNDER SECTION 9.3 OF THE CDISC INTELLECTUAL PROPERTY POLICY, ALL DRAFT STANDARDS AND FINAL STANDARDS, AND ALL CONTRIBUTIONS TO FINAL STANDARDS AND DRAFT STANDARDS, ARE PROVIDED "AS IS" WITH NO WARRANTIES WHATSOEVER, WHETHER EXPRESS, IMPLIED, STATUTORY, OR OTHERWISE, AND THE PARTICIPANTS, REPRESENTATIVES, THE CDISC PRESIDENT, THE CDISC BOARD OF DIRECTORS, AND CDISC EXPRESSLY DISCLAIM ANY WARRANTY OF MERCHANTABILITY, NONINFRINGEMENT, FITNESS FOR ANY PARTICULAR OR INTENDED PURPOSE, OR ANY OTHER WARRANTY OTHERWISE ARISING OUT OF ANY PROPOSAL, FINAL STANDARDS OR DRAFT STANDARDS, OR CONTRIBUTION.
Limitation of Liability
IN NO EVENT WILL CDISC OR ANY OF ITS CONSTITUENT PARTS (INCLUDING, BUT NOT LIMITED TO, THE CDISC BOARD OF DIRECTORS, THE CDISC PRESIDENT, CDISC STAFF, AND CDISC MEMBERS) BE LIABLE TO ANY OTHER PERSON OR ENTITY FOR ANY LOSS OF PROFITS, LOSS OF USE, DIRECT, INDIRECT, INCIDENTAL, CONSEQUENTIAL, OR SPECIAL DAMAGES, WHETHER UNDER CONTRACT, TORT, WARRANTY, OR OTHERWISE, ARISING IN ANY WAY OUT OF THIS POLICY OR ANY RELATED AGREEMENT, WHETHER OR NOT SUCH PARTY HAD ADVANCE NOTICE OF THE POSSIBILITY OF SUCH DAMAGES.
Limitation of Liability
IN NO EVENT WILL CDISC OR ANY OF ITS CONSTITUENT PARTS (INCLUDING, BUT NOT LIMITED TO, THE CDISC BOARD OF DIRECTORS, THE CDISC PRESIDENT, CDISC STAFF, AND CDISC MEMBERS) BE LIABLE TO ANY OTHER PERSON OR ENTITY FOR ANY LOSS OF PROFITS, LOSS OF USE, DIRECT, INDIRECT, INCIDENTAL, CONSEQUENTIAL, OR SPECIAL DAMAGES, WHETHER UNDER CONTRACT, TORT, WARRANTY, OR OTHERWISE, ARISING IN ANY WAY OUT OF THIS POLICY OR ANY RELATED AGREEMENT, WHETHER OR NOT SUCH PARTY HAD ADVANCE NOTICE OF THE POSSIBILITY OF SUCH DAMAGES.
Note: The CDISC Intellectual Property Policy can be found at: https://www.cdisc.org/system/files/all/article/application/pdf/cdisc_policy_003_intellectual_property_v201408.pdf.