Clinical Data Acquisition Standards Harmonization Implementation Guide for Human Clinical Trials
Version 2.0
Notes to Readers
- This is Version 2.0 of the Clinical Data Acquisition Standards Harmonization Implementation Guide for Human Clinical Trials.
- This document is intended to be paired with the CDASH Model.
Revision History
| Date | Version |
|---|---|
| 2017-09-20 | 2.0 Final |
© 2017 Clinical Data Interchange Standards Consortium. All rights reserved. See also Representations and Warranties, Limitations of Liability, and Disclaimers.
Contents
- 1 Orientation
- 1.1 Purpose
- 1.2 Organization of this Document
- 1.2.1 General Notes
- 2 How to Use the CDASH Standard
- 3 General Assumptions for Implementing CDASH
- 3.1 How CDASH and SDTM Work Together
- 3.2 Core Designations for Basic Data Collection Fields
- 3.3 Additional Information about CDASHIG Core Designations
- 3.4 How to Create New Data Collection Fields When No CDASHIG Field Has Been Defined
- 3.5 Explanation of Table Headers in the CDASH Model and CDASHIG Metadata Table
- 3.5.1 CDASH Model
- 3.5.2 CDASHIG Metadata Table
- 3.6 Collection of Dates
- 3.7 Mapping Relative Times from Collection to Submissions
- 3.8 CDISC Controlled Terminology
- 4 Best Practice Recommendations
- 5 Conformance to the CDASH Standard
- 6 Other Information
- 7 CDASH Special-Purpose Domains
- 8 General Observation Classes
- 8.1 CDASH Interventions Domains
- 8.2 CDASH Events Domains
- 8.2.1 General CDASH Assumptions for Events Domains
- 8.2.2 AE - Adverse Events
- 8.2.3 CE - Clinical Events
- 8.2.4 DS - Disposition
- 8.2.5 DV - Protocol Deviations
- 8.2.6 HO - Healthcare Encounters
- 8.2.7 MH - Medical History
- 8.3 CDASH Findings Domains
- 8.3.1 General CDASH Assumptions for Findings Domains
- 8.3.2 DA - Drug Accountability
- 8.3.3 DD - Death Details
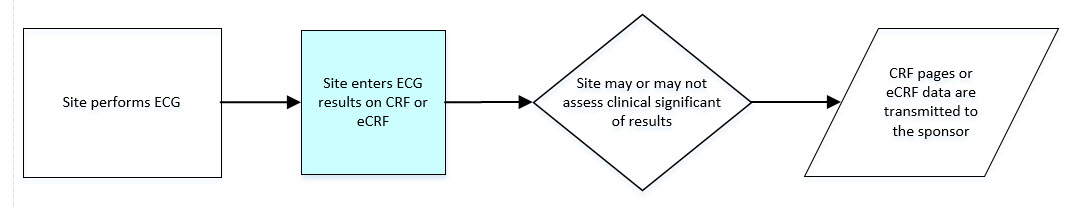
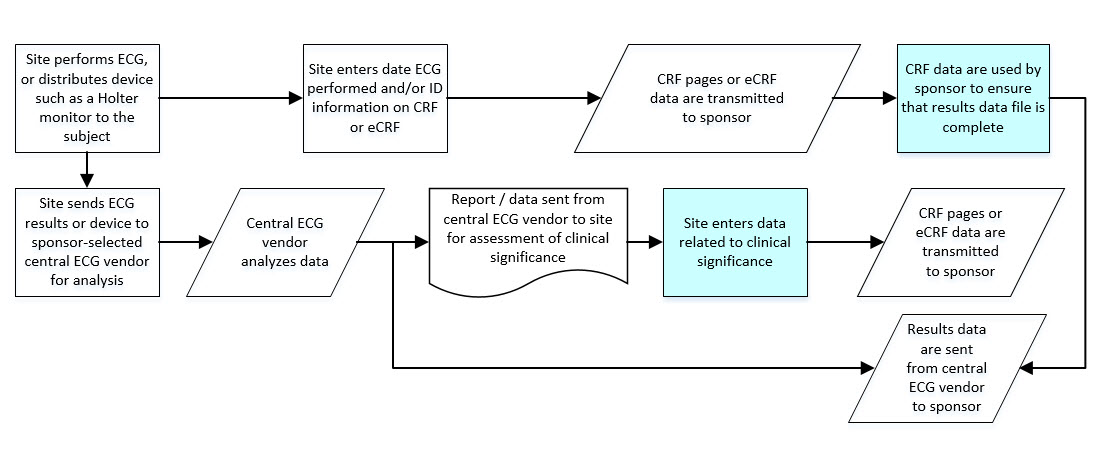
- 8.3.4 EG - ECG Test Results
- 8.3.5 IE - Inclusion/Exclusion Criteria Not Met
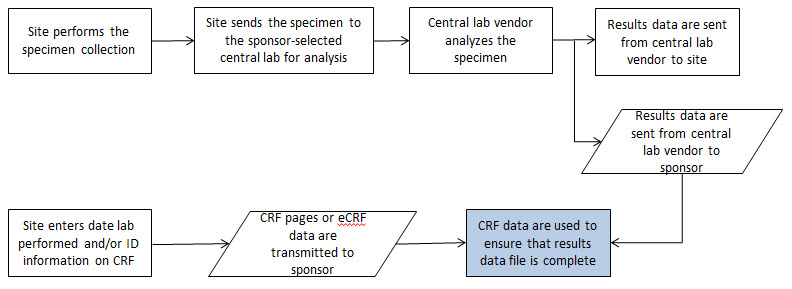
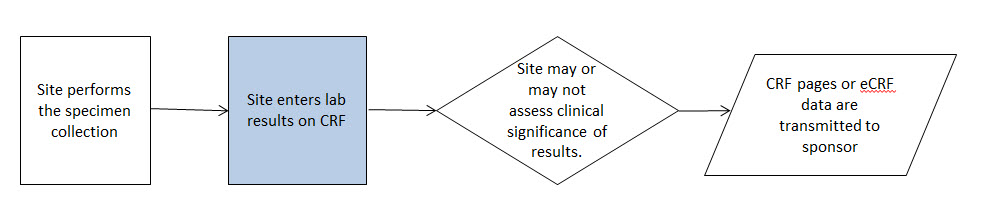
- 8.3.6 LB - Laboratory Test Results
- 8.3.7 MI - Microscopic Findings
- 8.3.8 PC - Pharmacokinetics Sampling
- 8.3.9 PE - Physical Examination
- 8.3.10 QRS - Questionnaires, Ratings and Scales
- 8.3.11 SC - Subject Characteristics
- 8.3.12 RP - Reproductive System Findings
- 8.3.13 SR - Skin Response
- 8.3.14 VS - Vital Signs
- 8.3.15 FA - Findings About
- Appendices
- Appendix A: CDASH Contributors
- Appendix A1: CDASH Co-Chairs
- Appendix A2: CDASH Model and CDASHIG Team Contributors
- Appendix B: Glossary and Abbreviations
- Appendix C: Revision History
- Appendix C1: Changes from CDASH v1.1 to CDASHIG v2.0
- Appendix D: Representations and Warranties, Limitations of Liability, and Disclaimers
- Appendix A: CDASH Contributors
1 Orientation
This implementation guide has been developed to assist in the following activities associated with the collection and compilation of data in a clinical trial.
"There is arguably no more important document than the instrument that is used to acquire the data from the clinical trial, with the exception of the protocol, which specifies the conduct of that trial. The quality of the data collected relies first and foremost on the quality of that instrument. No matter how much time and effort go into conducting the trial, if the correct data points were not collected, a meaningful analysis may not be possible. It follows, therefore, that the design, development and quality assurance of such an instrument must be given the utmost attention."
— Good Clinical Data Management Practices, Version 4, October 2005, Society for Clinical Data Management
1.1 Purpose
The Clinical Data Acquisition Standards Harmonization (CDASH) Model, the CDASH Implementation Guide (CDASHIG), and the CDASHIG Metadata Table define basic standards for the collection of clinical trial data and how to implement the standard for specific case report forms. CDASH establishes a standard way to collect data in a similar way across studies and sponsors, so that data collection formats and structures provide clear traceability of submission data into the Study Data Tabulation Model (SDTM), delivering more transparency to regulators and others who conduct data review. The CDASH standard is part of the Clinical Data Interchange Standards Consortium (CDISC) Technical Road Map, which is designed to realize the vision of a set of beginning-to-end harmonized standards for the representation of data from clinical studies throughout the data lifecycle. The CDASH standard directly supports the production of clinical data collection instruments. Through this support, the standard also contributes to:
- Efficient development of research protocols
- Streamlined processes within medical research
- Development of a corporate library of standardized CRFs
- Use of metadata repositories
- Reporting and regulatory submission
- Data warehouse population
- Data archiving
- Post-marketing studies/safety surveillance
For more information, click on the following link: http://www.cdisc.org/strategies-and-goals.
There is growing recognition around the globe that industry standards promote data interchange, which is essential to effective partnering and information exchange between and among clinicians and researchers. Clinical care can more easily reap benefits through medical research findings, and more clinicians will be interested in conducting research if the research process can be integrated into their clinical care workflow. CDISC encourages the adoption of its global standards for clinical research, which should continue to be harmonized with healthcare standards, to provide a means for interoperability among healthcare and research systems such that medical research can support informed healthcare decisions and improve patient safety.
This document is intended to be used by persons involved in the planning, collection, management, and analysis of clinical trials and clinical data, including Clinical Investigators, Medical Monitors, Clinical Research Associates (Monitors), Clinical Research Study Coordinators, Clinical Data Standards Subject Matter Experts (SMEs), Clinical Data Managers, Clinical Data and Statistical Programmers, Biostatisticians, Drug Safety, Case Report Form (CRF) Designers, and other functions tasked with the responsibility to collect, clean, and ensure the integrity of clinical trial data.
1.2 Organization of this Document
This document has been organized into the following sections:
- Section 1, Orientation
- Section 2, How to Use the CDASH Standard
- Section 3, General Assumptions for Implementing CDASH
- Section 4, Best Practice Recommendations
- Section 5, Conformance Rules
- Section 6, Other Information
- Section 7, CDASH Special-Purpose Domains
- Section 8, General Observation Classes
- Appendices
1.2.1 General Notes
- Paper CRFs vs. Electronic CRFs: The term "CRF" used throughout this document refers to both paper and electronic formats, unless otherwise specified.
- Fields vs. Variables: Data collection "fields" refers to terms that are commonly on the CRF. Data collection "variables" refers to what is in a clinical database.
- Study Treatment: The phrase "study treatment" has been used instead of investigational/medicinal product, study drug, test article, medical device, etc., in order to include all types of study designs and products.
- Mechanisms for Data Collection: Different data collection mechanisms can be used to control how data are collected (e.g., tick boxes, check boxes, radio buttons, drop-down lists, etc.). For the purposes of this document, these terms will be used interchangeably.
2 How to Use the CDASH Standard
2.1 The Three Components of the CDASH Standard
CDASH is composed of the CDASH Model and the CDASH Implementation Guide (CDASHIG), with its associated CDASHIG Metadata Table. A domain is a collection of data points related by a common topic, such as adverse events or demographics. CDASHIG domains are aligned with SDTMIG domains for beginning-to-end traceability.
CDASH Model
The CDASH Model v1.0 provides a general framework for creating fields to collect information on CRFs and includes the model metadata, which shows the standard variables in the model. The CDASHIG provides information on the implementation of the CDASH Model and includes the CDASHIG Metadata Table, which details additional specifications for data collection variables within each domain.
The CDASH Model v1.0 provides root naming conventions for CDASHIG variables that are intended to facilitate mapping to the SDTMIG variables. The variables defined in the CDASH model follow the same "--XXXX" naming convention as the SDTM model. The two dashes are replaced by the domain code when applied to create the CDASHIG variable. For example, --DOSFRQ is the CDASH Model variable name to for Dosing Frequency per Interval in the Interventions Class. When a domain abbreviation is applied (e.g., "CM"), CMDOSFRQ is the CDASHIG variable for the frequency of the concomitant medication use. The CDASH Model includes metadata for variables used in each of the SDTM general observation classes, Timing variables, Identifier variables, variables for Special Purpose domains, and domain-specific variables. See Section 3.5 for specific information on the CDASH Model content.
CDASHIG
The CDASHIG provides general information on the implementation of CDASH standards. The CDASH standards include the CDASH Model and the CDASHIG, which includes the supporting CDASHIG Metadata Table. The informative content of the CDASHIG and the normative content metadata table comprise the CDASHIG and must be referenced together.
CDASHIG Metadata Table
The CDASHIG Metadata Table includes only those variables commonly implemented by a significant number of the organizations/companies that provided information/examples (e.g., Medical History, Adverse Events). Implementers can add appropriate variables to their CDASHIG domain using the associated General Observation class within the CDASH Model. The CDASHIG Domain Metadata illustrates the use of Question Text and Prompts employed by many sponsors. Implementers should reference the CDASH Model to see all available options for Question Text and Prompt for parameters and verb tenses that may be substituted.
2.2 CDASHIG Metadata Table Attributes
The CDASHIG Metadata Table attributes provide building blocks for the development of a case report form and the underlying database or other data collection structure.
CRF and Data Management System Design Metadata
Certain metadata attributes are essential to CDASH conformance. Combined with the variable naming conventions discussed in Section 5.1 Conformance Rules, these metadata attributes will assist the designer of the CRFs and the underlying database structure to remain in conformance with the standard:
- Question Text (full sentence/question forms to prompt for data) OR Prompts (short phrases, often suitable as column headers, to prompt for data)
- CDISC Controlled Terminology lists and subsets of list values when applicable
- DRAFT CDASH Definition (to assist in understanding the purpose of each variable)
- CDASHIG and SDTMIG Core designations and implementation notes (which, when used together, can assist a designer in determining the complete set of data to be collected on a form)
SDTMIG Programming Metadata
Columns in the CDASHIG Metadata Table that will assist in developing programs to generate SDTM domain datasets from CDASHIG compliant data include:
- Domain
- CDASHIG Variable
- Maps to SDTMIG Variable
- Mapping Instructions
- Implementation Notes
Additional Metadata
The CDASHIG Metadata Table includes the column "Case Report Form Completion Instructions" to assist authors in creating this study level documentation for instructing sites how to complete the CRF fields.
2.3 CRF Development Overview
The key steps to developing CRFs using CDASH are as follows:
- Each organization may maintain a corporate library of standardized CRFs. Determine the requirements for data domains from these (if applicable) or the protocol data collection requirements for the study.
- Review the domains published in CDASHIG to determine which of the data collection domains and fields are already specified in the published domains.
- As much as possible, the standard domains should be used to collect data in a manner that will be effective for data collection. Develop the data collection tools using these published, standard domains first.
-
Using the root variables and other CDASH metadata in the CDASH Model, add any additional variables that are needed to meet the requirements of data collection. Follow CDISC Variable-Naming Fragments (see the CDASHIG Glossary and Abbreviations) conventions, and CDASH root variable naming conventions where they exist (e.g., --DAT for dates, --TIM for times, --YN for prompts as described in the CDASH Model).
Example: Replace "--" with the two-character domain code that matches the other variables in the same domain. For example, to add the --LOC variable to a Medical History CRF, the domain code is "MH", so the variable would become "MHLOC" in that domain. -
The Question Text and Prompt columns in the CDASH Model provide different variations in the recommended text for asking the question on a CRF. For each question, the sponsor may elect to either use the Question Text or the Prompt on the CRF. Some text is presented using brackets [ ], parentheses ( ), and/or incorporating forward slashes. These different formats are used to indicate how the Question Text or Prompt may be modified by the sponsor.
-
The text inside the brackets provides an option on the tense of the question, or text that can be replaced with protocol specific verbiage.
-
The text inside the parentheses provides options (e.g., singular/plural) or text that may be eliminated.
-
Text separated with a forward slash provides optional words which the sponsor may choose.
Example: The CDASH variable, --PERF, from the CDASH Model has the following Question Text and Prompt.
Question Text:
[Were any/Was the] [--TEST/ topic] [measurement(s)/test(s)/examination(s)/specimen(s)/sample(s)] [performed/collected]?
Prompt:
[--TEST/Topic] [Measurement(s)/Test(s)/Examination(s)/Specimen(s)/Sample(s)] [Performed/Collected]?
The sponsor wants to add a question to a CRF that asks whether a lab specimen was collected using a yes, no response.
a) The sponsor selects the CDASH variable --PERF and adds the appropriate domain code. LBPERF
b) Use either the Prompt or the full Question Text on the CRF.
Question Text: Was the laboratory specimen collected?
- In the first set of brackets, the text option "Was the" is selected as the study required only one lab test to be performed. [Were any/Was the]
- In the second set of brackets, the text used is "laboratory" which is the topic of interest. [--TEST/Topic (laboratory)]
- In the third set of brackets, the text option "specimen" without the optional "s" is selected. [measurement(s)/test(s)/examination(s)/specimen(s)/sample(s)]
- In the fourth set of brackets, the text option "collected" is selected. [performed/collected]
Prompt: Laboratory Specimen Collected
- In the first set of brackets, the text used is the topic of interest (i.e., laboratory). [--TEST/Topic (Laboratory)]
- In the second set of brackets, the text option "specimen" without the optional "s" is selected. [Measurement(s)/Test(s)/Examination(s)/Specimen(s)/Sample(s)]
- In the third set of brackets, the text option "collected" is selected. [Performed/Collected]
-
- Create custom domains based on one of the General Observation Classes in the CDASH Model.
The CDASHIG Metadata Table attributes provide building blocks for the development of a case report form and the underlying database or other data collection structure.
3 General Assumptions for Implementing CDASH
3.1 How CDASH and SDTM Work Together
- The Study Data Tabulation Model (SDTM) and the SDTM Implementation Guide (SDTMIG) provide a standard for the submission of data. CDASH is earlier in the data flow and defines a basic set of data collection fields that are expected to be present on the majority of CRFs. SDTM and CDASH are clearly related. The use of CDASH data collection fields and variables is intended to facilitate mapping to the SDTM structure. When the data are identical between the two standards, the SDTMIG variable names are presented in the CDASHIG Metadata Table and should be used to collect the data. In cases where the data are not identical or do not exist in the SDTMIG, CDASH has created standardized data collection variable names.
- The CDASHIG v2.0 content is based on the SDTMIG Version 3.2.
- All SDTMIG "Required" variables have been addressed either directly through data collection or by determining what needs to be collected to derive the SDTMIG variable. In some cases, SDTMIG variable values can be obtained from data sources other than the CRF or are populated during the preparation of the submission datasets (e.g., --SEQ values).
- CDASHIG Domains contain variables that may be used in the creation of the RELREC submission dataset. For example: CDASHIG variable CMAENO: "What [is/was] the identifier for the adverse event(s) related to this (concomitant) [medication/therapy]?" may be used to identify a relationship between records in the CM dataset and records in the AE dataset.
- The CDASH standard also includes some data collection fields that are not included in the SDTMIG (e.g., "Were any adverse events experienced?" or "Were any (concomitant) [medication(s)/therapy(ies)] taken?"). These data collection fields are intended to assist in the cleaning of data and in confirming that no data are unintentionally missing. To facilitate the use of these types of fields, variable names are provided (e.g., AEYN, CMYN) in the CDASHIG Metadata Table to denote that they are data collection variables, but the SDTMIG Variable Name column is listed as N/A, and the Mapping Instruction column indicates that these CDASHIG variables are not included in the SDTM datasets.
- The CDASHIG Findings domain (e.g., Drug Accountability (DA), ECG Test Results (EG) and Vital Signs (VS)) tables are presented in a structure that is similar to the SDTMIG, which is to list the variable names and some examples of the tests. Implementers are expected to include protocol-specific tests in a CRF presentation layout, using the appropriate values from the relevant CDISC Controlled Terminology codelists. For example, VSTEST values are used to name the test on the CRF, and the corresponding test code is determined from the VSTESTCD codelist. Implementers may use synonyms when the xxTEST values are long or not commonly recognized (e.g., ALT in place of Alanine Aminotransferase). Implementers should use the CDASHIG recommendations to identify the types of data to collect while referring to the SDTMIG and CDISC Controlled Terminology for additional metadata, (e.g., labels, data type, controlled terminology).
- The CDASH standard has intentionally not reproduced other sections of the SDTM standard and implementers are asked to refer to the SDTM Model and SDTMIG on the CDISC website for additional information (http://www.cdisc.org/sdtm).
- The CDASHIG data collection fields included in the CDASHIG Metadata Table are the most commonly used and should be easily identified by most implementers. Additional data collection fields may be necessary to capture therapeutic area (TA) specific data points as well as other data specified in the clinical study protocol or for local regulatory requirements. Reference the CDASH Model and CDISC Therapeutic Area User Guides for additional information.
- Use the CDASH recommendations to develop company standards, taking into consideration the stage of clinical development and the individual therapeutic area requirements. To gain the greatest benefit from using the CDASH standard, CRFs should not be developed on a trial-by-trial basis within the implementer organization, but rather be brought into a study from a library of approved CRFs based on the CDASH Model and Implementation Guide, whenever practicable.
- The CDASHIG is divided into sections of similar types of data and the CDASHIG Metadata Table is arranged in alphabetical order (by domain abbreviation) within their respective general observation class. CRF layout was not within the original scope of the CDASH project; however, to assist with standardization of CRF layout, data collection fields are presented within the CDASHIG Metadata Table in a logical order, and annotated example CRFs have been provided (if available). In addition, implementers are referred to Best Practice for Creating Data Collection Instruments (Best Practice Recommendations), for a discussion on best practices for ordering fields on a case report form.
3.2 Core Designations for Basic Data Collection Fields
In order to facilitate classification of the different types of data collection fields, the following categories were used:
- Highly Recommended (HR): A data collection field that should always be on the CRF (e.g., the data are needed to meet a regulatory requirement or are required to create a meaningful dataset).
- Recommended/Conditional (R/C): A data collection field that should be on a CRF based on certain conditions (e.g., complete date of birth is preferred, but may not be allowed in some regions; AE time should be captured only if there is another data point with which to compare it). For any recommended/conditional fields, the "condition" is described in the "Implementation Notes" portion of the CDASHIG Metadata Table.
- Optional (O): A data collection field that is available for use.
3.3 Additional Information about CDASHIG Core Designations
The CDASH team initially considered utilizing the SDTMIG Core Designations of Required, Expected, and Permissible to capitalize on prior understanding of these descriptive designations as well as to enable a consistent categorization across CDASH and SDTM standards. Yet, when the CDASHIG Metadata Table was constructed, it quickly became apparent that CDASHIG core designations would often differ from SDTMIG core designations due to the inherent differences between the manner in which data are collected (to ensure the most accurate data) and the structure in which data are reported and submitted. For example, a variable categorized as Required in the SDTMIG may not be required in the CDASHIG if it can be derived in the SDTM datasets (rather than be a field captured explicitly on a CRF). Also, the SDTMIG core designation of "Required" imposes a rule that the variable cannot be null. CDASHIG Core designations are not intended to impose any rules that require a field to be populated with data. They are only intended to designate which fields should be present on the CRF.
3.4 How to Create New Data Collection Fields When No CDASHIG Field Has Been Defined
Adding new collection fields is often constrained by business rules, as well as by clinical data standards SMEs, clinical data management processes, and EDC systems. The naming conventions and other variable creation recommendations in CDASHIG are designed to allow collection of data regardless of subsequent inclusion in SDTM, as well as to consistently facilitate transforming the collected data into submission datasets.
Prior to adding any new fields to current domain models, the CDASH Model should be reviewed to see if there is a root field that will work for the data collection need.
New data collection fields (not already defined in a CDASH Model) will fall under one of following categories.
- Fields used for data cleaning purposes only and not submitted in SDTM datasets (e.g., --YN). The field --YN with Question Text "Were there any [interventions/events/findings]?" can be added to a domain for this purpose. Replace the two dashes (--) with the two-character domain code, and create the Question Text or Prompt using generic Question Text or Prompt from the CDASH Model as a base. Always create custom data cleaning/operational variables using consistent naming conventions.
- Fields with a direct mapping to an SDTMIG variable. If a value can be collected exactly as it will be reported in the SDTM dataset (i.e., same value, same datatype, same meaning, same Controlled Terminology), the SDTMIG variable name should be used as part of the data collection variable name in the operational database to streamline the mapping process. Any collection variable whose meaning is the same as an SDTMIG variable should be a copy of the SDTMIG variable, and the meaning should not be modified for data collection.
- Fields without a direct one-to-one mapping to SDTM datasets. If a study requires a field that is not identical to an SDTMIG field, for example the collected data type is different from the data type in the corresponding SDTMIG variable, or the SDTMIG variable is derived from collected data, the operational database should use a variable with a different name from the SDTMIG variable into which it will be mapped.
- Example 1: A study collects Findings data in a denormalized format and then maps the data to the normalized SDTM structure. The --TESTCD values can be used as the CDASHIG variable names, and the corresponding --TEST value can be used as the prompt on the CRF (See Section 8.3 General CDASH Assumptions for Findings Domains for more information).
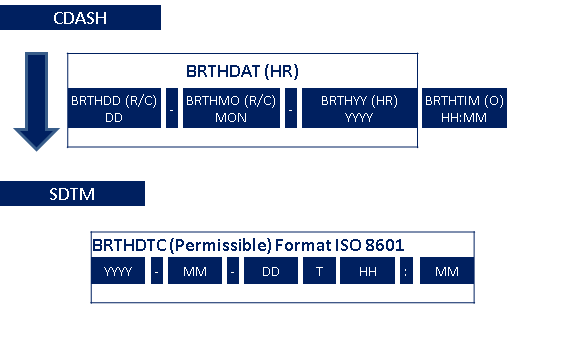
- Example 2: Dates and times are collected in a local format, familiar to the CRF users, and then reported in the SDTM-specified ISO 8601 format. In the operational database, the CDASH variables --DAT and --TIM (if collected) map into the single SDTM variable (--DTC).
- Example 3: If the mapping to SDTM is similar, but not direct, "C" can be included before the root variable name to indicate a "collected" version of the variable to which that data will map.
For example, if an injection is to be administered to a subject's LEFT THIGH, RIGHT THIGH, LEFT ARM, or RIGHT ARM, the sponsor may create the variable EXCLOC. The SDTM mapping would split these into EXLOC and EXLAT. That would avoid having to split the collection of the data into two fields on the CRF.
3.5 Explanation of Table Headers in the CDASH Model and CDASHIG Metadata Table
3.5.1 CDASH Model
This section provides an explanation of the columns used in the CDASH Model.
- Observation Class: This column contains the SDTM Class for the domain.
- Domain: This column contains the two-letter domain code.
- CDASH Variable: This column provides the CDASH root variable names (e.g., --ONGO, --DAT).
- CDASH Variable Label: This column contains a suggested root variable label that that may be used for the CDASHIG variable.
- Draft CDASH Definition: This column provides a draft definition of the root variable. This text may or may not mirror any text in the SDTM. Currently, there is a new CDASH/SDTM team creating variable definitions. Once these definitions are finalized, the CDASH definitions will be updated to harmonize with them.
- Question Text: This column in the CDASH Model contains the recommended question text for the data collection field. Question Text is a complete sentence. Some text is presented inside brackets [ ] or parentheses (). The text inside the brackets should be replaced with protocol-specified verbiage. The text inside the parentheses is optional. Text separated with a forward slash indicates optional wording from which the sponsor may choose.
- Prompt: This column in the CDASH Model contains the recommended prompt text for the data collection field. The Prompt is a short version of the question. Some text is presented inside brackets [ ] or parentheses (). The text inside the brackets should be replaced with protocol-specified verbiage. The text inside the parentheses is optional. Text separated with a forward slash indicates optional wording from which the sponsor may choose.
- Data Type: This column contains the simple data type of the CDASH variable (e.g., Char, Num, Date, or Time).
- SDTM Target: This column provides the suggested mapping to the SDTM root variable. When no direct mapping to an SDTM root variable is available, the column contains "N/A". When the column contains "SUPP--.QNAM", it means that the value represented in the CDASH variable shall be mapped to an SDTM Supplemental Qualifier. NOTE: CDASH variables noted as not having a direct map to SDTM variables (i.e., non standard variables) may have SDTM variable equivalents in future versions.
- Mapping Instructions: This column contains information on the suggested mapping of the root variable to the SDTM variable.
- Controlled Terminology Codelist Name: This column contains the Controlled Terminology (CT) codelist name {e.g., (LOC)} that is associated with the field. Certain variables (e.g., dates) use ISO formats as CT, however in CDASH these variables are generally not collected using the ISO CT. These variables are converted to the ISO format when the SDTM-based submission datasets are created.
- Implementation Notes: This column contains further information, such as rationale and implementation instructions, on how to implement the CRF data collection fields and how to map CDASH variables to SDTM variables.
Note: When multiple options are contained in a single cell, the options are separated by a semicolon.
3.5.2 CDASHIG Metadata Table
This section provides an explanation of the columns used in the CDASHIG Metadata Table.
- Observation Class: This column contains the SDTM Class for the domain.
- Domain: This column contains the two-letter domain code.
- Data Collection Scenario: This column in the CDASHIG Metadata Table identifies the different data collection options in CDASH for the same domain and is best used for filtering the table. The information in this column provides the context for the CDASHIG Core Designations, e.g., denoting which fields should be present on the CRF. When only one data collection scenario is provided for the domain, the column contains "N/A".
- Implementation Options: When this column contains "Horizontal-Generic", a sampling of the CDASHIG metadata is provided as a template for the metadata of the CRF in a denormalized structure. When this column contains "Horizontal-Example", it represents a specific execution of CRF metadata in a denormalized structure.
- Order Number: This column provides a suggested order of CDASHIG variables to be displayed on a CRF.
- CDASHIG Variable: This column provides the CDASHIG variable names (e.g., CMONGO, AEDAT).
- CDASHIG Variable Label: This column provides the CDASHIG variable label.
- Draft CDASHIG Definition: This column provides a draft definition of the CDASHIG variable. This text may or may not mirror any text in the SDTMIG. Currently, there is a new CDASH/SDTM team creating variable definitions. Once these definitions are finalized, the CDASH definitions will be updated to harmonize with them.
- Question Text: This column in the CDASHIG Metadata Table provides the suggested text for the specific Domain. Implementers should refer to the CDASH Model to create alternative question text that may be used that meets the CDASH conformance rules. Question Text is a complete sentence. Some text is presented inside brackets [ ] or parentheses (). The text inside the brackets should be replaced with protocol-specified verbiage. The text inside the parentheses is optional. Text separated with a forward slash indicates optional wording from which the sponsor may choose.
- Prompt: This column in the CDASHIG Metadata Table provides the suggested text for the specific Domain. Implementers should refer to the CDASH Model to create alternative prompt text that may be used that meets the CDASH conformance rules The Prompt is a short version of the question. Some text is presented inside brackets [ ] or parentheses (). The text inside the brackets should be replaced with protocol-specified verbiage.
- Data Type: This column contains the simple data type of the CDASH variable (e.g., Char, Num, Date, or Time).
- CDASHIG Core: This column contains the CDASHIG core designations for basic data collection fields (i.e., Highly Recommended (HR), Recommended/Conditional (R/C), Optional (O)). See Section 3.3 for definitions of CDASH core designations.
- Case Report Form Completion Instructions: This column contains recommended example instructions for the clinical site on how to enter collected information on the CRF.
- SDTMIG Target: This column provides the suggested mapping to the SDTMIG variable name. It may help facilitate the creation of the SDTMIG variables needed for submission. When no direct mapping to an SDTMIG variable is available the column contains "N/A". When the column contains "SUPP--.QNAM", it means that that the value represented in the CDASH field shall be mapped to an SDTM Supplemental Qualifier. NOTE: CDASHIG variables noted as not having a direct map to SDTMIG variables (i.e., non standard variables) may have SDTM variable equivalents in future versions.
- Mapping Instructions: This column contains information on the suggested mapping of the CDASHIG variable to the SDTMIG variable. Mapping instructions in the CDASHIG Metadata Table provide more complete guidance than those present in the CDASH Model. When domain level metadata are not available, consult the Model for SDTM Mapping Instructions.
- SDTMIG Core: This column contains the SDTMIG core designations (i.e., Required (Req), Expected (Exp), and Permissible (Perm)). The CDASHIG core designations differ from SDTMIG core designations. A Required variable in the SDTMIG may not be Highly Recommended in the CDASHIG.
- Controlled Terminology Codelist Name: This column contains the Controlled Terminology (CT) codelist name {e.g., (LOC)} that is associated with the field. The SDTMIG indicates that certain variables (e.g., dates) use ISO formats as CT, however in CDASH these variables are generally not collected using the ISO CT. These variables are converted to the ISO format when the SDTM-based submission datasets are created.
- Subset Controlled Terminology/CDASH Codelist Name: This column contains the CDISC Controlled Terminology or CDASH Subset Codelist name that may be used for that specific variable (e.g., EXDOSFRM).
- Implementation Notes: This column contains further information, such as rationale and implementation instructions, on how to implement the CRF data collection fields and how to map CDASHIG variables to SDTMIG variables.
Note: When multiple options are contained in a single cell, the options are separated by a semicolon.
3.6 Collection of Dates
Collection of Dates: Collect dates in such a way to allow the sites to record only the precision they know. The system should also store only the collected precision. Any incomplete dates must remain incomplete with no imputation and no "zero-filling" of missing components.
Data collection and database processes should allow for the possibility of partial dates and times, since a partial date may be the most precise information that can be collected for some data. An example of when it may be necessary or appropriate to collect partial dates is found in the DM domain. In some countries, collection of a complete date of birth is restricted under privacy rules, so only a year, or year and month of birth might be collected. Other examples of commonly collected partial dates are found in CM and MH, where the subject may not remember the complete date of when they started to take a medication or when a significant medical history condition began.
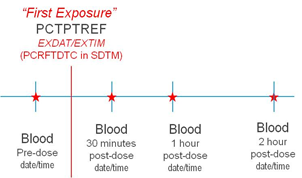
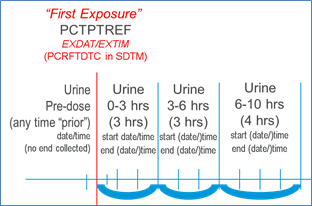
If a full date is collected, the CDASH variable --DAT or all three date components (--DATYY, --DATMO, and --DATDD) should be included on the collection tool. If a partial date can be collected in a single field, the CDASH --DAT should be used. If a partial date must be collected as separate database fields to collect year, month and day, refer to the CDASH Model for examples of standard naming fragments (--YY, --MO, --DD, --TIM). The capabilities of individual software systems (e.g., EDC) will determine which variable names are needed. CDASH uses separate data collection fields for dates and times. If times are collected, it is expected that they will be used with the appropriate collected date to derive the related SDTM date variable in ISO8601 format.
Conversion of Dates for Submission: See section SDTMIG v3.2 Sections 4.1.4.1 and 4.1.4.2 for detailed information about converting dates and times from the collection format to the submission format using ISO 8601. A specific example of mapping birth date is shown here.
The SDTM date format allows this partial date to be submitted so the reviewer can see what was collected.
Imputation of Dates: If the missing parts of the date are imputed for analysis purposes, the imputed dates will be generated in the analysis data (ADaM) but not in the SDTM submission data sets.
3.7 Mapping Relative Times from Collection to Submissions
Relative Timing variables are sets of variables that provide information about how the timing of the record relates to either the study reference period or another fixed point in time. The CDASH Relative Timing variables are collected for those observations where a date is either not collected or is not available. The CDASH set of variables serve as an indicator (or flag) that the observation's "start" was "prior" to the study reference period or prior to another fixed point in time OR that the observation's "end" was "after" or "ongoing" as of the study reference period or another fixed point in time. The CDASH variables of --PRIOR and --ONGO serve this purpose. How these CDASH "flags" are translated to SDTM (according to controlled terminology) depends on whether the comparison is against the protocol-defined study reference period or against another fixed point in time that may serve as the "reference" for the timing of the record. To emphasize, the collection of these CDASH relative timing variables are always dependent on the actual date either being prospectively "not collected" or not available. Much more information can be found under the "General Assumptions" for both Interventions and Events domains.
For all SDTM submissions, there is a defined period during which the subject is considered to be "on study". According to the SDTMIG v3.2, the start and end dates of the "on study" period are submitted in the variables RFSTDTC and RFENDTC. The defined period may be protocol-specific or set by company policy, SOPs, or other documented procedures. The "on study" period might be defined as being from the date/time of Informed Consent through the date/time of subject's completion of the study, or it might be from the date/time of first dose to the date/time of last dose. Regardless of how the "on study" period is defined, the dates (and optionally times) of the start and end of that period must be collected.
If there is a need to collect information about whether an observation of interest occurred prior to a reference point or milestone other than the beginning of the study, or was ongoing or continuing at some reference point or milestone in the study other than the end of the defined "on study" period, the date/time of that reference point or milestone should also be collected. If this date/time has been collected, reasonable comparisons can be made to that date/time with "prior", "coincident", "continuing", or "ongoing" questions.
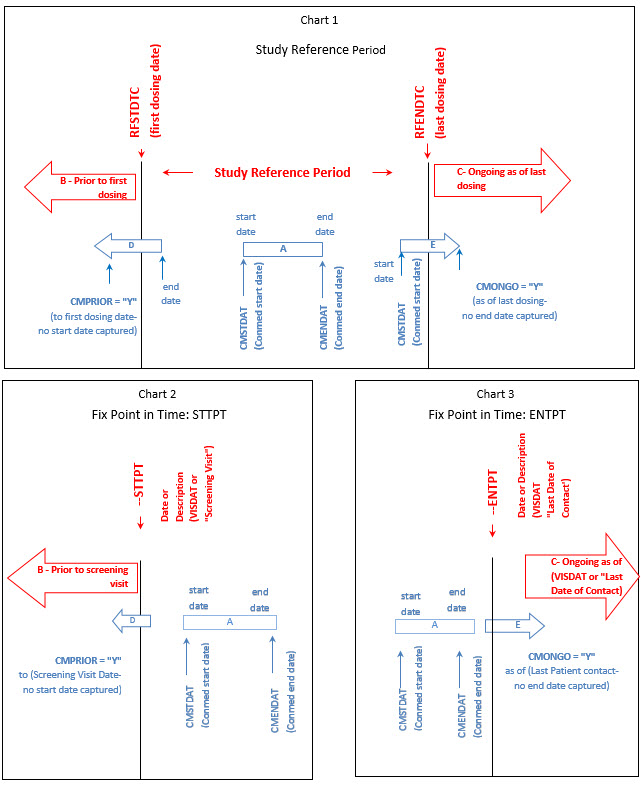
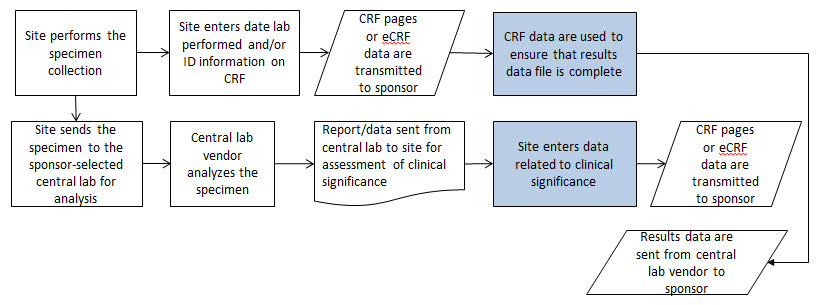
The following steps should be taken to ensure observations of interest that occur over time can be related to the study period or to fixed point in time or a milestone in a meaningful way. Chart 1 below provides a representation of an intervention as it relates to the study reference period, and Charts 2 and 3 provide a representation of comparisons as it relates to other fixed points in time or a milestone.
Study Reference Period (Chart 1)
-
Define the "on study" period.(B-C). Once the overall "on study" period has been defined (B-C), collect the dates (/times) of the start of the study reference period (e.g., date of informed consent, date of first dose) and end of the study reference period (e.g., date of last contact, date of last dose), as part of the clinical data with their respective domains (e.g., DS, EX). These dates will map into the RFSTDTC (B) (start of Study Reference Period) and RFENDTC (C) (end of Study Reference Period) variables in the SDTMIG DM dataset.
- Collected comparisons ((D, E) using CDASHIG variables (e.g., "prior", "ongoing") of when something started or ended in relation to the "on study" reference period (i.e., RFSTDTC-RFENDTC: (B-C). These CDASH variables are used to populate the SDTMIG variables--STRF and --ENRF variables when the SDTM-based datasets are created. (Note: these relative timing variables are only populated in the SDTM -based datasets when a date is not collected.).
Fix point in Time/Milestone (Charts 2, 3)
- Define the fix point in time or milestone (B or C).The fix point in time or milestone can be a date or a description. This will map into either the SDTMIG variables (–STTPT or --ENTPT) when the SDTM-based datasets are created .
- Collected comparisons (D or E) using CDASHIG variables (e.g., "prior", "ongoing") of when something started or ended in relation to the fixed point in time or milestone (B or C). These CDASH variables are used to populate the SDTMIG variables--STRTPT or --ENRTPT when the SDTM-based datasets are created.(Note: these relative timing variables are only populated in the SDTM -based datasets when a date is not collected).
For information about mapping what is collected in "prior", "ongoing", and "continuing" fields into the appropriate SDTMIG variables, see the SDTMIG V3.2 Section 4.1.4.7.
3.8 CDISC Controlled Terminology
Submission data standards are required by some global regulators, and controlled terminology (CT) is part of the requirement. Using CT from the start during data collection builds in traceability and transparency, and reduces the problems associated with trying to convert legacy codelists and variables to the submission standards. CT can be used in the following ways during data collection:
- To collect data using a standardized list of values (e.g., Mild, Moderate, Severe)
- To ask a specific question on the CRF (e.g., Temperature)
- To create a variable name in the database (e.g., TEMP for the collection of vital sign data when a unique variable name must be created for each vital sign result.)
Terminology applicable to CDASH data collection fields is either in production or under development by the CDISC Terminology Team. Production terminology is published by the National Cancer Institute's Enterprise Vocabulary Services (NCI EVS) and can be accessed via the following link: http://www.cancer.gov/cancertopics/terminologyresources/CDISC. The examples in this document use CDISC controlled terminology where possible, but some values that seem to be controlled terminology may still be under development at the time of publication, or even especially plausible "best guess" placeholder values. Do not rely on any source other than the CDISC value set in the NCI Thesaurus for controlled terminology.
In some cases it is more appropriate to use a subset of a published SDTM terminology list, rather than the entire list. To begin with an established subset of the SDTM terminology, go to https://www.cancer.gov/research/resources/terminology/cdisc and reference the CDASH terminology. These CDASH codelists have been subsetted from the complete SDTM terminology lists and are available to implementers as a way to quickly set up codelists for data collection. However, most implementers will also need to review the SDTM terminology to determine which other values are needed for their particular implementation. The CDASH terminology subset names are provided in the CDASHIG Metadata Table for easy reference.
Some codelists, such as Laboratory Test Codes (LBTESTCD), are extensible. This means that values that are not already represented in that list (either as a CDISC Submission Value, a synonym or an NCI preferred term) may be added as needed. Other codelists, such as AE Action Taken with Study Treatment, are non-extensible and must be used without adding any terms to the list. If gaps are indentified, sponsors should submit requests to add values to controlled terminology by using the New Term Request form found at http://ncitermform.nci.nih.gov/ncitermform/?version=cdisc.
In cases where a CDASH/CDASHIG variable has associated controlled terminology, the codelist is referenced in Controlled Terminology column in the CDASH Model and CDASHIG Metadata Table in this format: (codelist name).
4 Best Practice Recommendations
CDASH Best Practices describe operational recommendations to support data collection, suggested CRF development workflow, and methods for creating data collections instruments. The first section, Best Practices for Creating Data Collection Instruments, is part of conformance to the CDASH Standard.
4.1 Best Practices for Creating Data Collection Instruments
| Num | Best Practice Recommendation | Rationale |
| 1 |
When a binary response is expected, "Yes/No" responses are preferred over "Check all that apply", because a missing response could lead to a misinterpretation of critical data. For example, if AEs are determined to be serious based only upon checking the applicable serious criteria (e.g., Hospitalization, Congenital Anomaly, etc) failure to check a criterion would potentially delay identification of an SAE. If an assessment has composite responses (e.g., presence or absence of two or more symptoms), "Yes/No" questions for each component response (e.g., symptom) are preferred to "Check all that apply" questions. One exception to this recommendation might be assessments where the majority of options would be answered "No". An example would be the collection of ECG abnormality data where approximately 45 abnormalities may be listed, but only a few will apply. Another exception is when a validated instrument contains checkboxes. In this case, they should remain checkboxes in the CRF or eCRF. Another exception to this recommendation is when there are controlled terminologies governing the values being collected. For example, if collecting RACE using the "check all that apply" option, the RACE values defined by Controlled Terminology should be collected as individual check boxes, and not as a Yes/No response. In cases where the sponsor chooses to use "check all that apply" additional quality checks should be considered (e.g., SDV) to ensure the data collected in the CRF are correct and complete. |
"Yes/No" questions provide a definite answer. The absence of a response is ambiguous as it can mean "No", "None", or that the response is missing. In situations where there is no other dependent or related field by which to gauge the completeness of the field in question, a "Yes/No" response ensures that the data are complete. For example, when an AE End Date is blank, a "Yes" response to the question "Is the AE ongoing?" ensures that the data are complete. When the end date is provided it is not necessary to answer the question "No". |
| 2 |
The database should contain an indication that a planned exam/assessment was not performed. The mechanism for this may be different from system to system or from paper to EDC. For example, the data collection instrument/CRF could contain a field that allows the site to record an indication that a Vital Sign assessment was not performed (e.g., VSPERF = "N" or TEMP_VSSTAT = "NOT DONE") A "'Yes/No' – assessment completed" question is preferred over the "Check if not done" box, unless the "Check if not done" field can be compared to a completed data field using a validation check to confirm that one or the other has data. |
This will provide a definitive indicator that a data field has missing data and has not been overlooked. This will prevent unnecessary data queries to clarify whether an assessment has been performed. The use of the "Yes/No" format helps to eliminate ambiguity about whether an assessment has been completed. In situations where there is no other dependent or related field to gauge the completeness of the field in question, a "Yes/No" response format should be used to eliminate ambiguity. When another related field is present the "Yes/No" response is optional. For example, when a value for temperature is missing, a simple "Not Done" box may be checked. It is not necessary to respond "Done" when a temperature value is present. |
| 3 |
Data cleaning prompts should be used to confirm that blank CRFs are intentionally blank. Usually this will be a "Yes/No" question (e.g., AEYN) but it may be a "check if blank" if a validation check can be used to confirm that either the "check if blank" box is checked, or that there are data recorded in the CRF. |
This will provide a definitive indicator that a CRF is blank on purpose and has not been overlooked. This will prevent unnecessary data queries. |
| 4 | The same data (i.e., the same information at the same time) should not be collected more than once. |
Collecting the same data more than once:
|
| 5 |
A "Check if ongoing" question is recommended to confirm ongoing against an end date. This is a special use case of "Yes/No" where the data entry field may be presented as a single possible response of "Yes" in conjunction with an End Date variable. If the box is checked, the operational variable may contain "Yes". If the box is not checked and the End Date is populated, the value of the variable may be set to "No". For some EDC systems, it may be better to display the possible responses to the "check if ongoing" question as radio buttons. Conditional logic can then be used to solicit the collection of the end date only if the answer to the "Ongoing" question is "N" (No). | For the use case of "Check if ongoing", for the data to be considered "clean", one of the two responses must be present and the other response must be blank. So, the presence of the end date provides confirmation that the event is not ongoing. |
| 6 |
CRFs should use a consistent order of responses (e.g., "Yes/No") from question to question, for questions with response boxes or other standardized lists of values. Exceptions to this would be cases where a validated instrument (e.g., a standardized assessment questionnaire) is used. | A consistent order of response boxes promotes ease of use of the CRF to help reduce data entry errors and to avoid introducing bias or leading the investigator to a desired response. |
| 7 | CRF questions and completion instructions should be unambiguous, and should not "lead" the site to answer the question in a particular way. | Data should be collected in a way that does not introduce bias or errors into the study data. Questions should be clear and unambiguous. This includes making sure that the options for answering the question are complete such as providing options for "other" and "none" when applicable. |
| 8 |
Collection of dates should use an unambiguous format, such as DD-MON-YYYY, where each part of the date is a unique format: "DD" is the day as a 2-digit numeric value; "MON" is the month as a 3-character letter abbreviation in English, or similar character abbreviation or representation in the local language; and "YYYY" is the year as a 4-digit numeric value. For electronic data capture (EDC), the user may be able to select a date from a calendar, and this would also meet the recommendation for an unambiguous date. If the recommended approach is not adaptable to the local language, a similarly unambiguous format should be used. The method for capturing date values should allow the collection of partial dates, and should use a consistent method or convention for collecting the known date parts to facilitate standard mapping to SDTM. Reference the CDASH Model for standard date variable names. |
Using this data collection format (i.e., DD-MON-YYYY) will provide unambiguous dates. For example, the date "06/08/02" is ambiguous because it can be interpreted as "June 8, 2002" or "August 6, 2002". If subject-completed CRF pages are translated into a local language, the CDASH recommended date format for collection may make translation of the documents easier. Dates are collected in this format, but reformatted and submitted in ISO 8601 format. See the SDTMIG and CDASHIG Section 3.6 for more information about the ISO 8601 format. |
| 9 |
To eliminate ambiguity, times should be collected with the use of a 24-hour clock, using the HH:MM:SS format for recording times. Use only as many of the HH:MM:SS elements as are needed for a particular field. Sites should be cautioned not to "zero-fill" the time components if they are not known (for example 21:00:00 means "exactly 9 PM", but if the site did not know how many seconds after 9 PM, they should not record the seconds). Subject-completed times may be recorded using a 12-hour clock and an A.M. or P.M. designation. The time would then be transformed to a 24-hour clock in the database. | When times are collected using a 24-hour clock, this eliminates ambiguity and eliminates the need to convert the values from 12-hour to 24-hour clock time when they are converted to ISO 8601 date/time for the SDTM-based datasets. |
| 10 |
Manually calculated fields should not typically be recorded within the CRF when the raw data on which the calculation is based are recorded in the CRF. An exception is when a treatment and/or study conduct decision should be made based on those calculations. In those cases it may be useful for the calculated field to be recorded within the CRF. It may also be useful to provide the site a step-by-step worksheet to calculate this data. |
Data items that can be calculated from other data captured within the CRF are more accurately reported if they are calculated programmatically using validated algorithms. The noted exception may be in cases where it's important to show how the investigator determined a protocol-defined endpoint from collected raw data. |
| 11 |
Questions with free text responses should be limited to cases of specific safety or therapeutic need in reporting or analysis, such as Adverse Events, Concomitant Medications, or Medical History, generally in cases where the data will be subsequently coded. Questions should be specific and clear rather than open-ended. Instead of free text comment fields, a thorough review of the CRF by the protocol development team should be performed to maximize the use of pre-defined lists of responses. Refer to the Comments Domain for additional recommendations. |
The collection and processing of free text requires significant resources for data entry: It requires CDM resources to review the text for safety information and for inconsistencies with other recorded data and is of limited use when analyzing and reporting clinical data. Another risk is that sites may enter data into free text fields that should be recorded elsewhere. |
| 12 | Subject-specific data should be collected and recorded by the site and should not be pre-populated in the CRF/eCRF. | The CRF is a tool to collect subject-level data. However, pre-population of some identifying (e.g., investigator name, site identification, protocol number) or timing (e.g., Visit Name) information prevents errors and reduces data entry time.
Fields on the CRF or in the database that are known to be the same for all subjects may be pre-populated (e.g., measurements for which there is only one possible unit, such as Respiratory Rate or Blood Pressure). The units can be displayed on the CRF and populated in the database |
| 13 | The anatomical location of a measurement, position of subject, or method of measurement should be collected only if the protocol specifies the allowable options, or if the parameter is relevant to the consistency or meaning of the resulting data. |
When a parameter, such as location, position, or method, is specified in a protocol and is part of the analysis, the CRF may include the common options for these parameters to ensure the site can report what actually happened and protocol deviations can be identified. If the parameter is pre-populated on the CRF and other options are not available, then the site should be directed to not record data that was not collected per protocol specifications. Taking measurements in multiple anatomical locations may impact the value of the measurement and/or the ability to analyze the data in a meaningful way (e.g., when data obtained from different locations may bias or skew the analysis). In this case, collecting the location may be necessary to ensure consistent readings. For example, temperature obtained from the ear, mouth, or skin may yield different results. If there is no such rationale for collecting location, position, method, or any other value, it would be considered unnecessary data. Reference Section 4.3, Organizational Best Practices to Support Data Collection, Num 1. |
| 14 | Sites should record verbatim terms for non-solicited adverse events, concomitant medications, or medical history reported terms. Sites should not be asked to select a preferred term from a coding dictionary as a mechanism for recording data. |
When the site records information about spontaneously reported adverse events or medical history, it is best to ask the sites to record responses verbatim, so that no information is omitted. Sites are not expected to be coding experts and are probably not familiar with the coding dictionaries used in clinical research. Having the sites record adverse events from a standardized list is the same as having them code these events. Having multiple sites "coding" data will result in inconsistencies in the coding across sites. See Section 6, Other Information, for more information about collecting data for coding purposes. |
| 15 |
CRF questions should be as self-explanatory as possible, thereby reducing the need for separate instructions. If required, short instructions may be placed on the CRF page, especially if the Prompt is not specific enough. More detailed instructions may be presented in a CRF completion guideline. All instructions should be concise. Instructions should be standardized as much as possible. |
Putting short instructions and prompts on the CRF increases the probability that they will be read and followed, and can reduce the number of queries and the overall data cleaning costs. Having standard instructions supports all sites using the same conventions for completing the fields. Providing short instructions and prompts on the CRF and moving long instructions to a separate instruction booklet, facing page, or checklist will decrease the number of CRF pages, with the following benefits:
|
| 16 | An SDTMIG variable name should only be used as a data collection/operational variable name if the collected value will directly populate the SDTMIG variable with no transformation (other than changing case). Otherwise, create a "collected" version of the variable and write a standard mapping to the SDTMIG variable. | This will provide clearer traceability from data collection to submission, and will facilitate a more automated process of transforming collected data to the standardized data tabulations for submission. |
4.2 CRF Design Best Practices
The recommendations are general principles that may be implemented during CRF form design and/or database set up in different ways, depending on the systems used.
Providing the clinical site with a consistent and clinically logical order of these fields will reduce data entry time and result in more reliable data. Therefore, the CRF should be quick and easy for site personnel to complete.
Clinical Operations staff should review the CRF for compatibility with common site workflow and site procedures.
| Num | Best Practice Recommendation |
| 1 | Headings – Place fields that routinely appear on multiple forms at the top of the form. For example, if the collection date and time are both asked, they should appear first and second, respectively, on each form where they are used. |
| 2 | Clinical flow – Fields should be placed on the form in the order that they are expected to be collected during the clinical assessment. It is acceptable to include fields from different domains on the same form if consistent with the clinical flow. |
| 3 | Group related fields for a single clinical encounter together, although multiple time points or visits may appear together on one form. For example, if heart rate and temperature are taken every hour for four hours on Study Day 1, the form can collect the data for Hour 1 (e.g., heart rate result and unit, temperature result and unit), followed by the data for Hour 2, Hour 3, and Hour 4. In this scenario, there would be labels indicating each time point within Study Day 1. |
| 4 |
Group related fields together. Test results and their associated units should always appear next to each other. For example, the results of the "TEMP" should be followed by its allowable units of "F" and "C". In some cases, the result might have only one applicable unit. For example, the only applicable unit for "PULSE" is "beats/min". The unit should be displayed on the CRF and databased. |
| 5 |
Data fields that are dependent on other data fields should be placed in the CRF in such a way that this dependence is obvious. For example, if there is a question in a paper CRF where "Other, specify" is an option, the text box used to collect what is being specified should be placed in proximity to the "Other" question to indicate that it is a sub-part of the "Other" question. An example of this in an EDC system that requires a specific response in order to render one or more additional, related questions. |
| 6 | Lists of values that have a logical order should be provided on the CRF in that logical order. For example, the values of "Low", "Medium", and "High" are logically placed in this order. Do not list "Medium" first, "Low" second, and "High" third. |
4.3 Organizational Best Practices to Support Data Collection
| Num | Best Practice Recommendation | Rationale |
| 1 |
Collect necessary data only. CRFs should focus on collecting only the data that support protocol objectives and endpoints. The protocol should clearly state which data will be collected in the study |
Usually, only data that will be used for efficacy analysis and to assess safety of the investigational product should be collected on the CRF, due to the cost and time associated with collecting and fully processing the data. However some fields on a CRF may be present to support the eDC functionality and/or review and cleaning of data through automated edit checks. The protocol (and SAP when it is prepared in conjunction with the protocol) should be reviewed to ensure that the parameters needed for analysis are collected and can be easily analyzed. The statistician is responsible for confirming that the CRF collects all of the data necessary to support the analysis. |
| 2 |
CRF development should be a controlled, documented process that incorporates (as applicable):
CRF development should be controlled by SOPs covering these topics, as well as site training. | A controlled process for developing CRFs will help ensure that CRFs comply with company standards and processes. |
| 3 |
The CRF design process should include adequate review and approval steps, and each reviewer should be informed on the scope of the review they are expected to provide. The team that designs the data collection instruments for a study should be involved in the development of the protocol and should have appropriate expertise represented on the CRF design team, including the following: Medical and Scientific Experts should provide sufficient information to ensure Clinical Data Standards, Standards Subject Matter Experts, and Clinical Data Management (CDM) staff understand the background, context, and medical relevance of the efficacy and/or safety data. Clinical Data Management, Standards Subject Matter Experts, and CRF Designers should review the protocol to ensure that proposed data can be collected, and should ensure that appropriate standards are used to develop the CRF. Statisticians should review the CRF against their planned analyses to make sure all required data will be collected in an appropriate form for those analyses. Clinical Operations Staff should review the CRF to make sure the questions are unambiguous and that requested data can be collected. Programmers should review the CRF to ensure that the manner in which the data are collected on the CRF are consistent with relevant metadata standards. Regulatory Experts should review the CRF for compliance with all applicable regulations. Data Entry Staff should review the CRF to ensure that the data are collected in a form that can be entered accurately. Pharmacovigilance personnel should review to ensure appropriate data capture and process to support expedited reporting. Ideally, the CRF should be developed in conjunction with the protocol (and the SAP if it is available). All research-related data on the CRF should be addressed in the protocol to specify how and when it will be collected. |
Reviewers from different functions increase the probability that the CRF will be easier to complete and support the assessment of safety and efficacy as defined in the protocol and SAP. The CRF design team should ensure that the data can be collected in a manner that is consistent with the implementer's processes and easy for the site to complete. |
| 4 | Translations of CRFs into other languages should be done under a controlled process by experts who understand both the study questions and the language and culture for which the CRF is being translated. The translation should be a parallel process following the same set of steps with separate reviews and approvals by the appropriate experts. Translations may require author approval and a separate validation of the translated instrument. |
CRFs that are translated into other languages should follow the same development process as the original CRF to ensure the integrity of the data collected. Consideration of translation should be part of the CRF development process. Avoid the use of slang or other wording that would complicate or compromise translation into other languages. Cultural and language issues should be addressed appropriately during the process of translating CRFs to ensure the CRF questions have consistent meaning across languages. |
| 5 |
Data that are collected on CRFs should usually be databased. For some fields, such as "Were there any Adverse Events", the response—in this case "Yes/No"—may need to be databased, but will not be included in the submission data. Some fields, such as Investigator's Signature, can be verified by the data entry staff, but an actual signature may not be databased unless there is an e-signature. |
If certain data are not required in the CRF, but are needed to aid the investigator or monitor, those data should be recorded on a site worksheet (e.g., an entry criteria worksheet or a dose titration worksheet). All such site worksheets should be considered source documents or monitoring tools, and should be maintained at the site with the study files. |
| 6 | Establish and use standardized case report forms. |
Using data collection standards across compounds and TAs saves time and money at every step of drug development. Using standards:
|
When a binary response is expected, "Yes/No" responsesare preferred over "Check all that apply", becausea missing response could lead to a misinterpretation of critical data. For example, if AEsare determined to be serious based only upon checking the applicable serious criteria (e.g., Hospitalization, Congenital Anomaly, etc.), failure to check a criterion would delay identification of an SAE.
5 Conformance to the CDASH Standard
5.1 Conformance Rules
Conformance means that:
- Core designations must be followed: All Highly Recommended and applicable Recommended/Conditional Fields must be present in the CRF or available from the operational database.
- CDISC Controlled Terminology must be used: The CDISC Controlled Terminology that is included in the CDASHIG Metadata Table must be used to collect the data in the CRF. All codelists displayed in the CRF must use or directly map to the current published CDISC Controlled Terminology submission values, when it is available. Subsets of published Controlled Terminology, such as those provided in CDASH terminology, can be used.
- In Findings domains, values from the relevant CDISC Controlled Terminology lists must also be used to create appropriate Question Text, Prompts and/or variable names (e.g., If the question is about the subject's height, incorporate the value of "Height" from the VSTEST codelist as the Prompt on the CRF, and incorporate "HEIGHT" from VSTESTCD in the variable name).
- Best practices must be followed: The design of the CRF must follow the Best Practices for Creating Data Collection Instruments and CRF Design Best Practices.
- The wording of the CRF questions should be standardized: CDASH Question Text or Prompt must be used to ask the question.
- In cases where the data collection is done in a denormalized presentation on the CRF, the relevant CDISC controlled terminology should be used in the Question Text or Prompt as much as possible. It is acceptable to use synonym text that will directly map to one CDISC Submission Value, including an NCI Preferred Term, if the CDISC Submission Value is not appropriate for data collection. For example, "ALT" may be better than "Alanine Aminotransferase" as the prompt for this lab test. If there is no CDISC Controlled Terminology available, the Question Text or Prompt must be standardized by the implementing organization and used consistently. One of the basic purposes of CDASH is to reduce unnecessary variability between CRFs and to encourage the consistent use of variables to support semantic interoperability; therefore, Question Text and Prompt must be used verbatim.
- Similarly, where SDTMIG variables may exist in the operational database and the value conforms to Controlled Terminology, it is permissible to use a familiar synonym on the CRF without affecting conformance. An example may be on the Demographics page where SEX may be displayed as "Male" or "Female", while in the operational database, the controlled terminology values of "M" and "F" would be used.
- In some cases, CDASH Questions Text and Prompt allow for flexibility while still being considered conformant. See section 2.3 CRF Development Overview for further details on the usage of Question Text and Prompt.
- The CDASH Model Question Text may contain options for the tense, but if the option is not provided, the tense of the Question Text may be modified to reflect the needs of the study.
- For cases where the Question Text or Prompt cannot be used due to culture or language, or a CRF must be translated for language or cultural reasons, the implementer must ensure the translation is semantically consistent with the CDASH Question Text and Prompt in the CDASHIG Metadata Table.
- In cases where a more specific question needs to be asked than what is provided by Question Text or Prompt, CDASH recommends the use of a brief CRF Completion Instruction, as long as the instruction clarifies the data required by the study without altering the meaning of variable as defined by the standard. For example "Sex at birth" is not the same question as "Sex" (which is loosely defined as "reported sex").
- Variable Names: The CDASHIG variable naming conventions should be used in the operational database, using a consistent syntax that includes the root variable name and/or controlled terminology, and any other standardized concepts that are needed to support efficient mapping of the collected value to SDTM datasets. The goals are to have beginning-to-end traceability of the variable name from the data capture system to the SDTM datasets, and to support automating electronic data capture (EDC) setup and downstream processes.
- It is recognized that particularly in an EDC system, the variable name of a data collection field, as well as the name in the underlying database, may have various "system" components that become part of the item's identifier. EDC systems, prior to exporting data in a defined format, may require the variable name to include such database "references" as the EDC page name, the item "group" name, or perhaps a combination.
- In cases where the data collection is done in a denormalized way, appropriate CDISC controlled terminology must be used when it is available. For example, when collecting Vital Signs results in a denormalized eCRF, the variable names can be created by using terms from the Vital Signs Test Code codelist. For example, Temperature result can be collected in a variable called TEMP or TEMP_VSORRES, Systolic Blood Pressure result, can be collected in a variable called SYSBP or SYSBP_VSORRES. When a particular system's constraints limit the variable name to 8 characters, a similar, consistent implementation that preserves either the normalized root variable (e.g., ORRES) or the controlled terminology (e.g., --TESTCD value) should be implemented.
- Whereas all CDASHIG defined variable names are 8 characters or less to accommodate SDTM limits on variable names, QNAMs, and --TESTCDs, the maximum length of a variable name that may be implemented is determined by the data management system used, not by CDASH.
- Data Values and Format: Because an SDTM data programmer should be able to assume that data in an SDTMIG variable is SDTMIG compliant, the data output by the operational database into an SDTMIG variable ideally requires no additional processing. Minimal processing (e.g., changing case) is still conformant. This helps to ensure a quality deliverable, even if the SDTM data programmer is unfamiliar with data capture practices.
- Questionnaires: In order to maintain the validity of a validated instrument, studies that include validated questionnaires, ratings, or scales must present the questions and reply choices in the manner in which these were validated. (Reference QRS - Questionnaires, Ratings and Scales).
- In some cases, this may result in CRFs that do not conform to CDASH Best Practices; however, restructuring these questionnaires should not be done because it could invalidate them.
- The use of such questionnaires in their native format should not be considered to affect conformance to CDASH.
Implementers must determine what additional data fields to add to address study-specific and therapeutic area requirements, and applicable regulatory and business practices. Refer to CDASHIG Section 3.4 (How to Create New Data Collection Fields When No CDASHIG Field Has Been Defined) for more information on how to create data collection fields that have not already been described in this implementation guide.
6 Other Information
6.1 Form Level CRF Instructions
Whenever possible, details related to the completion of a single field should be placed with the field itself on the CRF. If this is not possible due to the medium and/or system being used to create the CRFs, then it is permissible to include the field-level instructions at the top of the form, in what is generally considered the form-level instruction area. In some cases, such as when the form-level instructions are very lengthy or include graphics or flowcharts, a separate CRF completion instruction guideline may be required.
General Content Considerations for Completion Instructions
When creating form-level instructions for a CRF, the following points should be considered:
- The instructions should include clear references to the time period for which data are to be reported for the study, or to specific time windows that are allowed.
- The instructions should provide references to protocol sections for the specifics of and/or limitations on the data to be reported.
- The instructions should include any special instructions for additional reporting or actions required beyond what is collected on the CRF.
- The instructions should include considerations on how data collected on one CRF might have an impact on data that are reported on a different CRF.
- The instructions should refer to any other forms that are related to the CRF being completed.
6.2 General Recommendations on Screen Failures
Using CDASH, the minimum data to be collected should include a subject identifier and reason(s) for screen failure. Typically, there is a reason on the End of Study form indicating "Screen Failure". This information allows overall summarization of all subjects screened/enrolled and when captured, provides easy subject accountability for the Clinical Study Report. Other data may be considered for collection, such as date of informed consent, sex, race, date of birth or age, or other data to further describe the reason for ineligibility (e.g., the lab value that was out of range).
SDTMIG does not provide a separate domain specifically for screen failure data and does not require that the screen failure data be included in SDTM. Data for screen failure subjects, if submitted, should be included in the appropriate SDTMIG domains. Refer to the SDTMIG for further guidance on submitting Screen Failure data.
6.3 Standardized Coding of Collected Data
Adverse Events, Medical History, and Prior and Concomitant Medications are often coded to standard dictionaries (thesauri). There are many coding dictionaries, but this section will focus on the Medical Dictionary for Regulatory Activities (MedDRA®) and the World Health Organization Drug Dictionary (WHO-DD) as examples, since these are common coding dictionaries.
The SDTMIG variable AEDECOD is the dictionary-derived text description of AETERM (the reported term for the adverse event) or AEMODIFY (the modified reported term). When coding with MedDRA®, the AEDECOD is the Preferred Term and is a required variable. Corresponding SDTMIG variables CMDECOD (for medications) and MHDECOD (for medical history items) are permissible SDTMIG variables. These are the equivalent of the preferred term in the dictionary used for coding, and when data are coded these SDTMIG variables should be provided.
These --DECOD variables are not necessarily collected on CRFs. They are often determined from other collected variables (e.g., AETERM, CMTRT, and MHTERM). Conventions adopted in the collection of these reported terms can have an impact on the resulting --DECOD variables. If collected on a CRF, --DECOD values would be selected from sponsor-defined or CDISC controlled terminology.
CRF Designers should consult with medical coders, review relevant documentation, and ensure that all elements needed to facilitate the coding process are collected.
Coding Adverse Events and Medical History Items
Data managers are encouraged to enter into discussion with coding specialists and medical staff to develop guidance to sites in accordance with applicable coding conventions and other company/project agreements and requirements.
Reported terms are often coded without other information for the subject. Therefore, sites should be advised that "nothing can be assumed", and that the reported term should include all information relevant to the event being reported. For example, if "Congestion" is reported as an adverse event for a particular subject, together with several other pulmonary events, the coder cannot assume that the congestion is "Lung congestion", rather than congestion of some other organ (e.g., nose, ear, etc.). The reported term "Congestion" will need to be queried before it can be coded.
Medications
With regard to medications, the CDASH standard offers some guidance on the recording of medication names and on the use of additional Recommended/Conditional data collection fields (e.g., CMROUTE, CMINDC) to facilitate coding. See CDASHIG Section 8.1.1, Assumptions for Interventions Domains.
The purpose of coding medications is usually to provide a "Standardized Medication Name" (CMDECOD) and a "Medication Class" (CMCLAS). Most dictionaries facilitate the derivation of the Standardized Medication Name on identification of the medication that was taken and the reason taken.
It would be preferable to collect all active ingredients of a particular medication. In a clinical trial, however, this is impractical. There are numerous CRF design possibilities. When designing a collection tool, it is critical to ensure that the details appropriate to the trial and the sponsor's coding requirements are included. For example, betamethasone dipropionate is used topically; however, if the site records only betamethasone (which can be administered orally, as drops, or inhaled), the topical route of the drug will be lost. In this case, collecting route of administration (CMROUTE) or the indication (CMINDC) would provide the additional information needed to code this medication.
In summary, when medications are to be coded, the indication (CMINDC) and route (CMROUTE) or anatomical location (CMLOC) should be collected along with the medication name.
7 CDASH Special-Purpose Domains
The SDTMIG includes three types of special-purpose datasets:
- Domain datasets (Demographics (DM), Comments (CO), Subject Elements (SE), and Subject Visits (SV) which contain subject-level data.
- Trial Design Model (TDM) datasets which contain trial-level data.
- Relationship datasets
These datasets are described in SDTMIG Section 2.3. CDASH does not currently contain information on Trial Design Model, or Relationship datasets. CDASH standards are for collection of subject-level data, therefore the collection of Trial Design domains is out of the scope for CDASH. CDASHIG contains information on these Special-Purpose Domains: Demographics (DM), and Comments (CO).
7.1 General CDASH Assumptions for Special-Purpose Domains
- Each study must include the Demographics domain.
- CDASH does not currently contain metadata information on SDTM Special-Purpose Domains (Subject Elements (SE) and Subjects Visits (SV)). The SDTM SE and SV domains are commonly derived/created during the SDTM dataset creation process. Each implementer has to determine the best practice within their organization for creating visits and collecting the information on any unplanned visits. See the SDTMIG Section 5 Subject Elements and Subject Visits for more information.
7.2 CO - Comments
Description/Overview for the CDASHIG CO - Comments Domain
The CDASH IG Comments (CO) domain describes free text collected alongside other data on typical case report form (CRF) pages such as Adverse Events, when there is not a specified variable for the free text. The CDASHIG CO domain has no mandatory data elements for inclusion in a separate Comments CRF, and the recommendation is to avoid the creation of a General Comments CRF.
Specification for the CDASHIG CO - Comments Domain
Comments (CO)
Assumptions for the CDASHIG CO - Comments Domain
Solicited Comments versus Unsolicited Comments
Solicited comments are defined as those comments entered in free-text data collection fields (such as "Please comment") that are intentionally included on the CRFs. These data collection fields provide the site with a pre-defined space to further explain or clarify an associated record within the CRF. For example, the Vital Signs CRF may include a solicited comment data collection field that enables recording of free text, such as "reason for performing assessment out of window".
Unsolicited comments are those comments entered outside of pre-defined data collection fields (also referred to as "marginal" comments as they are sometimes written in margins of paper CRFs). These may include marginal CRF comments written by site staff, notes written by the subject on paper diaries, or additional information collected through an eDC system function which collects comments that are not included as data collection fields on the eCRF. Although such comments may be intended to reduce queries, in practice they often lead to clinical data not being entered into the correct data collection field and may cause additional work in the data management process. The collection of unsolicited comments should be discouraged. If they are allowed, they should be reviewed and resolved during the conduct of the study.
Some unsolicited comments may be intended to avoid queries, for example "subject visit was delayed due to his holidays", and may not be regarded as clinical data. When these comments are permitted during data collection, the sponsor should have a process by which the comments are reviewed and processed. This should include a method to query and move relevant data to the appropriate form.
The Investigative site should be trained to enter clinical data in the appropriate data collection fields rather than making marginal notes on the CRF, and to use appropriate methods and tools to communicate to the monitor information that should not be included in the clinical data.
Free Text versus Value List Fields
Clinical data must be entered in appropriate data collection fields; otherwise, there is a potential for data that should be entered on other CRFs to be hidden within the comment. For example, if a general comment of "subject visit was delayed as he had the flu" was captured, this would necessitate that someone review the data and query the site to enter "flu" in an Adverse Event CRF and not leave it as a comment. An additional concern with free text comments is the potential for inappropriate, or sensitive, information to be included within general comments data collection fields. For example, a comment could contain a subject's name or may have unblinding information.
Free text comments are also inefficient for processing due to their variable and unstructured nature; they offer limited or no value for statistical analysis, as they cannot be tabulated.
CRF development teams are encouraged to strive for data collection methods that maximize the use of pre-defined lists of responses rather than relying solely on free text comment fields. The recommendation is that CRF development teams consider what additional questions may be needed within a specific CRF, and what the typical responses would be. They can then create a standardized list of responses for those questions, and make the data collected more useful for analysis.
Considerations Regarding Usage of a General Comments CRF
Solicited comments often used to be collected on a General Comments CRF. In recent years though, most organizations have discontinued this practice.
The CDASHIG CO domain has no mandatory data elements and is not intended to encourage the creation of a General Comments CRF. The CDASHIG recommends against the use of a General Comments CRF. This recommendation is not meant to discourage investigators from providing unsolicited comments where they are appropriate, nor to discourage solicited free-text comment data collection fields that may appear within any CRF. Free text comment fields should be used to solicit comments where they are needed. When comments are collected, they should use a variable naming convention that is conformant to CDASH (e.g., COVAL may be used in any CRF because it is part of the SDTMIG specification).
Example CRF for the CDASHIG CO - Comments Domain
This CRF shows the use of a targeted comment to collect the reason a PK sample was drawn more than 5 minutes late.
Example 1
Example CRF Completion Instructions
- Record the actual collection times
- If the sample was drawn more than 5 minutes late, provide an explanation in the appropriate comment area.
| Were blood sample draws performed? PCPERF |
Ο Yes NOT SUBMITTED
Ο No PCSTAT = NOT DONE
|
| Collection Date PCDTC PCDAT | _ _ / _ _ _ / _ _ _ _ |
| Pre-Dose (Defaulted) PCTPT | Sponsor Defined |
| Pre-Dose Time PCDTC PCTIM |
_ _ : _ _ If late, comment _______________ |
| 30 Minutes Post Dose (Defaulted) PCTPT | Sponsor Defined |
| 30 Minutes Post Dose Time PCDTC PCTIM |
_ _ : _ _ If late, comment _______________ |
| 90 Minutes Post Dose (Defaulted) PCTPT | Sponsor Defined |
| 90 Minutes Post Dose Time PCDTC PCTIM |
_ _ : _ _ If late, comment _______________ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG CO - Comments Domain
Examples are not available at this time.
7.3 DM - Demographics
Description/Overview for the CDASHIG DM - Demographics Domain
The Demographics (DM) CDASHIG domain includes essential data collection fields that describe each subject in a clinical study. The collection of some demographics data is useful to perform simple analyses based upon population stratification.
Privacy concerns surrounding the DM & SC data were taken into account when these domains were created. For example, there are optional CDASHIG variables to collect the components of birthdate (e.g., BRTHDD, BRTHMO, and BRTHYY); therefore, limited elements of birthday may be collected and later mapped to the SDTMIG variable BRTHDTC. This approach provides flexibility in categorizing some variables to facilitate compliance with local privacy issues.
Collection of Age versus Date of Birth
It is recognized that sponsors may collect the Age or Date of Birth of the subject. In studies that are multi-regional, sponsors may need to enable the collection of either in order to be compliant to local regulations. But only one or the other should be collected for any given subject. When only AGE is collected, the sponsor is left with a window of uncertainty of, at most, 365 days. Knowing the precise date of birth provides the ability to calculate accurately an age for any date, however a precise (and complete) date of birth may be seen as "personally identifying information" for some privacy oversight boards or governmental regulators.
Collect the date of birth to the extent that the local regulatory authorities will allow.
- The best method is to collect a complete date of birth, and derive age.
- When there are privacy concerns with collecting the complete date of birth, the recommendation is to collect year of birth at a minimum.
- In cases when neither of the above can be implemented (e.g., cultural or regional considerations) then Age and Age Unit should be collected, and Date of Collection should be collected or derived from the Visit Date.
- Only use the AGETXT variable in very rare cases when only an age range or age description can be determined.
Date of Birth should be implemented such that incomplete dates are enterable, as allowed by the data capture system.
See Section 3.6 Collection of Dates for more information.
Collection of Sex
The collection of some demographics data is useful to perform simple analyses based upon population stratification.
Collection of Ethnicity and Race
Within the United States of America, collect Ethnicity before Race, per FDA requirement. A secondary analysis is often made using the phenotypic race of the subject. Collect race if required for the protocol and not prohibited by local laws and regulations.
In 2016, the U.S. Office of Management and Budget (OMB) issued its revised recommendations for the collection and use of race and ethnicity data by Federal agencies. FDA follows this directive and now recommends "the use of the standardized OMB race and ethnicity categories for data collection in clinical trials for two reasons. The use of the recommended OMB categories will help ensure consistency in demographic subset analyses across studies used to support certain marketing applications to FDA and across data collected by other government agencies".
The Race values listed in the FDA Guidance are:
- American Indian or Alaska Native
- Asian
- Black or African American*
- Native Hawaiian or Other Pacific Islander
- White
*For studies where data are collected outside the U.S., the recommended categories are the same except for "Black" instead of "Black or African American".
CDASH provides only one variable for Race. When the sponsor captures more than one race, the sponsors will need to create non-standard variables to store the collection of the multiple Races and map appropriately to the SDTMIG DM domain.
Race Other has been included as a free-text field to capture responses. The use of this variable is optional and should be used with caution because, when submitting data to the FDA, the FDA requires that all races be mapped to the five standard races recognized by the FDA, and providing an Other, Specify field might lead to mapping errors or difficulties. RACE is R/C because some sponsors prefer to derive values that are compliant with the codelist RACE (e.g., as derived from values collected in CRACE).
The category of ethnicity is similar to race but, as defined by the CDC, is an arbitrary classification based on cultural, religious, or linguistic traditions; ethnic traits, background, allegiance, or association. In a fairly heterogeneous country, such as in the U.S., ethnicity data might be useful only to confirm that ethnic groups are not being discriminated against by being unfairly excluded from clinical research. Other regulatory bodies may expect the reporting of ethnicity values (different than the U.S. FDA) which more appropriately reflect the population of their areas (e.g., Japanese for MHLW reporting to Japan). These may be collected using the CETHNIC variable with its corresponding codelist, ETHNICC.
If more detailed information on race or ethnicity are required to further characterize the study subject, it is recommended that the presented choices be "collapsible" up to one of the five designations for race, as well as the two categories for representing ethnicity, as needed for reporting to FDA under its guidance. When these more detailed categorizations are desired, the use of race and ethnicity vocabulary tables located within Health Level Seven's Reference Information Model Structural Vocabulary Tables is recommended, as they are designed to collapse up in this manner. For the collection of this added detail or granularity, as the sponsor may require, CDASH provides the variables CRACE and CETHNIC, respectively.
Source: Collection of Race and Ethnicity Data in Clinical Trials; October 26, 2016.
Collection of Special Optional Fields in Demographics
The CDASHIG allows for collection of the Date of Informed Consent using the variable RFICDAT. If a sponsor chooses to collect Informed Consent using this variable, the data should not also be collected using DSSTDAT from the DS Domain. The data from RFICDAT would then be mapped to the SDTMIG variable DSSTDTC and the companion variables (e.g., DSTERM, DSDECOD) must be populated accordingly.
The CDASH Model also defines a field for Death Date (DTHDAT) as a timing variable. It may be collected on any CRF deemed appropriate by the sponsor. The SDTMIG variables DTHDTC and DTHFL are mapped to the DM domain during the SDTM submission dataset creation process. The CDASH field Death Date may be mapped to other SDTMIG domains, as deemed appropriate by the sponsor (e.g., DS).
See Best Practice in Section 4.1.2 of the CDASHIG for additional guidance recommending that the same data not be collected more than one time per subject.
Specification for the CDASHIG DM - Demographics Domain
Demographics (DM)
Assumptions for the CDASHIG DM - Demographics Domain
The CDASHIG DM domain is a special-purpose domain that collects specific data elements that are mapped to the SDTMIG DM domain. Additional data elements that are not within the scope of the Demographics must be mapped to other domains.
Example CRF for the CDASHIG DM - Demographics Domain
Example 1
| Birth Date BRTHDTC BRTHDD BRTHMO BRTHYR BRTHDAT | _ _ / _ _ _ / _ _ _ _ |
| Sex SEX | Ο Male Ο Female |
| Do you consider yourself Hispanic/Latino or not Hispanic/Latino? ETHNIC |
Ο Hispanic or Latino
Ο Not Hispanic or Latino
Ο Not Reported
|
| Which of the following five racial designations best describes you? More than one choice is acceptable. RACE |
|
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 2
| Birth Date BRTHDTC BRTHDD BRTHMO BRTHYR BRTHDAT | _ _ / _ _ _ / _ _ _ _ |
| Sex SEX | Ο Male Ο Female |
| Ethnicity ETHNIC | Ο Hispanic or Latino CETHNIC1-NN SUPPDM.QVAL WHERE QNAM = "CETHNIC1-NN"
Ο Not Hispanic or Latino
Ο Not Reported
|
| Race RACE | CRACE1-NN SUPPDM.QVAL WHERE QNAM = "CRACE1-NN"
|
| Specify Other SUPPDM.QVAL WHERE QNAM = "RACEOTH" RACEOTH | __________________ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG DM - Demographics Domain
Examples are not available at this time.
8 General Observation Classes
This section reflects common practices implemented by a significant number of the organizations/companies that provided information/examples. Most subject-level data collected during a study are represented using one of the three SDTM general observation classes. Section 8.1 includes the CDASH Intervention domains, Section 8.2 includes the CDASH Events domains, and Section 8.3 includes the CDASH Findings Domains. Within each domain class, the domains are presented in alphabetical order. Readers should refer to Section 6 of the SDTMIG for additional information on these classes. Within each domain, a link is provided to the CDASHIG Domain Metadata table. The CDASHIG Domain Metadata tables include the variables that are commonly used by a significant number of the organizations/companies that provided information/examples. Implementers may add other variables from the CDASH model as needed. Other variables can be added if needed following the instructions in Section 3.4, How to Create New Data Collection Fields When No CDASHIG Field Has Been Defined.
8.1 CDASH Interventions Domains
The Interventions class includes CDASH domains that define collection standards for investigational treatments, therapeutic treatments, and procedures that are intentionally administered to the subject either as specified by the study protocol (e.g., exposure), coincident with the study assessment period (e.g., concomitant medications), or self-administered by the subject (such as alcohol, tobacco, or caffeine).
8.1.1 Assumptions for Interventions Domains
- CDASH --YN variables with the question text "Were there any interventions?" (e.g., "Were there any procedures?" or "Were there any concomitant medications?") are intended to assist in the cleaning of data and in confirming that there are no missing values. These variables are not included as part of the SDTM Intervention Domains for submission and are annotated as NOT SUBMITTED on the CRF.
- CDASH --CAT and/or -SCAT are generally not entered on the CRF by the sites. Implementers may pre-populate and display these category values to help the site understand what data should be recorded on the CRF. Implementers may also pre-populate hidden variables with the values assigned within their operational database. Categories and subcategories are determined per protocol design, and could be populated during the SDTM submission dataset creations.
- Date and Time variables
- CDASH date variables (e.g., --DAT, --STDAT, --ENDAT) are concatenated with the CDASH Time variables (e.g., --TIM, --STTIM, --ENTIM (if time is applicable) into the appropriate SDTM --DTC variables (e.g., --DTC, --STDTC, --ENDTC) using the ISO 8601 format.
- Collecting the time an intervention was started is only appropriate if it can be realistically determined and if there is a scientific reason for needing to know this level of detail. An example is where the subject is under the direct care of the site at the time the intervention was started, and the study design is such that it is important to know the intervention start time with respect to dosing.
- CDASH variable -- REASND is used with SDTM variable --STAT only. The value "NOT DONE" in --STAT indicates that the subject was not questioned about the intervention or data was not collected. It does not mean that the subject had no interventions.
- Coding
- When free text intervention treatments are recorded, the location may be included in the --TRT variable to facilitate coding (e.g., Liver Biopsy). Location is collected when the sponsor needs to identify the specific anatomical location of the intervention. This location information does not need to be removed from the verbatim --TRT when the SDTMIG submission datasets are created, but the --LOC variable will need to be populated with the anatomical location. Reference the SDTMIG for more information.
- --ATC1 through --ATC5, --ATC1CD through --ATC5CD. These Non-Standard (or SUPPQUAL) Variables are used only when the intervention is coded using the Anatomical Therapeutic Chemical Classification System. The numbers indicate the anatomical main group: "1" = the anatomical main group, "2" = the therapeutic main group, "3" = the therapeutic/pharmacological subgroup, "4" = chemical/therapeutic/pharmacological subgroup, "5" = chemical substance.
- The implementer may also add the MedDRA coding elements as Non-Standard Variables to the Intervention domain if this dictionary is used for coding.
- Location (--LOC) and related variables (--LAT, --DIR, -- PORTOT)
- Because the complete lists of controlled terminology for these variables may be too extensive to be relevant for a particular study CRF, sponsors may choose to include only subsets of the controlled terminology on the CRF.
- --LOC could be a defaulted or hidden field on the CRF for pre-specified [--TRT]/Intervention Topic].
- Relative Timing Variables (See SDTMIG Section 4.1.4.7 for more information and details)
- For each study, the sponsor defines the study reference period in the DM domain using SDTMIG variables RFSTDTC and RFENDTC. Other sponsor-specified reference time points can be defined for other data collection situations. The CDASH variables --PRIOR and --ONGO may be collected in lieu of a "start date" or an "end date".
- The CDASH variable --PRIOR is used to indicate if the --TRT (the topic item) started prior to either the study reference period or another sponsor-defined reference time point. When the study reference period is used as the anchor, --PRIOR may be used to derive a value (from the controlled terminology codelist STENRF) into the SDTM relative timing variable --STRF. When populating --STRF, if the value of --PRIOR is "Y", the value of "BEFORE" may be mapped to --STRF. The value in DM.RFSTDTC serves as the anchor for --STRF.
When a reference time point is used instead of the study reference period, --PRIOR may be used to derive a value into the SDTM relative timing variable --STRTPT. If the value of --PRIOR is "Y", the value of "BEFORE" may be derived into --STRTPT. Note: --STRTPT must refer to the "time point anchor" as described in --STTPT. The value in --STTPT can be either text (e.g., "VISIT 1") or a date (in ISO 8601 format). - The CDASH variable --ONGO is used to indicate if the value in --TRT is continuing beyond the study reference period or beyond another sponsor-defined reference time point. When the study reference period is used as the anchor, --ONGO may be used to derive a value into the SDTM relative timing variable --ENRF. If the value of --ONGO = "Y", the value of "AFTER" may be mapped to --ENRF.
When a reference time point is used instead of the study reference period, --ONGO may be used to derive a value into the SDTM relative timing variable --ENRTPT. If the value of --ONGO is "Y", the value of "ONGOING" may be mapped to --ENRTPT. Note: --ENRTPT must refer to the "time point anchor" as described in --ENTPT. The value in --ENTPT can be either text (e.g., "TRIAL EXIT") or a date (in ISO 8601 format).
8.1.2 CM - Prior and Concomitant Medications
Description/Overview for the CDASHIG CM - Prior and Concomitant Medications Domain
The same basic data collection variables should be collected for all medications/treatments/therapies (Prior, General Concomitant Medications, and Medications of Interest). If additional fields are needed to collect other data about a medication of interest, those should be added as nonstandard fields. Note: Sponsor may use terms like "concomitant medications", "treatments", or "therapies" as appropriate for the study. The text below may use one of these terms, but sponsors can always use the term most appropriate for their study.
The term "Prior" refers to medications/treatments that were started before study participation, since limited information may be available on prior medications taken by a subject; the core requirements were constrained to reflect this limitation.
Sponsors should define the appropriate collection period for prior and concomitant medications/treatments in the study protocol.
Specification for the CDASHIG CM - Prior and Concomitant Medications Domain
Concomitant Medications (CM)
Assumptions for the CDASHIG CM - Prior and Concomitant Medications Domain
- General medications/treatments are defined as any medications/treatments reported by a subject when asked if they have taken any medications in an open-ended way that does not ask about any specific drug. Additional information might be sourced by referring to a subject's medical record.
- Medications of interest are defined as any medications or classes of drugs specifically mentioned in the protocol and were not the primary focus for determining the CDASHIG Core designations for the domain (e.g., excluded medications, drugs requiring a washout period prior to dosing in study, or rescue medications).
- As with all the data collection variables recommended in the CDASH standard, it is assumed that sponsors will add other data variables as needed to meet protocol-specific and other data collection requirements (e.g., TA-specific data elements and others as required per protocol, business practice and operating procedures).
- The CMOCCUR field provides a structure for capturing the occurrence of specific medications of interest.
- If sponsors wish to capture non-pharmacological therapies (e.g., Surgery procedures, aromatherapy, massage, acupuncture), they can use the PR domain.
Example CRF for the CDASHIG CM - Prior and Concomitant Medications Domain
Example 1
| Were any concomitant medications taken? NOT SUBMITTED CMYN |
Ο Yes
Ο No
|
| What was the medication? CMTRT | ___________ |
| For what indication was the medication taken? CMINDC | ___________ |
| What was the individual dose of medication? CMDOSE | ___________ |
| Dose Unit CMDOSU |
Ο mg
Ο g
Ο mL
|
| What was the frequency of the medication? CMDOSFRQ |
Ο PRN
Ο BID
Ο QD
|
| What was the route of administration of the medication? CMROUTE |
Ο ORAL
Ο SUBCUTANEOUS
Ο TOPICAL
|
| What was the medication start date? CMSTDTC CMSTDAT | _ _ / _ _ _ / _ _ _ _ |
| What was the medication end date? CMENDTC CMENDAT | _ _ / _ _ _ / _ _ _ _ |
| Was the medication still ongoing? CMENRTPT / CMENRF CMONGO |
Ο Yes
Ο No
|
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG CM - Prior and Concomitant Medications Domain
Examples are not available at this time.
8.1.3 EC - Exposure as Collected and EX - Exposure
Description/Overview for the CDASHIG EC - Exposure as Collected and the CDASHIG EX - Exposure Domains
In the SDTMIG, the Exposure (EX) domain is used to represent exposure to study treatment as described in the protocol. The Exposure as Collected (EC) domain is used to represent the data as collected on the CRF. The CDASHIG EC domain is used in a study when the SDTMIG EX domain cannot be directly populated with the data collected on the CRF.
CDASHIG EC is used when:
- An alias for the actual treatment name is used (e.g., the study is masked) rather than the actual treatment name (e.g., the study is open label).
- Exposure data are collected in non-protocol-specified units (e.g., tablets).
- Scheduled and/or missed doses are collected.
- Planned doses (e.g., a calculation based on body weight) are collected in addition to actual doses given.
A sponsor may choose to always collect exposure data using the CDASHIG EC domain.
Extensive discussion of the use of the EC and EX domains, including examples of data collection, is available in the SDTMIG.
Specification for the CDASHIG EC - Exposure as Collected and the EX - Exposure Domains
Exposure as Collected (EC)
Exposure (EX)
- If the SDTMIG EC dataset would be an exact duplicate of the SDTMIG EX dataset, then the sponsor may choose to collect data using either the CDASHIG EC or EX domain. (See the SDTMIG for additional information on submission.)
- The collected --TRT value is the name of the study treatment that is known to the subject or investigative site (e.g., ECTRT = "TABLET A" when EXTRT= <treatment name>).
-
If dosing data are collected in non-protocol-specified units, the data are collected in CDASHIG EC dataset, then programmatically converted to the protocol-specified units when the data are mapped to the SDTMIG EX domain.
Dosing units specified in the protocol Dosing information as collected
on the CRF and represented in ECRepresentation in EX 200 mg tablet 2 tablets 400 mg 100 mg capsule 1 capsule 100 mg 5 mg/kg 99 mL 5 mg/kg - If a treatment is such that start and stop times are not required, and only one dosing date is collected, then the collected dosing date will map to both the start date (--STDTC) and end date (--ENDTC) in the submitted exposure dataset(s).
- If scheduled dosing is collected, the ECMOOD variable may be used to distinguish between "SCHEDULED" and "PERFORMED" dosing records. The sponsor may choose to use this variable to capture multiple scheduled dosing records, if needed.
- ECOCCUR:
- ECOCCUR value of "N" indicates a dose was not taken, not given, or missed.
- ECOCCUR is generally not applicable for Scheduled records.
- ECOCCUR = "N" is the standard representation of the collected doses not taken, not given, or missed.
- Dose amount variables (e.g., ECDOSE, ECDOSTXT) must not be set to zero (0) as an alternative method for indicating doses not taken, not given, or missed.
Example CRFs for the CDASHIG EC- Exposure as Collected and the CDASHIG EX - Exposure Domains
Example 1
| Treatment Phase (Defaulted) EPOCH | Sponsor Defined |
| Study Treatment Name (Defaulted) ECTRT | Sponsor Defined |
| Study Treatment Label ID ECREFID | _______________ |
| Lot Number ECLOT | _______________ |
| Fasting ECFAST |
Ο Yes
Ο No
|
| Start Date ECSTDTC ECSTDAT | _ _ / _ _ _ / _ _ _ _ |
| Start Time ECSTDTC ECSTTIM | _ _ : _ _ |
| End Date ECENDTC ECENDAT | _ _ / _ _ _ / _ _ _ _ |
| End Time ECENDTC ECENTIM | _ _ : _ _ |
| Dose ECDOSTXT ECDSTXT ECDOSE | _______________ |
| Unit ECDOSU |
Ο Tablet
Ο Capsule
|
| Frequency ECDOSFRQ |
Ο QD
Ο BID
Ο TID
|
| Route ECROUTE |
Ο Oral
Ο Sublingual
|
| Was the dose adjusted? NOT SUBMITTED ECDOSADJ |
Ο Yes
Ο No
|
| Reason for dose adjustment ECADJ | _______________ |
In this CRF example, the unit was not blinded to the site and hence the actual unit and dosage form were collected.
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 2
| Was study treatment administered? (Defaulted) ECOCCUR | N |
| Study Treatment Category (Defaulted) ECCAT | MISSED DOSE |
| Study Treatment Name (Defaulted) ECTRT | Sponsor Defined |
| Line Number ECSPID | _________ |
| What was the intended dose date? ECSTDTC ECSTDAT | _ _ / _ _ _ / _ _ _ _ |
| Reason Treatment Not Administered SUPPEC.QVAL WHERE QNAM = "ECREASOC" ECREASOC | _________________________ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 3
| Treatment Phase (Defaulted) EPOCH | Sponsor Defined |
| Study Treatment Name (Defaulted) EXTRT | Sponsor Defined |
| Start Date EXSTDTC EXSTDAT | _ _ / _ _ _ / _ _ _ _ |
| Start Time EXSTDTC EXSTTIM | _ _ : _ _ |
| End Date EXENDTC EXENDAT | _ _ / _ _ _ / _ _ _ _ |
| End Time EXENDTC EXENTIM | _ _ : _ _ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG EC - Exposure as Collected Domain
Examples are not available at this time.
8.1.4 PR - Procedures
Description/Overview for the CDASHIG PR - Procedures Domain
The Procedures domain should be used to collect details describing a subject's therapeutic and diagnostic procedures conducted before, during, and/or after the study. Measurements obtained from procedures are to be represented in the appropriate Findings domain(s). For example, the details of an endoscopy (e.g., date and time of start and stop) are represented in the PR domain, while microscopic results would be represented in the Microscopic (MI) domain.
Specification for the CDASHIG PR - Procedures Domain
Procedures (PR)
Assumptions for the Procedures CDASHIG Domain Model
- Information on procedures is generally collected in one of two ways, either by recording free text or using a pre-specified list of terms.
- Since the solicitation of information on specific therapeutic and diagnostic procedures may affect the frequency at which they are reported, the fact that a specific procedure was solicited may be of interest to reviewers. PROCCUR is used to indicate whether a pre-specified procedure occurred. A value of "Y" indicates that the procedure occurred and "N" indicates that it did not. If a procedure was not pre-specified, the value of PROCCUR should not be collected.
Example CRFs for the CDASHIG PR - Procedures Domain
Example 1
| OUTPATIENT PROCEDURES | |
| Procedures Category (Defaulted) PRCAT | OUTPATIENT PROCEDURES |
| Procedure Name (Defaulted) PRTRT | PULMONARY ANGIOGRAM |
| Was a pulmonary angiogram performed? PROCCUR |
Ο Yes
Ο No
|
| Was the intervention pre-specified? (Defaulted) PRPRESP | Y |
| Start Date PRSTDTC PRSTDAT | _ _ / _ _ _ / _ _ _ _ |
| For what indication was the procedure performed? PRINDC | ______________________ |
| Adverse Event ID ASSOCIATE WITH RELATED AE RECORD VIA RELREC PRAENO | ______________________ |
| INPATIENT PROCEDURES | |
| Procedures Category (Defaulted) PRCAT | INPATIENT PROCEDURES |
| Procedure Name (Defaulted) PRTRT | CARDIAC CATHETERIZATION |
| Was a cardiac catheterization performed? PROCCUR |
Ο Yes
Ο No
|
| Start Date PRSTDTC PRSTDAT | _ _ / _ _ _ / _ _ _ _ |
| Was the procedure interrupted? NOT SUBMITTED PRITRPYN |
Ο Yes
Ο No
|
| Interruption Duration SUPPPR.QVAL WHERE QNAM = PRINTRP PRITRPD | ______________________ |
| Interruption Duration Unit SUPPPR.QVAL WHERE QNAM = PRINTRPU PRITRPDU |
Ο HOURS
Ο min
|
| For what indication was the procedure performed? PRINDC | ______________________ |
| Adverse Event ID ASSOCIATE WITH RELATED AE RECORD VIA RELREC PRAENO | ______________________ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 2
| Were any surgical, therapeutic or diagnostic procedures performed? NOT SUBMITTED PRYN |
Ο Yes
Ο No
|
| Procedure Name PRTRT | ______________________ |
| Indication PRINDC | ______________________ |
| Start Date PRSTDTC PRSTDAT | _ _ / _ _ _ / _ _ _ _ |
| Start Time PRSTDTC PRSTTIM | _ _ : _ _ |
| End Date PRENDTC PRENDAT | _ _ / _ _ _ / _ _ _ _ |
| End Time PRENDTC PRENTIM | _ _ : _ _ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG PR - Procedures Domain
Examples are not available at this time.
8.1.5 SU - Substance Use
Description/Overview for the CDASHIG SU - Substance Use Model
This domain is used to collect information on substance use when this information is relevant to the assessment of the efficacy and safety of therapies. The amount of information collected for this domain depends upon the sponsor's protocol. In many protocols, only information on the use of the substance is required. In this case, many of the variables in this domain would not be collected (e.g., duration, amount).
CDASH recommends the use of the more descriptive CDASHIG variable SUNCF with responses of "Never", "Current", or "Former" for each substance use type, rather than a simple "No/Yes" response. Based on the wide variability of protocol definitions of use, the specific definitions and timeframes for the SUNCF responses would be sponsor/protocol-defined. By using the SUNCF response categories for usage, a number of questions about use and frequency can be collapsed, in turn decreasing the number of data points required in the SU Domain. More detailed information about duration, amount, and start and end dates are optionally captured.
Specification for the CDASHIG SU- Substance Use Model
Substance Use (SU)
Assumptions for the CDASHIG SU- Substance Use Model
- Categories SUCAT and SUSCAT
- Sponsors may require different types of substance use data (e.g., illicit drug use, cigarettes, etc.) to be collected; the value for category may be pre-printed on the CRF.
- SUCAT and SUSCAT should not be redundant with SUTRT. For example, if a more detailed type of substance usage is collected on the CRF (e.g., "CIGARETTES", "CIGARS"), SUCAT should be "TOBACCO" and SUTRT could be "CIGARETTES", "CIGARS". If the sponsor does not solicit responses about specific types of substance used, on the CRF (e.g., "CIGAR", "CIGARETTE"), the value of SUTRT is the more general description of the substance, (e.g., "TOBACCO") and SUCAT is generally null. This practice avoids assigning the same value to both SUTRT and SUCAT. However for consistency across studies, the sponsor may elect to repeat the values of SUTRT in SUCAT.
- The SDTMIG variable SUPRESP should be pre-populated to the value of "Y" when information about the use of a specific substance is solicited on the CRF.
- Relative Timing Variables
- Relative timing variables are used to represent collected data in the SDTM tabulations in those cases where a Start Date or an End Date has not been collected, but some indication of when/if the intervention or event started or ended has been collected. In the CDASHIG SU domain, if the CDASHIG variable SUNCF (with the possible responses of "Never", "Current", or "Former") is used, the collected values may be used to derive a value into an SDTMIG relative timing variable to represent when the person started or stopped using the substance relative to either a time point or to a period of time in the study.
For example, if the value collected in SUNCF is "Current", the value of "ONGOING" may be represented in the SDTMIG Variable SUENRTPT to indicate that the person was still using cigarettes as of the time point described in SUENTPT. It is recommended that the sponsor collect either a date or a description of a time point that will be used in conjunction with relative timing variables. See SDTMIG Section 4.1.4.7 for more information about relative timing variables.
If the actual, complete start date or end date of the substance use has been collected, there is no need to use relative timing variables.
- Relative timing variables are used to represent collected data in the SDTM tabulations in those cases where a Start Date or an End Date has not been collected, but some indication of when/if the intervention or event started or ended has been collected. In the CDASHIG SU domain, if the CDASHIG variable SUNCF (with the possible responses of "Never", "Current", or "Former") is used, the collected values may be used to derive a value into an SDTMIG relative timing variable to represent when the person started or stopped using the substance relative to either a time point or to a period of time in the study.
- Start and End Dates
- Start and end dates can be collected if this level of detail is required by the protocol. Partial dates can be collected where the subject may not remember the complete date of when substance use started or ended. The sponsor may choose to capture a complete date or any variation thereof (e.g., month & year or year, etc.).
- Sponsors may elect to capture only a start date, or only an end date, and use the associated SDTMIG relative timing variables to represent information about the date not collected.
- If the sponsor is only interested in collecting whether or not the subject is consuming a particular substance, start and end dates are optional and may be omitted, and SUNCF may be collected as described above.
- Coding
- Coding may be performed if deemed necessary by the sponsor. The SDTMIG variable SUDECOD is a permissible variable in the SDTMIG SU Domain.
- Coding variables are not usually displayed on CRFs. If a sponsor chooses to display coding on the form, CDASH does not advocate using that as a field for entry by site personnel.
Example CRF for the CDASHIG SU- Substance Use Model
Example 1
Example CRF Completion Instructions
- Record the substance(s) used by the subject.
| Substance Use Category (Defaulted) SUCAT | Recreational Drugs |
| Were any recreational drugs used? NOT SUBMITTED SUYN |
Ο Yes
Ο No
|
| What was the substance used? SUTRT | ______________________ |
| Usage SUNCF |
Ο Current SUOCCUR = "Y" WHEN SUNCF = "CURRENT"
Ο Former SUOCCUR = "Y" WHEN SUNCF = "FORMER"
|
| What was the amount of substance used? SUDOSE AND/OR SUDOSTXT SUDSTXT | ______________________ |
| What was the duration? SUDUR SUCDUR | ______________________ |
| Duration Unit SUDUR SUCDURU |
Ο QD
Ο EVERY WEEK
Ο QM
Ο PA
|
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 2
Example CRF Completion Instructions
- The amount of each type of alcohol consumed should be an integer. The following description should be used in determining the amount consumed:
1 Beer Unit = 12 oz or 360 ml
1 Wine Unit = 5 oz or 150 ml
1 Spirits Unit = 1.5 oz or 45 ml
| What was the category of the substance used? (Defaulted) SUCAT | ALCOHOL |
| Has the subject ever consumed alcohol? ALCOHOL_SUNCF |
Ο Never SUOCCUR = "N" WHEN SUNCF = "NEVER"
Ο Current SUOCCUR = "Y" WHEN SUNCF = "CURRENT"
Ο Former SUOCCUR = "Y" WHEN SUNCF = "FORMER"
|
| Start Date SUSTDTC ALCOHOL_SUSTDAT | _ _ / _ _ _ / _ _ _ _ |
| End Date SUENDTC ALCOHOL_SUENDAT | _ _ / _ _ _ / _ _ _ _ |
| What was the amount of beer consumed? SUDOSE WHERE SUTRT = "BEER" BEER_SUDOSE | ____________ |
| Unit (Defaulted) SUDOSU WHERE SUTRT = "BEER" BEER_SUDOSU | UNIT |
| Frequency SUDOSFRQ WHERE SUTRT = "BEER" BEER_SUDOSFRQ |
Ο QD
Ο EVERY WEEK
Ο QM
Ο PA
|
| What was the amount of wine consumed? SUDOSE WHERE SUTRT = "WINE" WINE_SUDOSE | ____________ |
| Unit (Defaulted) SUDOSU WHERE SUTRT = "WINE" WINE_SUDOSU | UNIT |
| Frequency SUDOSFRQ WHERE SUTRT = "WINE" WINE_SUDOSFRQ |
Ο QD
Ο EVERY WEEK
Ο QM
Ο PA
|
| What was the amount of spirits consumed? SUDOSE WHERE SUTRT = "SPIRITS" SPIRTS_SUDOS | ____________ |
| Unit (Defaulted) SUDOSU WHERE SUTRT = "SPIRITS" SPIRTS_SUDOSU | UNIT |
| Frequency SUDOSFRQ WHERE SUTRT = "SPIRITS" SPIRITS_SUDOSE |
Ο QD
Ο EVERY WEEK
Ο QM
Ο PA
|
In this example CRF, the following syntax was used to create denormalized variable names: "SUTRT value" _DOMAIN ROOT VARIABLE.
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG SU- Substance Use Model
Examples are not available at this time.
8.2 CDASH Events Domains
The Events class includes CDASH domains that define standards for the collection of occurrences or incidents occurring during a trial (e.g., "adverse events" or "disposition") or prior to a trial (e.g., "medical history").
8.2.1 General CDASH Assumptions for Events Domains
- CDASH --YN variables with the question text "Were there any <events>?" (e.g., "Were there any adverse events?" or "Were there any healthcare encounters?") are intended to assist in the cleaning of data and in confirming there are no missing values. These questions can be added to any CRF in order to capture this information.
- CDASH --CAT and/or -SCAT are generally not entered on the CRF by the sites. Implementers may pre-populate and display these category values to help the site understand what data should be recorded on the CRF. Implementers may also pre-populate hidden variables with the values assigned within their operational database. Categories and subcategories are typically self-evident from the protocol design, and could be populated during the SDTM dataset creation.
- Date and Time variables
- CDASH date variables (e.g., --DAT, --STDAT,--ENDAT) are concatenated with the CDASH Time variables (e.g., --TIM, --STTIM, --ENTIM (if time is collected) into the appropriate SDTM --DTC variables (e.g., --DTC, – STDTC, --ENDTC) using the ISO 8601 format.
- Collecting the time of an event is only appropriate if it can be easily obtained and if there is a scientific reason, such as the need to know the order of events (i.e., the adverse event started after dosing). An example of this would be a study where the subject is confined to a Phase 1 unit and under the direct care of the unit staff at the time that the event started or using time to tie together dosing and PK sample collection.
- CDASH --COCCUR variable is used only if a specific event is solicited (pre-printed) on the CRF and the CRF elicits a response from the CDASH Codelist --COCCUR. As of the time of publication, the CDASH Controlled Terminology includes values that indicate that the data was not collected (Not Done). Whereas the SDTM Controlled Terminology for --OCCUR only includes the "N"," Y", and "UNKNOWN" responses. If the CDASH variable --OCCUR was used, the CRF would require a second question to indicate that the data was not collected. In STDM, the CDASH --COCCUR variable will be mapped to the SDTM variable --OCCUR and --STAT variables. Responses of "Y" or "N" map directly to SDTM --OCCUR. Responses of "NOT DONE" or "NOT COLLECTED" map to SDTM --STAT as "NOT DONE". --COCCUR is not used if the events are not pre-specified.
- CDASH variable --REASND is used in conjunction with SDTM variable --STAT. The value "NOT DONE" in --STAT indicates that the subject was not questioned about the event or data was not collected. It does not mean that the subject had no events.
- Coding
- The CDASH variables used for coding are not data collection fields that will appear on the CRF itself. Sponsors will populate these through the coding process.
- When free text event terms are entered, the location may be included in --TERM to facilitate coding and further clarify the event. This location information does not need to be removed from the verbatim term when the SDTM submission datasets are created.
- The CDASH variables --LLT, --LLTCD, --PTCD, --HLT, --HLTCD, --HLGT, --HLGTCD, --SOC, and --SOCCD are only applicable to events coded in MedDRA.
- Location (--LOC, --LAT, --DIR, --PORTOT)
- Location is collected when the sponsor needs to identify the specific anatomical location of the event.
- Implementers may collect the location information using a subset list of CT on the CRF. Location variables can be pre-populated as needed. There is currently some overlap across the LOC, LAT, and DIR variables for controlled terminology. While the overlap exists, ensure that this overlap for these variables is not part of database design.
8.2.2 AE - Adverse Events
Description/Overview for the CDASHIG AE- Adverse Events Domain
The Adverse Events dataset includes clinical data describing "any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have to have a causal relationship with this treatment" (ICH E2A). In consultation with regulatory authorities, sponsors may extend or limit the scope of adverse event collection (e.g., collecting pre-treatment events related to trial conduct, not collecting events that are assessed as efficacy endpoints). The events included in the AE dataset should be consistent with the protocol requirements. Adverse events may be captured either as free text or via a pre-specified list of terms. The structure of the SDTMIG AE domain is one record per adverse event per subject. It is the sponsor's responsibility to define an event. This definition may vary based on the sponsor's requirements for characterizing and reporting product safety and is usually described in the protocol.
As with all the data collection variables recommended in CDASH, it is assumed that sponsors will add other data variables as needed to meet protocol-specific and other data collection requirements (e.g., TA-specific data elements and others as required per protocol, business practice, and operating procedures). Sponsors should define the appropriate collection period for adverse events.
Specification for the CDASHIG AE- Adverse Events Domain
Adverse Events (AE)
Assumptions for the CDASHIG AE- Adverse Events Domain
- CDASHIG AEYN variable with the question text "Were any adverse events experienced?" is intended to assist in the cleaning of data and in confirming that there are no missing values. This CDASHIG variable is not included as part of the SDTMIG AE domain for submission. This question is annotated as "NOT SUBMITTED" on the CRF.
- Categories AECAT and AESCAT
- AECAT and AESCAT should not be redundant with the dictionary coding provided by AEDECOD and AESOC (i.e., they should provide a different means of defining or classifying AE records).
- AECAT and AESCAT are intended for categorizations that are defined in advance. Forexample, a sponsor may have a separate CRF page for AEs of special interest and then another page for all other AEs. In cases where a category of AEs of special interest resembles a part of the dictionary hierarchy (e.g., "CARDIAC EVENTS"), the categorization represented by AECAT and AESCAT may differ from the categorization derived from the coding dictionary.
- Presence or Absence of Events
- Adverse Events are most often collected as free-text, spontaneously reported adverse events. There may be cases where the occurrences of specific adverse events are solicited, per protocol requirements. In that case, the pre-specified adverse events would be listed on the CRF with a "Yes/No" question (AEOCCUR) asking about the occurrence of each one.
- The CDASHIG variable AEOCCUR does not map directly to an SDTM variable. Since the SDTM AE domain is intended to hold only Adverse Events that actually happen, all values collected in AEOCCUR for pre-specified AEs should be submitted in a Findings About Adverse Events data set (FAAE) where FAORRES=the value of AEOCCUR where FATESTCD="OCCUR". In addition, where AEOCCUR="Y", there should be a corresponding record in the AE domain.
- It's important to reiterate the distinction between Adverse Events and Clinical Events, especially if there may be pre-specified terms. In consulting with regulatory authorities, certain events (e.g., events associated with protocol endpoints) may simply be Clinical Events. (See the CDASHIG Section 8.2 Clinical Events for more details.)
- Coding
- AEDECOD is the preferred term derived by the sponsor from the coding dictionary. It is a required SDTMIG variable and must have a value. It is expected that the reported term (AETERM) will be coded using a standard dictionary such as MedDRA. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the define.xml external codelist attributes.
- AEMODIFY is a permissible SDTMIG variable and should be included if the sponsor's coding procedure permits modification of a verbatim term. The modified term is listed in AEMODIFY. The variable should be populated as per the sponsor's coding procedure.
- The CDASHIG elements AELLT, AELLTCD, AEPTCD, AEHLT, AEHLTCD, AEHLGT, AEHLGTCD, AEBDSYCD, AESOC, and AESOCCD are only applicable to events coded in MedDRA. These items are not expected for non-MedDRA coding.
- Relative Timing Variables
- AEONGO field does not map directly to an SDTMIG variable. It may be used to derive a value into an SDTMIG relative timing variable such as AEENRF or AEENRTPT when no end date is recorded. When populating AEENRF, if the value of AEONGO is "Y", a value (from the values "DURING", "AFTER" or "DURING/AFTER" as is appropriate to the study) may be derived. When populating AEENRTPT, if the value of AEONGO is "Y", the value of "ONGOING" may be derived. Note: AEENRTPT must refer to a "time point anchor" described in AEENTPT.
- Note AEONGO is a special use case of "Yes/No" where the question is usually presented as a single possible response of "Yes" in conjunction with another question, as in this case, an end date. AEONGO is completed to indicate that the AE has not resolved at the time of data collection, and thus no end date is collected. In some cases the ongoing status may be determined from AE Outcome. The purpose of collecting this field is to help with data cleaning and monitoring, since this field provides further confirmation that the end date was deliberately left blank.
- CDASHIG Action Taken Variables
- CDASHIG variables AEACN, AECONTRT, AEACNDEV, AEACNOYN, and AEACNOTH are used to collect the action taken as the result of an AE.
- CDASHIG AEACN describes changes to the study treatment as a result of the event. AEACN is specifically for the relationship to study treatment using controlled terminology. It is expected that a response would be provided for this question for all AEs.
- AEACNOTH describes Other Action(s) taken in response to the adverse event that are unrelated to study treatment dose changes or other non-study treatment given because of this adverse event. This field is usually collected as a free text field. Example: Treatment Unblinded, Primary Care Physician Notified. If possible/desired, the sponsor can create sponsor-defined controlled terminology. The CDASHIG variable AEACNOYN is used in conjunction with AEACNOTH to assist in the cleaning of data and in confirming that AEACNOTH is not missing. AEACNOYN is not included as part of the SDTMIG AE Domain for submission and is annotated as NOT SUBMITTED on the CRF. The CDASHIG variable AEACNOYN should only be used on the AE CRF.
- AECONTRT indicates if any non-study treatments were received because of this adverse event using the NY Controlled terminology. If "Yes" is answered for this question, any drugs used are recorded on the Concomitant Medications CRF and any procedures performed are recorded on the Procedure CRF. On the Concomitant Medications CRF, the CDASHIG variable CMAENO or on the Procedures CRF the CDASHIG variable PRAENO can be collected to identify the adverse event associated with this treatment by recording the appropriate AESPID. RELREC can be used to identify this relationship.
- AEACNDEV describes action taken with respect to a device in a study, which may or may not be the device under study. This field is usually collected as a free text field. If possible/desired, the sponsor can create sponsor-defined controlled terminology. FDA Medical Device Reporting (MDR) guidelines provide CT for the reporting of device problems.
- Serious Adverse events
- If the details regarding a Serious AE are collected in the clinical database, then it is recommended that a separate "Yes/No" variable be defined for each Serious AE type (AESCAN, AESCONG, AESDISAB, AESDTH, AESHOSP, AESLIFE, AESOD, AESMIE).
- The sponsor should check if the regulatory agencies to which the data will be submitted require the collection of this data (see FDA Study Data Technical Conformance Guide). The serious categories "Involves cancer" (AESCAN) and "Occurred with overdose" (AESOD) are not part of the ICH definition of a serious adverse event, but these categories are available for use in studies conducted under guidelines that existed prior to the FDA's adoption of the ICH definition.
- CDASHIG variables AESEV, AETOXGR
- In studies using a standard toxicity scale such as Common Terminology Criteria for Adverse Events v3.0 (CTCAE), published by the NCI (National Cancer Institute) at http://ctep.cancer.gov/reporting/ctc.html, AETOXGR should be used instead of AESEV. In most cases, either AESEV or AETOXGR is populated but not both.
- There may be cases when a sponsor may need to populate both variables. Whichever is used, the sponsor is expected to provide the dictionary name and version or standard toxicity scale name and version used to map the terms utilizing the define.xml external codelist attributes.
- CDASHIG variable DTHDAT
- The CDASH Model allows the Date of Death to be collected on any CRF deemed appropriate by the Sponsor. Death Date is mapped to other SDTMIG domains, as deemed appropriate by the sponsor (e.g., DM, DS).
- The death date should only be collected on one form.
Adverse Events are most often collected as free text, spontaneously reported adverse events. There may be cases where the occurrences of specific adverse events are solicited, per protocol requirements. In that case, the pre-specified adverse events would be listed on the CRF with a "Yes/No" question (AEOCCUR) asking about the occurrence of each one.
Example CRF for the CDASHIG AE- Adverse Events Domain
Example 1
Example CRF Completion Instructions
- Record all AEs except <list of protocol-defined exceptions> on the AE CRF after informed consent is obtained.
- All Serious Adverse Events (AEs), regardless of relationship to study drug, must be reported <via telephone or fax> within 24 hours of discovery.
- Safety information (e.g., AE, SAE) identified for all subjects must be recorded on source documents from the time informed consent is obtained.
| Were any adverse events experienced? NOT SUBMITTED AEYN |
Ο Yes
Ο No
|
| Adverse Event Category: Defaulted AECAT | Sponsor Defined |
| Adverse Event Subcategory: Defaulted AESCAT | Sponsor Defined |
| What is the adverse event identifier? AESPID | ____________________ |
| What is the adverse event term? AETERM | ____________________ |
| Start Date AESTDTC AESTDAT | _ _ / _ _ _ / _ _ _ _ |
| Is the adverse event ongoing? AEENRF / AEENRTPT AEENTPT AEONGO |
Ο Yes
Ο No
|
| End Date AEENDTC AEENDAT | _ _ / _ _ _ / _ _ _ _ |
| What is the severity of the adverse event? AESEV |
Ο MILD
Ο MODERATE
Ο SEVERE
|
| Was the adverse event serious? AESER |
Ο Yes
Ο No
|
| Was the adverse event associated with a congenital anomaly or birth defect? AESCONG |
Ο Yes
Ο No
|
| Did the adverse event result in disability or permanent damage? AESDISAB |
Ο Yes
Ο No
|
| Did the adverse event result in death? AESDTH |
Ο Yes
Ο No
|
| Death Date DS.DSSTDTC DM.DTHDTC DM.DTHDAT | _ _ / _ _ _ / _ _ _ _ |
| Did the adverse event result in initial or prolonged hospitalization of the subject? AESHOSP |
Ο Yes
Ο No
|
| Was the adverse event life threatening? AESLIFE |
Ο Yes
Ο No
|
| Did the adverse event require intervention to prevent permanent impairment or damage due to the use of a medical device? SUPPAE.QVAL WHERE QNAM = "AESINTV" AESINTV |
Ο Yes
Ο No
|
| Was the adverse event a medically important event not covered by other serious criteria? AESMIE |
Ο Yes
Ο No
|
| Action Taken with Study Treatment AEACN |
Ο DRUG WITHDRAWN
Ο DOSE REDUCED
Ο DOSE INCREASED
Ο DOSE NOT CHANGED
Ο UNKNOWN
Ο NOT APPLICABLE
|
| Relationship to Study Treatment AEREL |
Ο NOT RELATED
Ο UNLIKELY RELATED
Ο POSSIBLY RELATED
Ο RELATED
|
| Outcome AEOUT |
Ο RECOVERING / RESOLVING
Ο NOT RECOVERED / NOT RESOLVED
Ο RECOVERED / RESOLVED
Ο RECOVERED / RESOLVED WITH SEQUELAE
Ο FATAL
|
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG AE- Adverse Events Domain
Examples are not available at this time.
8.2.3 CE - Clinical Events
Description/Overview for the CDASHIG CE - Clinical Events Domain
The Clinical Events domain includes clinical events of interest that would not be classified as adverse events. The clinical events included in the CE dataset should be consistent with the protocol requirements. Clinical events may be captured either as free text or via a pre-specified list of terms. The structure of the CE domain is one record per clinical event per subject. It is the sponsor's responsibility to define a clinical event and the event is usually described in the protocol.
As with all the data collection variables recommended in CDASH it is assumed that sponsors will add other data variables as needed to meet protocol-specific and other data collection requirements (e.g., TA-specific data elements and others as required per protocol, business practice and operating procedures). Sponsors should define the appropriate collection period for clinical events.
Specification for the CDASHIG CE - Clinical Events Domain
Clinical Events (CE)
Assumptions for the CDASHIG CE - Clinical Events Domain
- The determination of events to be considered clinical events versus adverse events should be done carefully and with reference to regulatory guidelines or consultation with a regulatory review division. Please note that all reportable adverse events that would contribute to AE incidence tables in a clinical study report must be included in the AE domain. Adverse event data should not be commingled with clinical event data. The collection of write-in events on a Clinical Events CRF should be considered with caution.
- Events considered to be clinical events may include episodes of symptoms of the disease under study (often known as signs and symptoms), or about events that do not constitute adverse events in themselves, though they might lead to the identification of an adverse event. For example, in a study of an investigational treatment for migraine headaches, migraine headaches may not be considered to be adverse events per protocol. The occurrence of migraines or associated signs and symptoms might be reported in CE.
- Some clinical events may escalate (e.g., increase in duration, severity) and require additional collection in AE. This should be clearly defined in the protocol.
- The CDASHIG variable, CEYN, with the question text "Were any clinical events experienced?" is intended to assist in the cleaning of data and in confirming that no data are unintentionally missing. This CDASHIG variable is not included as part of the SDTM Event Domain for submission. This field is annotated as NOT SUBMITTED on the CRF.
- Categories CECAT and CESCAT should not be redundant with the domain code or dictionary classification provided by CEDECOD and CESOC (i.e., they should provide a different means of defining or classifying CE records).
- Presence or Absence of Events: The CDASHIG variable CEOCCUR is used to record whether a subject had a pre-specified clinical event. Example: "Does the subject have a fever?"
- Coding
- CEDECOD is the preferred term derived by the sponsor from the coding dictionary. It is permissible to code CETERM using a standard dictionary such as MedDRA. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the define.xml external codelist attributes.
- CEMODIFY is a permissible variable and should be included if the sponsor's coding procedure permits modification of a verbatim term. The modified term is listed in CEMODIFY. The variable should be populated as per the sponsor's coding procedure. The CDASHIG elements CELLT, CELLTCD, CEPTCD, CEHLT, CEHLTCD, CEHLGT, CEHLGTCD, CEBDSYCD, CESOC, and CESOCCD are only applicable to events coded in MedDRA. These items are not expected for non-MedDRA coding.
- Relative Timing Variables
- CEONGO field does not map directly to an SDTMIG variable. It may be used to derive a value into an SDTMIG relative timing variable such as CEENRF or CEENRTPT when no end date is recorded. When populating CEENRF, if the value of CEONGO is "Y", a value (from the values "DURING", "AFTER" or "DURING/AFTER" as is appropriate to the study) may be derived.
- When populating CEENRTPT, if the value of CEONGO is "Y", the value of "ONGOING" may be derived. CEENRTPT must refer to a "time point anchor" described in CEENTPT.
- CEONGO is a special use case of Yes/No where the question is usually presented as a single possible response of Yes in conjunction with another question, as in this case, an end date. CEONGO is completed to indicate that the CE has not resolved at the time of data collection, and thus no end date is collected. The purpose of collecting this field is to help with data cleaning and monitoring, since this field provides further confirmation that the end date was deliberately left blank.
Example CRF for the CDASHIG CE- Clinical Events Domain
See Example CRF 5: Hypoglycemia as referenced in CDISC Therapeutic Area Data Standards User Guide for Diabetes (Version 1.0)
http://www.cdisc.org/sites/default/files/members/standard/ta/diabetes/taug-diabetesv101.pdf
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
CRF annotated to show mapping. Variables defined in the SDTMIG are in RED. If the CDASHIG variable differs from the one defined in the SDTMIG, the CDASHIG variable is in BLUE. Data collected, but not submitted in SDTM-based datasets, are denoted as NOT SUBMITTED.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
8.2.4 DS - Disposition
Description/Overview for the CDASHIG DS - Disposition Domain
The DS domain includes disposition events and protocol milestones (e.g., informed consent obtained, randomized).
Specification for the CDASHIG DS - Disposition Domain
Disposition (DS)
Assumptions for the CDASHIG DS - Disposition Domain
- Sponsors may choose which disposition events and milestones to collect for a study. A milestone is a protocol-specified "point-in-time" that is not assigned to an Epoch. A disposition event describes whether a subject completed the study or portion of a study (Epoch) or the reason they did not complete. A sponsor may collect one disposition event for the trial as a whole, or they may collect disposition for each Epoch of the trial.
- Disposition data may be collected on a form dedicated to disposition data, or it may be collected across several forms that also contains data that are not DS. When disposition data are collected on forms also containing data that are not DS, the disposition should be mapped to the appropriate DS SDTMIG variable (e.g., DSCAT, DSSTDAT, DSTERM, and DSDECOD). The same disposition data should not be collected on both domain-specific forms and on a disposition form.
- The CDASHIG DS domain does not specify where to collect protocol milestones within the CRF. Protocol milestones may be included in convenient places in the CRF. For example, Informed Consent Date or Randomization Date may be collected on the same CRF page as demographicsdata and mapped for submission to the SDTMIG DS domain.
- The CDASH Model allows for collection of the Date of Death to be collected on any CRF deemed appropriate by the sponsor and mapped for submission to the SDTMIG DS domain. Or, the Date of Death may be collected as part of a Disposition form. Consideration should be given to design the CRF to collect the Date of Death only once in the study.
- The CDASHIG DM domain allows for collection of the Date of Informed Consent (RFICDAT) and Date of Death (DTHDAT). When these variables are used for data collection, the data should not also be collected in the CDASHIG DS domain, but should be mapped from the CDASHIG DM variables to the appropriate SDTMIG DSSTDAT, DSTERM, and DSDECOD variables.
- The Controlled Terminology (NCOMPLT) is focused on disposition events, and is used when DSCAT is "DISPOSITION EVENT". Because the complete list of controlled terminology may not be appropriate, sponsors may choose to include only subsets of the CT on the CRF. The choices that appear for a Disposition Event are dependent on the event itself, and the contents of the list can vary if data are collected for multiple epochs in a study. For example, "Non-Compliance with Study Drug" and "Lack of Efficacy" are not applicable choices prior to the start of treatment; "Failure to Meet Randomization Criteria" is an applicable choice only for the epoch that immediately follows the date of subject's randomization; etc.
- If DSUNBLND is "Yes", and the unblinding/unmasking resulted in the subject discontinuing the trial prematurely, DSTERM and DSDECOD capture the applicable details. If the unblinding/unmasking occurred due to an Adverse Event, DSTERM contains the text of the Adverse Event, and the AE "Were any other actions taken in response to this adverse event?" (AEACNOTH [optional]) free text may include text of "Treatment Unblinded". DSUNBLND may also be used to document intentional unblinding at a protocol defined point in the trial.
- DSCONT and DSNEXT data are used to aid in monitoring and data cleaning. Because the responses are about future plans, the validity of the responses cannot be ascertained until the subject enters the subsequent epoch or new study.
Example CRFs for the CDASHIG DS - Disposition Domain
Example 1
| Defaulted DSCAT | PROTOCOL MILESTONE |
| Defaulted DSTERM DSDECOD | INFORMED CONSENT OBTAINED |
| What was the Informed Consent date? DSSTDTC DSSTDAT | _ _ / _ _ _ / _ _ _ _ |
| What was the Informed Consent time? DSSTDTC DSSTTIM | _ _ : _ _ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 2
| What is the disposition category? Defaulted DSCAT | DISPOSITION EVENT |
| What is the trial period for this disposition event? Defaulted EPOCH | BLINDED TREATMENT |
| What was the subject's status? DSTERM DSDECOD |
Ο COMPLETED
Ο ADVERSE EVENT
Specify: ________________ |
| What was the date of study completion/discontinuation? DSSTDTC DSSTDAT | _ _ / _ _ _ / _ _ _ _ |
| What was the time of study completion/discontinuation? DSSTDTC DSSTTIM | _ _ : _ _ |
|
Was study treatment unblinded by the site? Note: See CDASH metadata for SDTM mapping information. |
Ο Yes
Ο No
|
| What is the next period the subject will continue to? NOT SUBMITTED DSNEXT |
Ο OPEN LABEL TREATMENT
Ο FOLLOW-UP
|
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 3
| What is the disposition category? (Defaulted) DSCAT | DISPOSITION EVENT |
| What is the trial phase for this disposition event? (Defaulted) EPOCH | TREATMENT |
| What was the completion/discontinuation date? DSSTDTC DSSTDAT | _ _ / _ _ _ / _ _ _ _ |
| What was the completion/discontinuation time? DSSTDTC DSSTTIM | _ _ : _ _ |
| What was the subject's status? DSDECOD |
Ο COMPLETED
Ο SCREEN FAILURE
Ο ADVERSE EVENT
Ο DEATH
Ο DISEASE RELAPSE
Ο FAILURE TO MEET RANDOMIZATION CRITERIA
Ο LACK OF EFFICACY
Ο LOST TO FOLLOW-UP
Ο NON-COMPLIANCE WITH STUDY DRUG
Ο PHYSICIAN DECISION
Ο PREGNANCY
Ο PROGRESSIVE DISEASE
Ο PROTOCOL DEVIATION
Ο RECOVERY
Ο SITE TERMINATED BY SPONSOR
Ο STUDY TERMINATED BY SPONSOR
Ο TECHNICAL PROBLEMS
Ο WITHDRAWAL BY PARENT/GUARDIAN
Ο WITHDRAWAL BY SUBJECT
Ο OTHER
|
|
If the subject's status is other: (In this example, OTHER is chosen for further details, but sponsor may define additional "subject's status" choices for which further details should be collected.) Specify: | ________________________________________ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG DS - Disposition Domain
Examples are not available at this time.
8.2.5 DV - Protocol Deviations
Description/Overview for the CDASHIG DV - Protocol Deviations Domain
The DV domain is intended to collect protocol deviations or violations that occur after enrollment. It is not intended to collect information about inclusion/exclusion criteria; that data should be collected in IE.
Considerations Regarding Usage of a Protocol Deviations CRF
Sponsors must employ a robust and systematic method for recording protocol deviations, and this may include the use of a dedicated CRF for this purpose, or the intentional inclusion of data collection fields throughout the entire set of CRFs that will detect protocol deviations.
The DV domain metadata and example CRF were developed as a guide that sponsors could use for designing a Protocol Deviations CRF and associated database, should they choose to do so.
Definitions of Protocol Deviation and Protocol Violation are available in the Glossary and Abbreviations appendix.
Specification for the CDASHIG DV - Protocol Deviations Domain
Protocol Deviation (DV)
Assumptions for the CDASHIG DV - Protocol Deviations Domain
If a sponsor decides to use a Protocol Deviations CRF, the sponsor should not rely on this CRF as the only source of protocol deviation information for a study. Rather, they should also utilize monitoring, data review, and programming tools to assess whether there were protocol deviations in the study that may affect the usefulness of the datasets for analysis of efficacy and safety.
Example CRF for the CDASHIG DV - Protocol Deviations Domain
Example 1
| Were there any protocol deviations? NOT SUBMITTED DVYN |
Ο Yes
Ο No
|
| What was the protocol deviation? DVDECOD |
Ο Codelist Item A
Ο Codelist Item B
Ο Codelist Item C
|
| Protocol deviation, Specify DVTERM | _______________ |
| Start date DVSTDTC DVSTDAT | _ _ / _ _ _ / _ _ _ _ |
| End Date DVENDTC DVENDAT | _ _ / _ _ _ / _ _ _ _ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG DV - Protocol Deviations Domain
Examples are not available at this time.
8.2.6 HO - Healthcare Encounters
Description/Overview for the CDASHIG HO - Healthcare Encounters Domain
The Healthcare Encounters dataset includes inpatient and outpatient healthcare events (e.g., hospitalizations, nursing home stay, rehabilitation facility stays, medical practitioner office visits).
The Healthcare Encounters (HO) domain is used to record information about the event (the type of healthcare encounter, the timing of the event, etc.). Other data about any interventions administered or any findings measured, observed or tested during the healthcare encounter will be collected for submission in the respective domains.
- Information about imaging (e.g., MRI or Endoscopic Retrograde Cholangiopancreatography, or ERCP, which is a combination of an upper GI endoscopy and X-ray) would be collected in the Procedures (PR) domain.
- Laboratory results would be reported in Lab Results (LB).
It may not be necessary to collect data for the HO domain if the planned reporting or analysis is about the interventions and/or findings data rather than to the number/types of encounters, the duration of encounters, etc.
Specification for the CDASHIG HO - Healthcare Encounters Domain
Healthcare Encounters (HO)
Assumptions for the CDASHIG HO - Healthcare Encounters Domain
None as of publication
Example CRFs for the CDASHIG HO - Healthcare Encounters Domain
Example 1
This example shows CRF fields for the following data: reason for the healthcare encounter (HOINDC), encounter duration (HOCDUR), and any linked adverse events.
| Has the subject reported any healthcare encounters? NOT SUBMITTED HOYN |
Ο Yes
Ο No
|
| Healthcare Encounters Category (Defaulted) HOCAT | OUTPATIENT ENCOUNTERS |
| Healthcare Encounter Name (Defaulted) HOTERM | PHYSICAL THERAPY CLINIC |
| Did the subject receive physical rehabilitation services? HOOCCUR |
Ο Yes
Ο No
|
| Reason for Healthcare Encounter SUPPHO.QVAL WHERE QNAM = "HOREAS" HOREAS | _________________ |
| Adverse Event Identifier LINKED TO RELATED AE VIA RELREC HOAENO | _________________ |
| Healthcare Encounters Category (Defaulted) HOCAT | INPATIENT ENCOUNTERS |
| Healthcare Encounter Name (Defaulted) HOTERM | INTENSIVE CARE UNIT |
| Was the subject admitted to the ICU? HOOCCUR |
Ο Yes
Ο No
|
| Start Date HOSTDTC HOSTDAT | _ _ / _ _ _ / _ _ _ _ |
| Duration HODUR HOCDUR | _________________ |
| Duration Unit HODUR HOCDURU | _________________ |
| Was the healthcare encounter still ongoing? HOENRF/HOENRTPT HOENTPT HOONGO |
Ο Yes
Ο No
|
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 2
This example shows the collection of end date/time and another (specify) datapoints.
| Has the subject reported any healthcare encounters? [NOT SUBMITTED] HOYN |
Ο Yes
Ο No
|
| OUTPATIENT ENCOUNTERS | |
| Healthcare Encounters Category Defaulted HOCAT | OUTPATIENT ENCOUNTERS |
| Healthcare Encounter Name HOTERM | _______________________ |
| Start Date HOSTDTC HOSTDAT | _ _ / _ _ _ / _ _ _ _ |
| INPATIENT ENCOUNTERS | |
| Healthcare Encounters Category Defaulted HOCAT | INPATIENT ENCOUNTERS |
| Healthcare Encounter Name Defaulted HOTERM | HOSPITAL |
| Healthcare Encounters Not Collected HOREASND HOSTAT = "NOT DONE" | Ο Not Asked |
| Did the subject have any hospitalizations? HOOCCUR |
Ο Yes
Ο No
|
| Start Date HOSTDTC HOSTDAT | _ _ / _ _ _ / _ _ _ _ |
| Start Time HOSTDTC HOSTTIM | _ _ : _ _ |
| Was the healthcare encounter still ongoing? HOENRF/HOENRTPT HOENTPT HOONGO |
Ο Yes
Ο No
|
| End Date HOENDTC HOENDAT | _ _ / _ _ _ / _ _ _ _ |
| End Time HOENDTC HOENTIM | _ _ : _ _ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG HO - Healthcare Encounters Domain
Examples are not available at this time.
8.2.7 MH - Medical History
Description/Overview for the CDASHIG MH - Medical History Domain
The Medical History dataset includes the subject's medical history at the start of the trial. The CDASH metadata tables contain the most common general medical history data collection fields. In cases where more indication-specific medical history is required by the protocol, sponsors should add fields as needed from the CDASH events model.
Specification for the CDASHIG MH - Medical History Domain
Medical History (MH)
Assumptions for the CDASHIG MH - Medical History Domain
- Medical History Collection Period
- Sponsors should define the appropriate collection period for medical history in the protocol. The evaluation interval may be provided in the SDTMIG variable MHEVLINT or MHEVINTX. These intervals are not recorded by the investigator, but are populated by the sponsor in the SDTMIG MH dataset. These intervals may be pre-printed on the CRF as instruction text.
- Medical History Coding
- Sponsors who code medical history should use appropriate dictionary variables for the coding. The sponsor is expected to provide the dictionary name and version used to map the terms utilizing the define.xml External Codelist attributes.
- Coding using MedDRA, though not required by the FDA, is recommended by CDASH. Coding of medical history is recommended, because it provides methodology to compare medical history to adverse events and facilitate the identification of unexpected safety concerns or potential relationships to past treatments. If coding is performed it is recommended that it be done during the execution phase of a study rather than after it is completed, as this facilitates efficient resolution of any coding queries.
- For un-coded medical history, a sponsor-defined categorization of medical history events is recommended. One approach is to use the MHCAT variable.
- Coding variables are not a data collection field that will appear on the CRF itself. Sponsors will populate this through the coding process. When MedDRA is used as the coding dictionary, the MedDRA coding variables are included in the SDTM dataset.
- Date of Collection (DAT)
- This is the date that the data was recorded, and not the date that the condition started or the event occurred. The date of collection can be "derived" from the date of the visit.
- Relative Timing Variables
- The date of data collection in conjunction with a collected time point anchor date and the MHONGO CDASHIG fields would determine how the SDTMIG relative Timing variables would be populated.
- MHONGO field does not map directly to an SDTMIG variable but it may be used to derive a value into an SDTM-based relative Timing variable such as MHENRF or MHENRTPT. When populating MHENRF, if the value of MHONGO is "Y", the value of "DURING", "AFTER" or "DURING/AFTER" may be derived. When populating MHENRTPT, if the value of MHONGO is "Y", the value of "ONGOING" may be derived. Note: MHENRTPT must refer to a "time point anchor" described in MHENTPT. See CDASHIG Section 3.7 Mapping Relative Times from Collection to Submissions and SDTMIG Section 4.1.4.7 for more information.
- MHONGO is a special-use case of "Yes/No", where the question is usually presented as a single possible response of "Yes" when there is no applicable end date at time of collection. In this case, if the box is checked and the end date is blank, MHONGO is "Yes". If the box is not checked and an end date is present, MHONGO is "No". MHONGO should not be submitted in the MH or SUPPMH dataset.
- MHPRIOR can be added to this domain from the CDASH Model and used when the sponsor elects not to collect start dates (even partial dates) on the MH CRF. The sponsor would derive a value into an SDTMIG relative Timing variable such as MHSTRF or MHSTRTPT. When populating MHSTRF, if the value of MHPRIOR is "Y", the value of "BEFORE" may be derived. When populating MHSTRTPT, if the value of MHPRIOR is "Y", the value of "BEFORE" may be derived. Note: MHSTRTPT must refer to a "time point anchor" as described in MHSTTPT.
- Start and End Dates
- Partial dates are commonly collected in MH where the subject may not remember the complete date of when a medical history condition started or ended. The sponsor may choose to capture a complete date or any variation thereof (e.g., month and year or year, etc.).
- Non-standard Supplemental Qualifier Variables
- Medical History Event Type (MHEVDTYP) is used to specify the aspect of the medical condition or event by which its start date is defined. This variable (MHEVDTYP) is only to be used in the MH domain. This variable is used when the CRF records "multiple" dates such as the date when the condition was diagnosed, when symptoms were first reported prior to diagnosis, when the subject had a relapse, or when the infection associated with the diagnosis was reported. Example values for MHEVDTYP include DIAGNOSIS, SYMPTOMS, RELAPSE, and INFECTION.
- Condition under control (MHCTRL) is used to indicate that the disease/symptoms are under control at the time of data collection. It is recommended that since this non-standard variable has an implied time frame, the sponsor should provide the MHDTC data in the SDTMIG MH domain when using this non-standard variable. Refer to Section 3.5.2, CDASHIG Metadata Table, for detailed mapping instructions.
Example CRFs for the CDASHIG MH - Medical History Domain
Example 1
Example CRF Completion Instructions
- Any relevant medical conditions or events including <allergies, diseases or disorders that start <protocol-specified time period>> must be recorded. Any relevant medical conditions or events that start after the <protocol-specific time period> must be recorded as an adverse event.
- If a medical condition or event listed on the Medical History page worsens in intensity and/or frequency after the start of <protocol-specific time period>, record on the Adverse Events page.
| Has the subject had any medical conditions or events? NOT SUBMITTED MHYN |
Ο Yes
Ο No
|
|
Medical History Category Defaulted | Sponsor Defined |
| What is the medical condition or event identifier? MHSPID | |
| What is the medical condition or event? Defaulted MHTERM | CIRRHOSIS |
| Was the medical condition or event pre-specified? Defaulted MHPRESP | Y |
| Does the subject have cirrhosis? MHOCCUR |
Ο Yes
Ο No
|
| Start Date MHSTDTC MHSTDAT | _ _ / _ _ _ / _ _ _ _ |
| Ongoing MHENRF / MHENRTPT MHENTPT MHONGO |
Ο Yes
Ο No
|
| End Date MHENDTC MHENDAT | _ _ / _ _ _ / _ _ _ _ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 2
Example CRF Completion Instructions
- Any relevant medical conditions or events including <allergies, diseases or disorders that start <protocol-specified time period>> must be recorded. Any relevant medical conditions or events that start after the <protocol-specific time period> must be recorded as an adverse event.
- If a medical condition or event listed on the Medical History page worsens in intensity and/or frequency after the start of <protocol-specific time period>, record on the Adverse Events page.
| Has the subject had any medical conditions or events? NOT SUBMITTED MHYN |
Ο Yes
Ο No
|
| Medical History Category Defaulted MHCAT | Sponsor Defined |
| What is the medical condition or event identifier? MHSPID | _______________ |
| What is the medical condition or event? MHTERM | _______________ |
| Start Date MHSTDTC MHSTDAT | _ _ / _ _ _ / _ _ _ _ |
| Was the medical condition or event ongoing? MHENRF / MHENRTPT MHENTPT MHONGO |
Ο Yes
Ο No
|
| End Date MHENDAT MHENDTC | _ _ / _ _ _ / _ _ _ _ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 3
| Category (Defaulted) MHCAT | PSYCHIATRIC HISTORY |
| Medical History Date Type (Defaulted) SUPPMH.QVAL MDD_MHEVDTYP | SYMPTOM |
| Major Depressive Disorder (Defaulted) MHTERM MDD_MHTERM | MAJOR DEPRESSIVE DISORDER |
| MDD Symptom Start Date MDD_MHSTDTC MDD_MHSTDAT | _ _ / _ _ _ / _ _ _ _ |
| Medical History Event Date Type (Defaulted) SUPPMH.QVAL MDDEP_MHEVDTYP | DIAGNOSIS |
| Medical History Term (Defaulted) MHTERM MDDEP_MHTERM | MAJOR DEPRESSIVE DISORDER |
| MDD Diagnosis Start Date MHSTDTC MDDEP_MHSTDAT | _ _ / _ _ _ / _ _ _ _ |
In this example CRF, the following syntax was used to create denormalized CDASH variable names: Abbreviated "MHTERM"_DOMAIN ROOT VARIABLE.
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG MH - Medical History Domain
Examples are not available at this time.
8.3 CDASH Findings Domains
The Findings class includes CDASH domains that define collection standards for results from evaluations such as physical examinations, laboratory tests, ECG testing, and responses to questionnaires.
8.3.1 General CDASH Assumptions for Findings Domains
- CDASH --CAT and/or --SCAT are generally not entered on the CRF by the sites. Implementers may pre-populate and display these category values to help the site understand what data should be recorded on the CRF. Implementers may also pre-populate hidden variables with the values assigned within their operational database. Categories and subcategories that are not collected and are self-evident from the protocol design are populated during the creation of the SDTM submission dataset.
- CDASH --PERF
- --PERF variables having the Question Text "[Were any/Was the] [--TEST/ topic] [measurement(s)/test(s) /examinations (s)/specimen(s) /sample(s) ] [performed/collected]?" are intended to assist in the cleaning of data and in confirming that there are no missing values. (Refer to the CDASH Model v1.0 for more information on creating and using --PERF variables.)
- --PERF may be used at the page, panel, or question level. --PERF may be used during the creation of the STDM submission datasets to derive a value into the SDTM variable --STAT. The implementer can use a combination of --CAT, --SCAT, with the --TESTCD= "--ALL" and --TEST= "<Name of the CRF module>" to represent what tests were not performed. Refer to the current SDTMIG for more information.
- The CDASH variable --REASND is used with SDTM variable --STAT only. The value NOT DONE in --STAT indicates that the findings test was not performed.
- The CDASH --SPID variable may be populated by the sponsor's data collection system. If collected, it can be beneficial to use an identifier in a data query to communicate clearly to the site the specific record in question. This field may be populated by the sponsor's data collection system.
- Date and time variables
- CDASH variables (--DAT, --TIM) are used in Findings domains to collect the date or date and time that the test was done or performed. The SDTM --DTC variable contains either a date or date and time when a specimen is collected or the start date or start date and time when a specimen is collected over time.
- Collecting the time is only appropriate if it can be realistically determined and if there is a scientific reason for needing to know this level of detail. An example is where the subject is under the direct care of the site at the time the test was performed and the study design is such that it is important to know the time the test was performed with respect to dosing time.
- Implementers must not use these elements to record a date that is the result of a test (e.g. date of last day on the job). The date of the last day on the job would be recorded in the CDASH variable --ORRES. See the SDTMIG Section 4.1.4.9 for more information.
- The date of collection of a test may be derived from the date of visit. If so, a separate date of observation field is not required to be present on the CRF.
- Horizontal (Denormalized) and Vertical Data Structures (Normalized)
- In the CDASHIG Metadata Table, many of the CDASH Findings Class Domains are presented in a normalized structure (one record for each test) that is similar to the SDTM, even though many data management systems hold the data in a denormalized structure (one variable for each test). When implementing CDASH in a denormalized structure, create variable names for the Findings --TEST and/or --TESTCD values. To do this, define the denormalized variable names using available CDISC Controlled Terminology for --TESTCD. Alternatively, CDASH variable names for data management systems allowing more than 8 character variable names can use CDASH variables using the following naming convention: <--TESTCD>_<-- SDTM variable name> where --TESTCD is the appropriate CT for the test code e.g., DIABP_VSORRES, DIABP_VSLOC.
- In the horizontal (denormalized) setting, SDTM variables like --PERF, --LOC , --STAT may be collected once for the whole horizontal record and applying the value to all of the observations on that record, or they can be collected by test using the CDASH variable like <--TESTCD>_--PERF. When the SDTM submission datasets are created, any variables collected for the entire horizontal record must be mapped to each vertical record (if appropriate).
- In the horizontal (denormalized) setting, an identifier (e.g., --GRPID) may be used to identify all --TESTCD collected on the same record. This may facilitate the transformation from the CDASH horizontal setting to the SDTM vertical setting and creation of RELRECs.
- Tests and Original Results
- The value in --TEST cannot be longer than 40 characters. The corresponding codelist value for the short test name (at most 8 characters) must also be populated in the SDTM variable --TESTCD.
- --TESTCD should be used to create a variable name and --TEST be used as the Prompt on the CRF. Both --TESTCD and --TEST are recommended for use in the operational database. See Section 5, conformance to the CDASH Standard for more details.
- CDASH variable --ORRES is used to collect test results or findings in the original units in character format.
- If the results are modified for coding, the --MODIFY variable contains any modified text.
- If normal or reference ranges are collected for results, the CDASH variables --ORNRLO and --ORNRHI and --NIND are used.
- CDASH does not define the SDTM variable used to standardize the findings results (e.g., --STRESC, --STRESN) or to standardize the normal/reference ranges (--STNRLO,--STNRHI, --STNRC). The standardization of the original findings results and normal/reference ranges is expected to be performed during the creation of the SDTM submission datasets. Extensive discussion on the standardization of findings results is provided in the SDTMIG.
- Location variables (--LOC, --LAT, --DIR, --PORTOT)
- These variables are used to collect the location of the test. SDTM standard acknowledges that the results themselves may not be at the same location as the test. This is a known issue with SDTM.
- Sponsors may collect the data using a subset list of Controlled Terminology on the CRF. --LOC could be a defaulted or hidden field on the CRF.
The Findings About Events or Intervention domains use the same root variables as the Findings domain with the addition of the --OBJ variable. CDASHIG Metadata Table contains a generic FA domain.
It is assumed that implementers will add other data variables as needed to meet protocol-specific and other data collection requirements (e.g., TA-specific data elements and others as required per protocol, business practice, and operating procedures) to Findings and Findings About domains.
8.3.2 DA - Drug Accountability
Description/Overview for the CDASHIG DA - Drug Accountability Domain
The CDASHIG Drug Accountability domain is used to collect information about dispensing and returning of study treatment materials used in a clinical trial.
The SDTMIG separates drug accountability from the Exposure domain, which contains the data about the subjects' actual exposure to study treatment.
SDTMIG definitions:
- "Drug Accountability is for data regarding the accountability of study drug, such as information on receipt, dispensing, return, and packaging" See the current SDTMIG for more information.
- "The Exposure domain model records the details of a subject's exposure to protocol-specified study treatment. Study treatment may be any intervention that is prospectively defined as a test material within a study, and is typically but not always supplied to the subject." Examples include but are not limited to placebo, active comparators, and investigational products. Treatments that are not protocol-specified should be recorded in the Concomitant Medications (CM) domain. See the current SDTMIG for more information.
Care should be taken not to confuse drug accountability with study treatment compliance or study drug exposure. Comparing the amount dispensed to the subject and the amount returned by the subject does not necessarily mean the difference equates to the amount of treatment consumed by the subject or the subject's compliance with the treatment plan. For example, the subject could have dropped 2 tablets down into the sink drain, which would not be reflected in the returned amount and could provide a false estimate of compliance.
Because the actual treatment name may not be known to the site at the time of dispensing or returning, the word "treatment" in the context of the CDASHIG Drug Accountability domain is referring to the identifier that references the treatment (e.g., Bottle A, Bottle B, Drug A, Drug B) rather than the actual (unblinded) treatment name.
The term "dispensed" refers to when the study treatment is provided to the subject, not when the subject uses or consumes the study treatment. The term "returned" refers to when the subject returns the unused study treatment to the investigational site.
In some cases sponsors may wish to link Drug Accountability data to Exposure data. This may be accomplished by using the appropriate identifier variables and the relationship (RELREC) dataset as described in the SDTMIG.
This domain, which is in the Findings class, has been modeled in both normalized and denormalized structures to provide users with examples of how each structure could be implemented. Findings domains are typically represented in the vertical/normalized structure as this is usually the easiest and quickest way to collect, process, and clean the data. However, users may have system constraints that would prevent them from collecting this data in the vertical/normalized manner. The horizontal/denormalized version provides them with the structure necessary to collect the variables in this manner.
Depending on the study design, the Drug Accountability CRF/eCRF may not be required.
Specification for the CDASHIG DA - Drug Accountability Domain
Drug Accountability (DA)
Assumptions for the CDASHIG DA - Drug Accountability Domain
- The Drug Accountability domain is only needed if this information will be collected for the study.
- There may need to be a clear understanding of how the drugs are identified (by subject, by masked-id, etc.) in order to set up the data collection in a manner that makes sense. This is a cross-functional team issue that needs to be addressed early in planning, if applicable.
- Drug Accountability may be implemented for an entire study or on a visit-by-visit basis depending on the most logical approach for each protocol.
- The Drug Accountability panel can be used for studies that allow dispensing different types of study treatment (e.g., study medication, rescue medication, run-in medication) by using the DACAT variable to differentiate treatment types.
Example CRFs for the CDASHIG DA - Drug Accountability Domain
Example 1
This example shows normalized data collection for drug accountability information.
| Was drug accountability performed? DAPERF |
Ο Yes NOT SUBMITTED
Ο No DASTAT = "NOT DONE" WHERE DATESTCD = "DAALL"
|
| Treatment Type (Defaulted) DACAT | STUDY MEDICATION |
| Treatment Name (Defaulted) DASCAT | Sponsor Defined |
| Date DADTC DADAT | _ _ / _ _ _ / _ _ _ _ |
| Treatment Label ID DAREFID | _______________ |
| What was the drug accountability being assessed? DATEST |
Ο Dispensed
Ο Returned
|
| Amount DAORRES | _______________ |
| Unit (Defaulted) DAORRESU |
Sponsor Defined |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 2
This example shows denormalized data collection for drug accountability information.
| What was the date the drug accountability was assessed? DADTC DADAT |
_ _ / _ _ _ / _ _ _ _ |
| Dispensed Drug | |
|---|---|
| Was dispensed amount collected? DISPAMT_DAPERF |
Ο Yes NOT SUBMITTED
Ο No DASTAT = "NOT DONE" WHERE DATESTCD = "DISPAMT"
|
| Treatment Type (Defaulted) DACAT DISPAMT_DACAT | STUDY MEDICATION |
| Dispensed Treatment Label ID DAREFID DISPAMT_DAREFID | _______________ |
| Drug Accountability Type (Defaulted) DATEST = "DISPENSED AMOUNT" DISPAMT_DATESTCD | DISPENSED AMOUNT |
| Amount DAORRES WHERE DATESTCD = "DISPAMT" DISPAMT_DAORRES | _______________ |
| Unit (Defaulted) DAORRESU WHERE DATESTCD = "DISPAMT" DISPAMT_DAORRESU | Sponsor Defined |
| Returned Drug | |
| Was returned amount collected? RETAMT_DAPERF |
Ο Yes NOT SUBMITTED
Ο No DASTAT = "NOT DONE" WHERE DATESTCD = "RETAMT"
|
| Drug Accountability Type (Defaulted) DATEST = "RETURNED AMOUNT" | RETURNED AMOUNT |
| Returned Treatment Label ID DAREFID RETAMT_DAREFID | _______________ |
| Treatment Type (Defaulted) DACAT RETAMT_DACAT | STUDY MEDICATION |
| Amount DAORRES WHERE DATESTCD = "RETAMT" RETAMT_DAORRES | _______________ |
| Unit (Defaulted) DAORRESU WHERE DATESTCD = "RETAMT" RETAMT_DAORRESU | Sponsor Defined |
In this example CRF, the following syntax was used to create de-normalized variable names: <DATESTCD>_<ROOT VARIABLE>.
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG DA - Drug Accountability Domain
Examples are not available at this time.
8.3.3 DD - Death Details
Description/Overview for the CDASHIG DD - Death Details Domain
The Death Details domain is used to collect additional data that are typically collected when a death occurs, such as the official cause of death, when the death occurred, and whether it was witnessed.
The Death Details domain is not intended to replace or collate data collected on designated CRF pages such as the SAE details on the AE domain or details of disposition in the DS domain. Data on the Death Details domain may be linked to other domains that relate to death using the appropriate identifier variables and the related records (RELREC) dataset as described in the SDTMIG.
Specification for the CDASHIG DD - Death Details Domain
Death Details (DD)
Assumptions for the CDASHIG DD - Death Details Domain
- This domain captures information pertaining to the death of a subject, including the causes of death and findings of an autopsy directly related to the death itself (e.g., cause of death). If more than one cause of death is obtained, these may be separated into primary and secondary causes and/or other appropriate designations.
- This domain is not intended to include the details describing the autopsy (e.g., who conducted the autopsy or when). Autopsy information should be handled as per recommendations in the Procedures domain.
- An autopsy is a procedure from which there will usually be findings. Results of the autopsy not specific to the death itself will be represented in the appropriate Findings domain(s).
Example CRF for the CDASHIG DD - Death Details Domain
Example 1
| Were any death detail assessments collected? NOT SUBMITTED DDYN |
Ο Yes
Ο No
|
| What was the date the death detail assessments were collected? DDDTC DDDAT | _ _ / _ _ _ / _ _ _ _ |
| Death Date DSSTDTC DM.DTHDTC DTHDAT | _ _ / _ _ _ / _ _ _ _ |
| What is the primary cause of death? DDORRES WHERE DDTESTCD = "PRCDTH" PRCDTH_DDORRES | ________________________ |
| What is the secondary cause of death? DDORRES WHERE DDTESTCD = "SECDTH" SECDTH_DDORRES | ________________________ |
| What is the location of death? DDORRES WHERE DDTESTCD = "LOCDTH" LOCDTH_DDORRES | ________________________ |
| Who provided the death detail assessment information? DDEVAL |
Ο Adjudication Committee
Ο Health Care Professional
Ο Independent Assessor
|
In this example CRF, the following syntax was used to create denormalized variable names: DDTESTCD value_DOMAIN ROOT VARIABLE.
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG DD - Death Details Domain
Examples are not available at this time.
8.3.4 EG - ECG Test Results
Description/Overview for the CDASHIG EG - ECG Test Results Domain
EG domain metadata are provided for three different data collection scenarios.
- Scenario 1: Central Reading — In this scenario, results are captured directly by an electronic device and transmitted separately or read by a central vendor, not recorded on the CRF. The accession number and date collected on the CRF can be used as an aid in reconciliation of the electronic data.
- Scenario 2: Local Reading — In this scenario, subjects' ECGs are performed and analyzed, and then the results are recorded directly on the CRF.
- Scenario 3: Central Reading with Investigator Assessment of Clinical Significance Assessment and/or Overall Interpretation — In this scenario results are captured directly by an electronic device by a central vendor. The results are provided in an electronic file to the sponsor. In addition, the results are provided to the investigator for assessment of clinical significance for any abnormal values and that information is provided to the sponsor on the CRF.
Specification for the CDASHIG EG - EG Test Results Domain
ECG Test Results
Assumptions for the CDASHIG EG - EG Test Results Domain
- The ECG tests that should be collected are not specified by CDASH as this is a medical and scientific decision that should be based on the needs of the protocol.
- Sponsor should decide which scenario is appropriate for each protocol.
- As required or defined by the study protocol, clinically significant results may need to be reported on the Adverse Event CRF.
- As required or defined by the study protocol, changes that are worsening may need to be reported on the Adverse Event CRF.
- As depicted in Scenario 3, where CRF includes site assessment of clinical significance and/or overall interpretation, results are returned to the sites, and the sites complete a CRF page of clinical significance for any abnormal/unexpected values and/or record an overall interpretation of the results. The actual testing results are transmitted electronically, as in scenario 1, but the CRF includes the data necessary to identify and rate the clinical significance of the abnormal results.
Example CRFs for the CDASHIG EG - EG Test Results Domain
Example 1
This example shows a CRF layout that collects electrocardiogram data utilizing a central reader.
| Was the ECG performed? EGPERF |
Ο Yes NOT SUBMITTED
Ο No EGSTAT = "NOT DONE" WHERE EGTESTCD = "EGALL"
|
| What was the ECG reference identifier? EGREFID | _______________ |
| What was the ECG date? EGDTC EGDAT | _ _ / _ _ _ / _ _ _ _ |
| What was the ECG time? EGDTC EGTIM | _ _ : _ _ |
| What was the method used to measure ECG? EGMETHOD |
Ο 6-Lead Standard
Ο 12-Lead Standard
|
| What was the position of the subject during ECG measurement? EGPOS | Ο Supine
Ο Standing
|
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 2
This example shows a CRF layout that collects electrocardiogram data utilizing a local reader.
| Was the ECG performed? EGPERF |
Ο Yes NOT SUBMITTED
Ο No EGSTAT = "NOT DONE" WHERE EGTESTCD = "EGALL"
|
| What was the method used to measure ECG? EGMETHOD |
Ο 6-Lead Standard
Ο 12-Lead Standard
|
| What was the position of the subject during ECG measurement? EGPOS |
Ο Supine
Ο Standing
|
| What was the ECG date? EGDTC EGDAT | _ _ / _ _ _ / _ _ _ _ |
| What was the planned time point of the measurement? EGTPT | _______________ |
| What was the ECG time? EGDTC EGTIM | _ _ : _ _ |
| What was the ECG test name? EGTEST | _______________ |
| What was the result of the ECG? EGORRES | _ _ _ |
| What was the unit of the ECG result? EGORRESU |
_______________ |
| What was the interpretation of the ECG? EGORRES WHERE EGTESTCD = "INTP" INTP_EGORRES |
Ο Normal
Ο Abnormal
|
| Was the ECG clinically significant? SUPPEG.QVAL WHERE QNAM = "EGCLSIG" EGCLSIG |
Ο Yes
Ο No
|
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 3
This example shows a CRF layout that collects electrocardiogram data which is centrally processed.
| Was the ECG performed? EGPERF |
Ο Yes NOT SUBMITTED
Ο No EGSTAT = "NOT DONE" WHERE EGTESTCD = "EGALL"
|
| What was the ECG reference identifier? EGREFID | _______________ |
| What was the method used to measure ECG? EGMETHOD |
Ο 6-Lead Standard
Ο 12-Lead Standard
|
| What was the position of the subject during ECG measurement? EGPOS |
Ο Supine
Ο Sitting
|
| What was the ECG date? EGDTC EGDAT | _ _ / _ _ _ / _ _ _ _ |
| What was the ECG time? EGDTC EGTIM | _ _ : _ _ |
| What was the interpretation of the ECG? EGORRES WHERE EGTESTCD = "INTP" INTP_EGORRES |
Ο Normal
Ο Abnormal
|
| Was the ECG clinically significant? SUPPEG.QVAL WHERE QNAM = "EGCLSIG" EGCLSIG |
Ο Yes
Ο No
|
| Adverse Event Identifier LINKED TO RELATED AE VIA RELREC EGAENO | |
| Medical History Event Identifier LINKED TO RELATED AE VIA RELREC EGMHNO |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG EG - EG Test Results Domain
Examples are not available at this time.
8.3.5 IE - Inclusion/Exclusion Criteria Not Met
Description/Overview for the CDASHIG IE - Inclusion/Exclusion Criteria Not Met Domain
The CDASHIG IE domain is recommended for collecting only inclusion/exclusion exceptions, in other words those criteria that are "not met", since these are the data that are required to be in the SDTMIG IE domain. The CDASHIG IE domain is used to collect failures on or exceptions to the inclusion/exclusion criteria during the screening process before the subject is enrolled in the study. It is not intended to collect protocol deviations or violations that occur after enrollment. Protocol deviations are collected using the DV domain.
The recommendation is that sites be given an entry criteria worksheet to be used for each subject to record the results of their eligibility review. This worksheet should be considered a source document, used in monitoring activities, and maintained with the subject's site files. The worksheet should identify each criterion using a unique identifier, which can be easily recorded on the CRF if a subject does not meet that criterion. If criteria lists are numbered the same for both inclusion and exclusion criteria (e.g., inclusion 001-100, exclusion 001-100), then this identifier could include a means of identifying the TYPE of criterion (e.g., I001-I100, E001-E100). Alternatively, the criteria could be collected in two separate sections on the CRF labeled "Inclusion" and "Exclusion" and the output records could include the values of Inclusion or Exclusion on each record. These are only examples; an organization's numbering scheme may be different, but some method that captures both the Inclusion or Exclusion category and the unique criterion identifier should be used.
The recommended collection method has been simplified to require the site to record a single "Y/N" value in the IEYN variable to indicate whether or not the subject met all of the criteria. If any criteria are not met, the site then records only those "unmet" criteria in the CRF. The result value for each unmet criterion may then be derived in the SDTMIG IE domain from the collection of the specific criterion that was not met. In other words, if the collected criterion is an inclusion criterion that was not met, the value of "N" can be derived into IEORRES and IESTRESC for that record in the SDTMIG IE domain. If it is an exclusion criterion, then "Y" can be derived into IEORRES and IESTRESC to indicate that the subject met the conditions for that exclusion record in IE.
The rationale for the recommended collection method is that what is being collected in the IE CRF is aligned with the data that would be in the SDTMIG IE domain.
This design allows criteria to change over the life of a study or project, e.g., when adaptive trial designs are used or protocol amendments result in changes to the inclusion or exclusion criteria. If inclusion/exclusion criteria were amended during the trial, then each complete set of criteria must be included in the TI domain. TIVERS is used to distinguish between the versions of the eligibility criteria.
CDASH recommends the use of uniquely numbered entry criteria within a study to manage effectively protocol changes and to facilitate both the collection and submission of IE data. See the current SDTMIG for more details. The Inclusion/Exclusion worksheet may need to be updated and re-numbered/re-lettered whenever a protocol amendment changes one or more criteria. For example, if new versions of a criterion have not been given new numbers, separate values of IETESTCD might be created by appending letters, e.g., INCL003A, INCL003B.
A field could be added to the CRF to capture the version number of the criteria being used; this can be mapped to the SDTMIG variable TI.TIVERS. This enables the retrieval of the full text of the criterion from the code used on the CRF.
Alternatively, an implementer may choose to include the full text of each criterion (IETEST) with a result field (IEORRES) in the CRF and request the site to record explicitly the "Y" or the "N" for each criterion, but only the recommended, simplified method is presented in the IE example CRF below.
Specification for the CDASHIG IE - Inclusion/Exclusion Criteria Not Met Domain
Inclusion/Exclusion Criteria Not Met (IE)
Assumptions for the CDASHIG IE - Inclusion/Exclusion Criteria Not Met Domain
- The recommendation is for only those records for criteria that are not met to be collected in the IE CRF.
- The complete list of Inclusion/Exclusion criteria and the version number of each of the criteria are provided in the SDTMIG TI dataset. The IETEST and IETESTCD values used to collect data in the IE CRF should match the values in the TI dataset.
- Categories IECAT and IESCAT
- The SDTMIG variable IECAT must be populated with INCLUSION or EXCLUSION. This criterion category may be collected on the CRF in a checkbox format using the CDASHIG field IECAT or it may be included as part of the Criterion Identification and populated when the SDTM submission datasets are created.
- IESCAT may be used by the sponsor to further categorize the exception criteria within the larger categories of Inclusion or Exclusion (e.g., Major, Minor).
- These categories may be collected on the CRF or they may be used as titles on the CRF and hidden/defaulted in the operational system. If these categories are not collected on the CRF or created in the operational data management system, they are added when the SDTM submission datasets are created.
- There should be a unique IETESTCD for each unique criterion text in IETEST, and these values must match the values in the TI domain.
- It may be useful to collect the protocol version under which a subject was screened.
- Collection Date (IEDAT) - This is the date that the IE data were recorded for the study, and not the actual date the exception occurred. The Visit Date (VISDAT) may be used, instead, to populate the SDTMIG IEDTC variable.
- Result (IEORRES) - The result ("Y" or "N") for each SDTMIG IETESTCD is derived or inferred from the collection of the specific criterion not met. IEORRES must be populated in the SDTMIG IE domain because it is a Required variable. Sponsors will populate this in the operational data management system or in the creation of the SDTMIG submission datasets. When an Inclusion Criterion is not met, the SDTMIG variable IEORRES is populated with "N" and when an Exclusion Criterion is not met, IEORRES is populated with "Y".
Example CRFs for the CDASHIG IE - Inclusion/Exclusion Criteria Not Met Domain
Example 1
Example CRF Completion Instructions
- All procedures must be performed and the subject's eligibility determined within <protocol-specified time period prior to study medication administration>.
- (If applicable, include the following instructions) Complete the Inclusion/Exclusion Worksheet as source document by recording a "Yes" or "No" response to each criterion.
- Record the criterion identification that was an exception.
| Were all eligibility criteria met? IEYN NOT SUBMITTED |
Ο Yes
Ο No
|
| What was the category of the criterion? IECAT |
Ο Inclusion
Ο Exclusion
|
| What was the identifier of the inclusion criterion the subject did not meet or the exclusion criterion the subject met? IETESTCD | _______________ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG IE - Inclusion/Exclusion Criteria Not Met Domain
Examples are not available at this time.
8.3.6 LB - Laboratory Test Results
Description/Overview for the CDASHIG LB - Laboratory Test Results Domain
Laboratory test findings domain includes, but is not limited to hematology, clinical chemistry, and urinalysis data. This domain does not include microbiology or pharmacokinetic data, which are represented in separate domains. The actual lab parameters that should be collected are not specified by CDASH.
The section below describes three different data collection scenarios for laboratory test results. It is up to the sponsor to determine which data collection scenario best meets the study needs.
- Scenario 1: Central Processing — In this scenario, subject specimens are taken at site and sent out for processing. Results are provided in an electronic file and the sponsor has chosen to collect reconciliation data (e.g., LBDAT, LBTIM, VISITNUM, LBREFID) on the CRF. This scenario may also apply if the central lab results are imported into the sponsor's EDC system. The fields for test results are not defined here, as these data are not part of the CRF.
- Scenario 2: Local Processing — In this scenario, subject specimens are taken and analyzed, and then the results are recorded directly on the CRF.
- Scenario 3: Central Processing with Investigator Assessment of Clinical Significance Assessment for Abnormal Values — In this scenario, subject specimens are taken at site and sent to a central lab for processing. The results are provided in an electronic file to the sponsor. In addition, the results are provided to the investigator for assessment of clinical significance for any abnormal values and that information is provided to the sponsor on the CRF.
Specification for the CDASHIG LB - Laboratory Test Results Domain
Laboratory Test Findings (LB)
Assumptions for the CDASHIG LB - Laboratory Test Results Domain
- The lab parameters that should be collected are not specified by CDASH, as this is a medical and scientific decision that is based on the needs of the protocol.
- Sponsor should decide which scenario is appropriate for each protocol.
- As required or defined by the study protocol, clinically significant results may need to be reported on the Medical History or Adverse Event CRF.
- As required or defined by the study protocol, changes that are worsening may need to be reported on the Adverse Event CRF.
- All pertinent laboratory normal ranges/units and laboratory certification for all laboratories used during the study will be provided to the sponsor. This is required for regulatory and database purposes.
Example CRF for the CDASHIG LB - Laboratory Test Results Domain
There are no example CRFs.
Example Data for the CDASHIG LB - Laboratory Test Results Domain
Examples are not available at this time.
8.3.7 MI - Microscopic Findings
Description/Overview for the CDASHIG MI - Microscopic Findings Domain
The microscopic findings domain collects the histopathology findings and microscopic evaluations that do not have specialized domains (e.g., MB and MS) for their results.
MI domain metadata are provided for two different data collection scenarios. It is up to the sponsor to determine which data collection scenario best meets the study needs. Sponsors may implement Scenario 3: Central Processing with Investigator Assessment of Clinical Significance Assessment for Abnormal Values following the LB domain scenario example, even though such an example is not provided in the CDASHIG Metadata Table.
- Scenario 1: Central Processing — In this scenario, subject specimens are taken at site, sent out for processing, and results are provided directly to the sponsor and not recorded on the CRF. This scenario also applies when results are captured directly via an electronic device and not recorded on the CRF. CRF data are captured at the site for tracking/ header reconciliation. The fields for test results are not defined here, as these data are not part of the CRF.
- Scenario 2: Local Processing — In this scenario, subject specimens are taken and analyzed, and then the results are recorded directly on the CRF.
Specification for the CDASHIG MI - Microscopic Findings Domain
Microscopic Findings (MI)
Assumptions for the CDASHIG MI - Microscopic Findings Domain
- This domain is used for findings resulting from the microscopic examination of tissue samples. These examinations are performed on a specimen, which has been prepared with some type of stain. Some examinations of cells in fluid specimens such as blood or urine are classified as lab tests and should be represented in the LB domain. The tests classified as pathology or cytology should be represented in the MI domain. Biomarkers assessed by histologic or histopathological examination (by employing cytochemical/immunocytochemical stains) will be represented in the MI domain.
- Sponsor should decide which scenario is appropriate for each protocol.
- The variable MITSTDTL is used when biomarker tests are represented in the MI domain. It represents test parameter details descriptive of slide stain results (e.g., cells at 1+ intensity cytoplasm stain, H-Score, nuclear reaction score).
- These CDASHIG variables are generally not used for this domain: --POS, --MODIFY, --ORNRLO, --ORNRHI, --LEAD.
Example CRFs for the CDASHIG MI - Microscopic Findings Domain
Example 1
| Was the microscopic examination performed? MIPERF |
Ο Yes [NOT SUBMITTED]
Ο No MISTAT = "NOT DONE" WHERE MITESTCD = "MIALL"
|
| Collection Date MIDTC MIDAT | _ _ / _ _ _ / _ _ _ _ |
| Microscopic Test Name (Defaulted) MITEST | Sponsor Defined |
| Additional Test Name Details (Defaulted) MITSTDTL | Sponsor Defined |
| Result MIORRES | ______________ |
| Result Category MIRESCAT |
Ο Negative
Ο Positive
Ο Borderline
|
| Specimen Type MISPEC |
Ο Adipose Tissue
Ο Bone Marrow
Ο Circulating Tumor Cell
Ο Skeletal Muscle Tissue
Ο Striated Muscle Tissue
Ο Tumor Tissue
|
| Anatomical Location MILOC |
Ο Abdomen
Ο Chest Wall
Ο Femur
Ο Hip
Ο Leg
Ο Liver
Ο Pericardium
|
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG MI - Microscopic Findings Domain
Examples are not available at this time.
8.3.8 PC - Pharmacokinetics Sampling
Description/Overview for the CDASHIG PC - Pharmacokinetics Concentration Domain
Information about sampling for pharmacokinetic (PK) concentration is collected in CRFs with the goal to reconcile or link sampling information (e.g., collection timing and specimen volumes) with pharmacokinetic concentration results provided by the laboratory. SDTMIG PC records are compiled when joining CRF sampling information and pharmacokinetic concentration results. This is similar to CDASHIG LB Scenario 1.
The goals of the CDASHIG PC domain are:
- To standardize specimen collection details in the CRF for PK samples collected at fixed time points or over timed intervals..
- To provide CDASHIG examples as to the collection of data that is closely related to PK sampling, such as the subject's most recent exposure to study treatment or the exposure record that is considered to be the "reference" for the timed PK samples.
- To document the data flow from the CDASHIG CRF to the SDTMIG PC dataset..
The CDASHIG PC domain defines fields for:
- The date and time of PK sample collections
- Either at fixed defined time points (e.g., 4 HRS POSTDOSE) or across a collection interval (e.g., 2-4 HRS POSTDOSE).
- How the body metabolizes and clears the analyte often determines which of these sampling approaches may be required.
- Sample properties (e.g., pH, sample volume)
Samples collected to measure drug concentration at an instant in time are generally associated with specimen types such as plasma, serum, or whole blood. Samples collected over a timed interval are generally associated with specimen types such as urine or feces.
PK Sample Collection at Fixed Time Points
In the case of fixed time points, the date (PCDAT) and time (PCTIM) of collection for each sample is recorded in the CRF. The protocol defines the time points at which samples are to be collected in relation to an intervention such as a dose of study treatment. This "reference" is depicted below by the longer vertical line and would correspond to a date and time in the EC or EX domain.
PK Sample Collection over a Time Interval
Similarly, for PK specimens collected to measure drug excretion over a time interval, the PCDAT and PCTIM capture the start date and time of the interval collection. End date (PCENDAT) and end time (PCENTIM) capture the end of the timed interval collection. As with fixed time point collections, these timed intervals are performed in relation to an intervention such as a dose of study treatment. This "reference" is depicted below by the longer vertical line and would correspond to a date and time in the EC or EX domain.
CDASHIG-SDTMIG PK DATA FLOW:
The concept map below illustrates the data flow from PK sample collection at site through the tabulation of both PK concentration and PK parameter results.
Specification for the CDASHIG PC - Pharmacokinetic Concentrations Domain
Pharmacokinetics (PC)
Assumptions for the CDASHIG PC - Pharmacokinetic Concentrations Domain
- This domain contains details regarding the collection of the PK samples from subjects at the site (e.g., timing of sample collection and associated specimen properties). Typically, the CDASHIG PC domain does not include concentration results generated by the bioanalytical laboratory. However, if the sponsor has occasion to collect concentration results directly on the same CRF, variables may be created based on CDASH Model variables, using the rules previously discussed.
- Other data may be needed for PK analysis, e.g., Demographics, Vital Signs, Substance Use, and Exposure. Refer to the appropriate CDASHIG sections for each of these domains.
Example CRFs for the CDASHIG PC - Pharmacokinetic Concentrations Domain
Example 1: PK Sample Collection at Fixed Time Points
| Accession Number PCREFID | _______________ |
| Visit Name (Defaulted) VISIT | Day 1 |
| Planned Time Point Name PCTPT |
Ο Pre-dose
Ο Hour 1
Ο Hour 3
Ο Hour 5
Ο Hour 12
Ο Hour 18
|
| Indicate if the PK sample was not done PCSTAT | Ο Not Done |
| Reason Not Done PCREASND | _______________ |
| Check if this specimen was collected on the same date as the previous specimen [NOT SUBMITTED] PCDATFL | Ο |
| Collection Date PCDTC PCDAT | _ _ / _ _ _ / _ _ _ _ |
| Collection Time PCDTC PCTIM | _ _ : _ _ |
| Test Name PCTEST | Ο VOLUME |
| Result PCORRES | ______________ |
| Unit PCORRESU |
Ο mL
Ο L
|
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 2: PK Sample Collection over a Time Interval.
| Accession Number PCREFID | _________________ |
| Planned Time Point Name PCTPT |
Ο 0-4 Hours after dose
Ο 4-8 Hours after dose
Ο 8-12 Hours after dose
Ο 12-16 Hours after dose
Ο 16-20 Hours after dose
Ο 20-24 Hours after dose
|
| Indicate if the PK sample collection for this interval was not done PCSTAT | Ο Not Done |
| Collection Start Date PCDTC PCDAT | _ _ / _ _ _ / _ _ _ _ |
| Collection Start Time PCDTC PCTIM | _ _ : _ _ |
| Collection End Date PCENDTC PCENDAT | _ _ / _ _ _ / _ _ |
| Collection End Time PCENDTC PCENTIM | _ _ : _ _ |
| Test Name PCTEST | Ο VOLUME |
| Result PCORRES | ______________ |
| Unit PCORRESU |
Ο mL
Ο L
|
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG PC - Pharmacokinetic Concentrations Domain
Examples are not available at this time.
8.3.9 PE - Physical Examination
Description/Overview for the CDASHIG PE - Physical Examination Domain
The scope of the metadata for the PE domain is limited to general physical examinations as part of overall safety data collection. The data collection fields defined in the CDASHIG Metadata Tables may not fit the needs of targeted body system evaluations as part of therapeutic area specific assessments. Following are the three scenarios that may be used for collecting physical exam data.
- Scenario 1 — The PE CRF is used only to record whether or not the exam was performed, and if so, the date of the examination. Sites are instructed to record baseline abnormalities on a medical history form, a targeted medical history form (e.g., a study-specific form requesting assessment of a pre-defined set of medical and/or surgical history events), or a baseline conditions form. Sites are typically instructed to record any post-baseline abnormalities or baseline conditions that worsened post baseline on the AE form.
- Scenario 2 — The PE CRF is used at baseline and post-baseline visits.
- Scenario 3 — The PE CRF is used at baseline, but not at post-baseline visits. Sites are instructed to record any post-baseline abnormalities or baseline conditions that worsened post baseline on the AE form.
In Scenarios 2 and 3, similar fields are captured: date of exam, body system/code, normal/abnormal, and description of abnormality. Scenario 1 is recommended as the best practice for the following reasons:
- It eliminates collection and reconciliation of duplicate data by capturing abnormal data in one central location. Abnormalities identified during a physical examination must also be recorded on an AE form, a medical history form, or other similar form.
- It reduces number of queries, thus reducing workload for data managers and site personnel.
- It supports consistency and standardization for data reporting purposes. Physical examination data are rarely summarized, only tabulated in listing format. Any trend analysis or summarization of abnormalities is performed on AE data, and medical history data are available for reference.
- It reduces coding needs (if PE abnormalities are coded).
Specification for the CDASHIG PE - Physical Examination Domain
Scenario 1 is a change from the more traditional approach for the submission of physical examination data. In this approach, the SDTMIG PE domain is not submitted. The physical examination data are submitted in the appropriate SDTMIG domain. Typically, the screening/baseline data are submitted in the SDTMIG MH domain, while post-baseline abnormalities or baseline conditions that worsened are submitted in the SDTMIG AE domain. Sponsors may submit information on whether PE examinations were performed and when they were performed in the SDTMIG PR domain. There is no CDASH PE domain specification for this approach in the CDASHIG Metadata Table.
In Scenarios 2, and 3, the sponsor may elect to submit this PE data in the SDTMIG PE domain, since the more traditional approach for data collection is followed. The CDASHIG Metadata Table includes this PE traditional scenario.
Physical Exam (PE)
Assumptions for the CDASHIG PE - Physical Examination Domain
Best Practice Domain Model
- Since the data from a general physical examination are not required for safety or efficacy evaluations, a sponsor may decide not to collect them on a separate PE CRF. The data would be collected on other CRFs, typically the AE and MH CRFs.
- If the sponsor chooses to create a PE CRF to capture only the information on "Was the Physical Examination performed?" and Date/Time of Examination, these may be considered as optional fields intended for monitoring and data cleaning only. However, the sponsor could elect to submit this information in the SDTMIG PR domain.
Traditional Domain Model
- If the sponsor chooses to create a traditional PE CRF, PEYN is still considered an optional field intended for monitoring and data cleaning only. If a sponsor wants to document information about overall physical examination at the subject level that were not performed in the SDTM submission, this field is mapped to PESTAT. If PEPERF= "N", then PECAT= "GENERAL", PETEST= "PHYSICAL EXAMINATION", PETESTCD= "PEALL", and PESTAT = "NOT DONE". If PEPERF = "Y", then the actual Physical exam results would be reported by body system (see PERES).
- Original and Standardized Results
- The CDASHIG variable PERES is used to collect test results or findings in the original units in character format as well as to collect the information on what specific body systems were not examined.
- When the results of a test or examination are reported as Normal or Abnormal, a description of the abnormal finding may also be collected using the CDASHIG element PEDESC.
- The information on what body system reviews were not done are mapped to the appropriate SDTMIG variables (i.e., PETEST, PETESTCD, PESTAT, with the ORRES being NULL). The value of "NOT DONE" in CDASHIG variable PERES should not be mapped to the SDTMIG variable PEORRES.
- The standardization of the original findings results is expected to be performed when the SDTM submission datasets are created. The SDTM mapping rules are provided in the CDASHIG Metadata Table.
- Date of Collection is the date that the examination was performed. The SDTMIG variable PEDTC can be populated from the date of visit.
Example CRFs for the CDASHIG PE - Physical Examination Domain
Example 1
Example CRF Completion Instructions
If the physical finding/abnormality started prior to <protocol-specific time period>, then it must be recorded as medical history.
If the physical finding started after <protocol-specific time period>, it must be recorded as an Adverse Event.
| Was the physical examination performed? PROCCUR WHERE PRTRT = "PHYSICAL EXAM" PROCCUR |
Ο Yes
Ο No
|
| What was the reason the physical examination was not performed? SUPPPR.QVAL WHERE QNAM = "PRREASOC" PRREASOC | _______________ |
| What was the physical examination date? PRDTC PRDAT | _ _ / _ _ _ / _ _ _ _ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
8.3.10 QRS - Questionnaires, Ratings and Scales
Description/Overview for the CDASHIG QRS - Questionnaires, Ratings and Scales Domain
Questionnaires, ratings and scales (QRS) are standardized and often validated instruments, and the data collected using them are represented in SDTMIG domains including Questionnaires (QS), Disease Response and Clin Classification (RS),and Functional Tests (FT). Please refer to the SDTMIG or the QRS web page for complete information on these domains.CDISC publishes supplemental specifications called QRS Supplements, including example annotated CRFs for many of these instruments. There is also a CDISC Operating Procedure (COP-017) that describes the development methodology for new QRS terminology. The nature of QRS precludes implementers from modifying the published data collection structure, therefore the CDASHIG Metadata Table does not include specifications for QRS. Instead, implementers should refer to instrument-specific QRS Supplements (available from https://www.cdisc.org/foundational/qrs) for example annotated CRFs, instrument-specific assumptions, and data examples.
For definitions and descriptions of the different types of QRS, visit the QRS web page (https://www.cdisc.org/foundational/qrs).
The released QRS documentation is maintained on the CDISC QRS web page (https://www.cdisc.org/foundational/qrs).
Specification for the CDASHIG QRS - Questionnaires, Ratings and Scales Domain
Reference the QRS Supplements posted on the QRS web page (https://www.cdisc.org/foundational/qrs) and the specifications for specific domains (QS, RS, and FT) in the SDTMIG.
Assumptions for the CDASHIG QRS - Questionnaires, Ratings and Scales Domain
- CDISC standards for QRS include controlled terminology for test codes (--TESTCD), test names (--TEST), standard timing values, standard results for database values, and an annotated CRF with the SDTMIG domain variable names. These standards can be used to create an electronic data collection structure following the same conventions that would be used for any Findings class domain. The SDTMIG QS, RS, and FT domains utilize a normalized data structure, i.e., one variable (--TEST) is used to capture the test name and another variable (--ORRES) is used to capture the result. Even though these domain variables are presented as a normalized structure in the CDASHIG Metadata Table, implementers using a denormalized structure (one variable for each test) should create variable names that mirror the values in QRS controlled terminology (e.g., QSTESTCD, RSTESTCD, or FTTESTCD).
- Electronic representations of QRS instruments should reflect the title, subheadings, and exact numbering and wording of questions as they appear in original versions.
- Electronic response fields should allow either the original response (--ORRES) or coded value (--STRESC) to be input, but usually not both, to avoid discrepancies.
- Checkboxes that appear on validated QRS instruments should remain checkboxes in the CRF/eCRF.
- Copyrighted instruments may include the copyright notice on the eCRF/CRF. For more copyright Information about QRS instruments visit the QRS web page (https://www.cdisc.org/foundational/qrs).
- Instrument-specific assumptions are included in the QRS Supplements posted on the QRS web page (https://www.cdisc.org/foundational/qrs).
Example CRFs for the CDASHIG QRS - Questionnaires, Ratings and Scales Domain
For additional examples, reference the annotated CRFs that are part of the QRS Supplements on the QRS web page (https://www.cdisc.org/foundational/qrs).
Example Data for the CDASHIG QRS - Questionnaires, Ratings and Scales Domain
Reference the examples in the QRS Supplements posted on the QRS web page (https://www.cdisc.org/foundational/qrs).
8.3.11 SC - Subject Characteristics
Description/Overview for the CDASHIG SC - Subject Characteristics Domain
The Subject Characteristics domain describes protocol-specified characteristics of the study subjects and serves as an extension of the data contained in the Demographics domain. It is important to note that:
- Data in this domain arecollected only once per subject.
- Subject Characteristics consists of data that are collected once at the beginning of the trial and are not expected to change during the trial.
- Subject Characteristics contains data such as additional information about education level, marital status, and national origin.
- There is extensible CDASHIG controlled terminology for SCTEST. These data might be useful, for example, for risk-benefits analyses or quality of life analyses, or for sub-setting a subject population.
- The SDTMIG SC domain utilizes a normalized data structure, i.e., one variable (SCTEST) is used to capture the test name and another variable (SCORRES) is used to capture the result. Subject Characteristics are presented as a normalized structure in the CDASHIG Metadata Table, but implementers using a denormalized structure (one variable for each test) should create variable names that mirror the SCTESTCDs in controlled terminology.
Specification for the CDASHIG SC - Subject Characteristics Domain
Subject Characteristics (SC)
Assumptions for the CDASHIG SC - Subject Characteristics Domain
- The Subject characteristics that should be collected are not specified by CDASH as this is a medical and scientific decision that should be based on the needs of the protocol.
- The SDTMIG variable SCDTC can be determined from a collected date of the visit (VISDAT); in such cases a CDASH date of collection field is not required on the CRF.
Example CRF for the CDASHIG SC - Subject Characteristics Domain
Example 1
| Were subject characteristics collected? SCPERF |
Ο Yes NOT SUBMITTED
Ο No SCSTAT = "NOT DONE" WHERE SCTESTCD = "SCALL"
|
| What is the subject's employment status? SCORRES WHERE SCTESTCD = "JOBCLAS" JOBCLAS_SCORRES | Ο Full-time Ο Part-time Ο Not employed |
| What is the subject's highest education level achieved? SCORRES WHERE SCTESTCD = "EDULEVEL" EDULEVEL_SCORRES | Ο Did not complete Secondary School or Less than High School Ο Some Secondary School or High School Education Ο High School or Secondary School Degree Complete Ο Associate's or Technical Degree Complete Ο College or Baccalaureate Degree Complete Ο Doctoral or Postgraduate Education |
| What is the subject's marital status? SCORRES WHERE SCTESTCD = "MARISTAT" MARISTAT_SCORRES | Ο Never Married Ο Married Ο Legally Separated Ο Divorced Ο Widowed |
In this example CRF, the following syntax was used to create denormalized variable names: SCTESTCD value_DOMAIN ROOT VARIABLE.
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG SC - Subject Characteristics Domain
Examples are not available at this time.
8.3.12 RP - Reproductive System Findings
Description/Overview for the CDASHIG RP - Reproductive System Findings Domain
The Reproductive System Findings domain is used to collect all reproductive detail information about the subject, such as the subject's reproductive ability, reproductive history such as number of previous pregnancies and number of births, pregnancy during the study, etc. All Reproductive System Findings for a subject are contained in the RP domain rather than other SDTMIG domains. Sponsors previously may have reported this information in the SC domain, but this information is now consolidated into the RP domain.
Specification for the CDASHIG RP - Reproductive System Findings Domain
Reproductive System Findings (RP)
Assumptions for the CDASHIG RP - Reproductive System Findings Domain
Any information on medications related to reproduction such as contraceptives or fertility treatments should not be collected in the Reproductive System Findings domain, instead they will need to be collected in the CM domain.
Example CRF for the CDASHIG RP - Reproductive System Findings Domain
Example 1
| Were any reproductive system findings tests performed? RPPERF |
Ο Yes NOT SUBMITTED
Ο No RPSTAT = "NOT DONE" WHERE RPTESTCD = "RPALL"
|
| Reason Not Done RPREASND | _______________ |
| What was the subject's Menarche Age? RPORRES WHERE RPTESTCD = "MENARAGE" MENARAGE_ORRES | _______________ |
| Unit (Defaulted) RPORRESU WHERE RPTESTCD = "MENARAGE" MENARAGE_ORRESU |
YEARS |
| Number of Pregnancies RPORRES WHERE RPTESTCD = "PREGNN" PREGNN_ORRES | _______________ |
| Number of Live Births RPORRES WHERE RPTESTCD = "BRTHLVN" BRTHLVN_ORRES | _______________ |
| What was the subject's Menopause Age? RPORRES WHERE RPTESTCD = "MENOAGE" MENOAGE_ORRES | _______________ |
| Unit (Defaulted) RPORRESU WHERE RPTESTCD = "MENOAGE" MENOAGE_ORRESU |
YEARS |
| Collection Date RPDTC RPDAT | _ _ / _ _ _ / _ _ _ _ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG RP - Reproductive System Findings Domain
Examples are not available at this time.
8.3.13 SR - Skin Response
Description/Overview for the CDASHIG SR - Skin Response Domain
The Skin Response domain is a Findings About domain used to collect dermal responses to antigens.
Specification for the CDASHIG SR - Skin Response Domain
Skin Response Findings (SR).
Assumptions for the CDASHIG SR - Skin Response Domain
- The SR domain is to be used when the skin response is the primary response that is being assessed, as is typically the case for allergy test kits and TB Skin test, versus it being a secondary response to the actual injection.
- The method of assessment is typically a skin-prick test.
Example CRFs for the CDASHIG SR - Skin Response Domain
Example 1
| Skin Response Category: Defaulted SRCAT | Sponsor Defined |
| Was a skin response test performed? SRPERF |
Ο Yes NOT SUBMITTED
Ο No SRSTAT = "NOT DONE" WHERE SRTESTCD = "SRALL"
|
| What was the reason not done? SRREASND | ________________ |
| What intervention was performed to elicit the skin response? SROBJ |
________________ |
| What was the planned time point for skin response measurement? SRTPT | ________________ |
| What was the date of the intervention performed to elicit the skin response? SRRFTDTC SRRFTDAT | _ _ / _ _ _ / _ _ _ _ |
| What was the time of the intervention performed to elicit the skin response? SRRFTDTC SRRFTTIM | _ _ : _ _ : _ _ |
| What was the date of the skin response measurement? SRDTC SRDAT | _ _ / _ _ _ / _ _ _ _ |
| _ _ : _ _ : _ _ | |
| What was the anatomical location of the skin response measurement? SRLOC |
Ο ARM
Ο LEG
|
| What was the side of the anatomical location of the skin response measurement? SRLAT |
Ο RIGHT
Ο LEFT
|
| What was the directionality of the anatomical location of the skin response measurement? SRDIR |
Ο UPPER
Ο LOWER
Ο ANTERIOR
|
| What was the skin response test name? SRTEST |
Ο Flare Size
Ο Flare Mean Diameter
Ο Induration Longest Diameter
Ο Wheal Size
Ο Wheal Longest Diameter
Ο Wheal Mean Diameter
|
| What was the result of the measurement? SRORRES | _ _ _ . _ _ |
| What was the unit of the measurement? SRORRESU |
Ο mm
Ο cm
|
| How did the reported values compare within the reference or expected range? SRNRIND |
Ο NORMAL
Ο ABNORMAL
|
| Was the result clinically significant? SUPPSR.QVAL WHERE QNAM = "SRCLSIG" SRCLSIG |
Ο Yes
Ο No
|
| Who provided the skin response measurement information? SREVAL |
Ο INVESTIGATOR
Ο HEALTH CARE PROFESSIONAL
Ο STUDY SUBJECT
|
| What was the identifier of the evaluator providing the skin response measurement information? SREVALID | ________________ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 2
| Skin Response Category: Defaulted SRCAT | Sponsor Defined |
| Was a skin response test performed? SRPERF |
Ο Yes NOT SUBMITTED
Ο No SRSTAT = "NOT DONE" WHERE SRTESTCD = "SRALL"
|
| What was the reason not done? SRREASND | _________________ |
| What intervention was performed to elicit the skin response? SROBJ | _________________ |
| What was the date of the intervention performed to elicit the skin response? SRRFTDTC SRRFTDAT | _ _ / _ _ _ / _ _ _ _ |
| What was the time of the intervention performed to elicit the skin response? SRRFTDTC SRRFTTIM | _ _ : _ _ |
| What was the planned time point for skin response measurement? SRTPT | _________________ |
| What was the date of the skin response measurement? SRDTC SRDAT | _ _ / _ _ _ / _ _ _ _ |
| What was the time of the skin response measurement? SRDTC SRTIM | _ _ : _ _ |
| What was the anatomical location of the skin response measurement? SRLOC |
Ο ARM
Ο LEG
|
| What was the side of the anatomical location of the skin response measurement? SRLAT |
Ο RIGHT
Ο LEFT
|
| What was the directionality of the anatomical location of the skin response measurement? SRDIR |
Ο UPPER
Ο LOWER
Ο ANTERIOR
|
| What was the result of the Wheal Size measurement? SRORRES WHERE SRTESTCD = "WHEALSZ" WHEALSZ_SRORRES | _ _ _ . _ _ |
| What was the unit of the Wheal Size measurement? SRORRESU WHERE SRTESTCD = "WHEALSZ" WHEALSZ_SRORRESU |
Ο mm
Ο cm
|
| How did the reported values compare within the reference or expected range? SRNRIND WHERE SRTESTCD = "WHEALSZ" WHEALSZ_SRNRIND |
Ο NORMAL
Ο ABNORMAL
|
| Was the result clinically significant? SUPPSR.QVAL WHERE QNAM = "SRCLSIG" AND SRTESTCD = "WHEALSZ" WHEALSZ_SRCLSIG |
Ο Yes
Ο No
|
| What was the result of the Flare Size measurement? SRORRES WHERE SRTESTCD = "FLARESZ" FLARESZ_SRORRES | _ _ _ . _ _ |
| What was the unit of the Flare Size measurement? SRORRESU WHERE SRTESTCD = "FLAREZ" FLARESZ_SRORRESU |
Ο mm
Ο cm
|
| How did the reported values compare within the reference or expected range? SRNRIND WHERE SRTESTCD = "FLARESZ" FLARESZ_SRNRIND |
Ο NORMAL
Ο ABNORMAL
|
| Was the result clinically significant? SUPPSR.QVAL WHERE QNAM = "SRCLSIG" AND SRTESTCD = "FLARESZ" FLARESZ_SRCLSIG |
Ο Yes
Ο No
|
| What was the result of the Induration Longest Diameter measurement? SRORRES WHERE SRTESTCD = "IDRLDIAM" IDRLDIAM_SRORRES | _ _ _ . _ _ |
| What was the unit of the Induration Longest Diameter measurement? SRORRESU WHERE SRTESTCD = "IDRLDIAM" IDRLDIAM_SRORRESU |
Ο mm
Ο cm
|
| How did the reported values compare within the reference or expected range? SRNRIND WHERE SRTESTCD = "IDRLDIAM" IDRLDIAM_SRNRIND |
Ο NORMAL
Ο ABNORMAL
|
| Was the result clinically significant? SUPPSR.QVAL WHERE QNAM = "SRCLSIG" AND SRTESTCD = "IDRLDIAM" IDRLDIAM_SRCLSIG |
Ο Yes
Ο No
|
| Who provided the skin response measurement information? SREVAL |
Ο INVESTIGATOR
Ο HEALTH CARE PROFESSIONAL
|
| What was the identifier of the evaluator providing the skin response measurement information? SREVALID | _________________ |
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG SR- Skin Response Domain
Examples are not available at this time.
8.3.14 VS - Vital Signs
Description/Overview for the CDASHIG VS - Vital Signs Domain
In CDASH, Vital Signs are measurements including but not limited to blood pressure, temperature, respiration, body surface area, BMI, height, and weight.
Specification for the CDASHIG VS - Vital Signs Domain
Vital Signs (VS)
Assumptions for the CDASHIG VS - Vital Signs Domain
- Vital Signs may be collected using either a normalized or a denormalized horizontal data structure, depending on the functionality of the data management or data capture system being used.
- In a denormalized structure, Vital Signs are collected using a unique variable name for each test, resulting in a wide, horizontal dataset with multiple test results in each record. The SDTMIG VS structure is normalized, using one variable (VSTEST) for the name of the test and one variable (VSORRES) for the collected results, resulting in a vertical data structure in which there is one record for each test/result. CDASH recommendations are intended to facilitate moving denormalized data to the SDTM normalized data structure, because standard transformation programming can be written when the variable naming syntax is consistent and uses CDISC root variables and controlled terminology.
- The set of variables needed for a particular study may include only test result and result unit, or it may include other variables such as location, laterality, position, or method. The examples below show a couple of different ways that denormalized variable names can be created based on the general variable-naming rules of CDASH, using the root variables found in the CDASH Model in combination with the controlled terminology from the Vital Signs Test Code codelist (VSTESTCD) in a consistent syntax.
Example CRFs for the CDASHIG VS - Vital Signs Domain
Example 1
This example shows a vital signs data collection in a denormalized structure with variable names that could be 8 or more characters.
| Date VSDTC VSDAT | _ _ / _ _ _ / _ _ _ _ |
| Time VISTIM VISTIM | _ _ : _ _ |
| Temperature VSORRES WHERE VSTESTCD = "TEMP" TEMP_VSORRES | __ __ __.__ |
| Temperature Unit VSORRESU WHERE VSTESTCD = "TEMP" TEMP_VSORRESU |
Ο C Ο F |
| Respiratory Rate VSORRES WHERE VSTESTCD = "RESP" RESP_VSORRES | __ __ __ |
| Respiratory Rate Unit VSORRESU WHERE VSTESTCD = "RESP" RESP_VSORRESU | breaths/min |
| Systolic Blood Pressure VSORRES WHERE VSTESTCD = "SYSBP" SYSBP_VSORRES | __ __ __ |
| Systolic Blood Pressure Unit VSORRESU WHERE VSTESTCD = "SYSBP" SYSBP_VSORRESU | mmHg |
| Diastolic Blood Pressure VSORRES WHERE VSTESTCD = "DIABP" DIABP_VSORRES | __ __ __ |
| Diastolic Blood Pressure Unit VSORRESU WHERE VSTESTCD = "DIABP" DIABP_VSORRESU | mmHg |
In this example CRF, the following syntax was used to create denormalized variable names: VSTESTCD value_DOMAIN ROOT VARIABLE.
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 2
This example shows a vital signs data collection in a denormalized structure with variable names 8 characters or less.
| Date VSDTC VSDAT | _ _ / _ _ _ / _ _ _ _ |
| Time VISTIM VISTIM | _ _ : _ _ |
| Temperature VSORRES WHERE VSTESTCD = "TEMP" TEMP | __ __ __.__ |
| Temperature Unit VSORRESU WHERE VSTESTCD = "TEMP" TEMPU |
Ο C Ο F |
| Respiratory Rate VSORRES WHERE VSTESTCD = "RESP" RESP | __ __ __ |
| Respiratory Rate Unit VSORRESU WHERE VSTESTCD = "RESP" RESPU | breaths/min |
| Systolic Blood Pressure VSORRES WHERE VSTESTCD = "SYSBP" SYSBP | __ __ __ |
| Systolic Blood Pressure Unit VSORRESU WHERE VSTESTCD = "SYSBP" SYSBPU | mmHg |
| Diastolic Blood Pressure VSORRES WHERE VSTESTCD = "DIABP" DIABP | __ __ __ |
| Diastolic Blood Pressure Unit VSORRESU WHERE VSTESTCD = "DIABP" DIABPU | mmHg |
In this example CRF, the following syntax was used to create denormalized variable names: VSTESTCD value.
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example Data for the CDASHIG VS - Vital Signs Domain
Examples are not available at this time.
8.3.15 FA - Findings About
Description/Overview for the CDASHIG FA - Findings About Domain
The Findings class also includes a sub-type "Findings About" which is used to record findings related to observations in the Interventions or Events class.
Specification for the CDASHIG FA - Findings About Domain
Findings About (FA)
Assumptions for the CDASHIG FA
- The Findings About Events or Intervention domains use the same root variables as the Findings domain with the addition of the --OBJ variable
Example CRFs for the CDASHIG FA - Findings About Domain
Example 1
| Clinical Event Term CETERM | MIGRAINE |
| What was the start date of the migraine? CESTDAT | _ _ / _ _ _ / _ _ _ _ |
| What was the start time of the migraine? CESTTIM CEDTC | _ _ : _ _ |
| Findings About Category (Defaulted) FACAT | MIGRAINE SYMPTOMS |
| What was the severity of the migraine? MRGSEV_FAORRES FAORRES WHERE FATESTCD = "SEV" AND FAOJB = "MIGRAINE" |
Ο Mild
Ο Moderate
Ο Severe
|
| Did sensitivity to light occur with the migraine? SENSLGHT_FAORRES FAORRES WHERE FATESTCD = "OCCUR" AND FAOJB = "SENSITIVITY TO LIGHT" |
Ο No
Ο Yes
|
| Did sensitivity to sound occur with the migraine? SENSOUND_FAORRES FAORRES WHERE FATESTCD = "OCCUR" AND FAOJB = "SENSITIVITY TO SOUND" |
Ο No
Ο Yes
|
| Did nausea occur with the migraine? NAUSEA_FAORRES FAORRES WHERE FATESTCD = "OCCUR" AND FAOJB = "NAUSEA" NAUSEA_FAORRES |
Ο No
Ο Yes
|
| Did aura occur with the migraine? AURA_FAORRES FAORRES WHERE FATESTCD = "OCCUR" AND FAOJB = "AURA" AURA_FAORRES |
Ο No
Ο Yes
|
In this example CRF, the following syntax was used to create denormalized CDASH variable names: "Sponsor abbreviated name"_DOMAIN ROOT VARIABLE.
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Example 2
| Date of Daily Assessment FADAT FADTC | _ _ / _ _ _ / _ _ _ _ |
| Did the subject have the following symptoms of GERD? | |
| Vomiting (Defaulted) FAOBJ | VOMIT_FAORRES FAORRES WHERE FATESTCD="OCCUR" AND FAOBJ="VOMITING" Ο No
Ο Yes
Ο Not Done
|
| What is the volume of the vomit? VOL_FAORRES FAORRES WHERE FATESTCD="VOL" AND FAOBJ="VOMITING" | ______ mL |
| What is the number of episodes? NUMEPISD_FAORRES FAORRES WHERE FATESTCD="NUMEPISD" AND FAOBJ="VOMITING" | _______ |
| What is the maximum severity? SEV_FAORRES FAORRES WHERE FATESTCD="SEV" AND FAOBJ="VOMITING" |
Ο Mild
Ο Moderate
Ο Severe
|
| Diarrhea (Defaulted) FAOBJ | DIAR_FAORRES FAORRES WHERE FATESTCD="OCCUR" AND FAOBJ="DIARRHEA" Ο No
Ο Yes
Ο Not Done
|
| What is the number of episodes? NUMEPISD_FAORRES FAORRES WHERE FATESTCD="NUMEPISD" AND FAOBJ="DIARHEA" | ______ |
| What is the maximum severity? SEV_FAORRES FAORRES WHERE FATESTCD="SEV" AND FAOBJ="DIARRHEA" |
Ο Mild
Ο Moderate
Ο Severe
|
| Nausea (Defaulted) FAOBJ | NAUS_FAORRES FAORRES WHERE FATESTCD="OCCUR" AND FAOBJ="NAUSEA"
Ο No
Ο Yes
Ο Not Done
|
| What is the number of episodes? NUMEPISD_FAORRES FAORRES WHERE FATESTCD="NUMEPISD" AND FAOBJ="NAUSEA" | ______ |
| What is the maximum severity? SEV_FAORRES FAORRES WHERE FATESTCD="SEV" AND FAOBJ="NAUSEA" |
Ο Mild
Ο Moderate
Ο Severe
|
In this example CRF, the following syntax was used to create denormalized CDASH variable names: <DATESTCD>_<ROOT VARIABLE> or <Sponsor Abbreviation>_<ROOT VARIABLE>
This CRF is only an example and is not meant to imply that any particular layout or collection plan is preferable over another.
Annotated CRFs are best understood in conjunction with their respective metadata. Consult the CDASHIG Metadata Table for mapping details.
The example CRFs do not include the Highly Recommended header variables. The population of these values are usually determined by each sponsor's data management system.
Sponsors are responsible for understanding and implementing CDISC Controlled Terminology where applicable.
Appendices
Appendix A: CDASH Contributors
Appendix A1: CDASH Co-Chairs
| Name | Institution/Organization |
|---|---|
| Michael J. Ward | Eli Lilly & Company |
| Lorraine P. Spencer | Takeda Pharmaceuticals |
| Trisha Simpson | UCB |
Appendix A2: CDASH Model and CDASHIG Team Contributors
The following table lists volunteers who actively contributed to the development of the CDASH Model 1.0 and CDASHIG 2.0:
| Name | Institution/Organization |
|---|---|
| Benjamin C. Shim | Eli Lilly & Company |
| Dan Crawford | Accenture |
| Dawn Kaminski | Accenture |
| Deborah Rittenhouse | CSL Behring |
| Éanna Kiely | inVentiv Health, Clinical Division |
| Elizabeth Kelchner | RHO World |
| Gary Walker | Quintiles |
| Guang-Liang Wang | Otsuka |
| Jerry J. Salyers | Accenture |
| Judy Tran | Medidata Solutions |
| Kathleen Mellars | CDISC Fellow |
| Kim Truett | KCT Data Inc. |
| Kit Howard | CDISC |
| Lorraine P. Spencer | Takada Pharmaceuticals |
| Lucinda Winterbourne | Gilead Sciences |
| Melanie Migliore | PRA Health Sciences |
| Melissa Binz | Pfizer |
| Michael J. Ward | Eli Lilly & Company |
| Mikenlette Avent | UCB |
| Natalia Khelmer | Johnson & Johnson |
| Nikki Flores | Gilead Sciences |
| Rachael Zirkle | Eli Lilly & Company |
| Roopa Kandukuri | SOA |
| Tasneem Shahmalak | Chiltern |
| Shannon Labout | CDISC |
| Sharon L. Powell | CDISC Fellow |
| Trisha Simpson | UCB |
Appendix B: Glossary and Abbreviations
The following abbreviations and terms are used in this document. Additional definitions can be found in the CDISC Glossary available at https://www.cdisc.org/standards/semantics/glossary.
| 21 CFR | Title 21 of the Code of Federal Regulations (CFR). Title 21 of the CFR is reserved for rules of the Food and Drug Administration. |
| AE | Adverse event, also refers to the Adverse Events domain |
| ATC code | Anatomic Therapeutic Chemical code from WHO Drug |
| AMIA | American Medical Informatics Association, a Collaborative Group Member |
| ACRO | Association of Clinical Research Organizations, a Collaborative Group Member |
| ACRP | Association of Clinical Research Professionals, a Collaborative Group Member |
| BID | Twice a day (Latin: bis in die) |
| BIO | Biotechnology Industry Organization, a Collaborative Group Member |
| BRIDG | Biomedical Research Integrated Domain Group |
| CDASH | Clinical Data Acquisition Standards Harmonization Project. The name for the project that delivers basic data collection fields. |
| CDISC | Clinical Data Interchange Standards Consortium, a Collaborative Group Member |
| CDM | Clinical Data Management |
| Clinical Database | A repository of the study results data collected in a clinical trial. The format and structure of this repository may vary across sponsors and vendors. |
| Collaborative Group | Group of organizations that support the CDASH project |
| CM | Prior and Concomitant Medications domain |
| CMAX | Concentration maximum; used in pharmacokinetics and bioequivalence testing to indicate maximum plasma concentration for a drug. |
| CO | Comments domain |
| Collected | Within this document collected refers to information that is recorded and/or transmitted to the sponsor. This includes data entered by the site on CRFs/eCRFs as well as vendor data such as core lab data. This term is a synonym for "captured". |
| CRF | Case report form (sometime case record form) A printed, optical, or electronic document designed to record all required information to be reported to the sponsor for each trial subject. |
| CTCAE | Common Terminology Criteria for Adverse Events |
| DA | Drug Accountability domain |
| Databased | To put (data) into a database |
| Dataset | A collection of structured data in a single file |
| Denormalized | The organization of data such that multiple observations (results) are presented in a single row of data. For example, the result values for PULSE, HEIGHT, WEIGHT would be presented in the same row of data with PULSE, HEIGHT, and WEIGHT as column headers. This is also called a horizontal data structure. |
| Derived | Within this document, "derived" refers to information that is not directly entered into the specific data field by the investigator site or by a core lab. This category includes autoencoded data, calculated data, and similar electronically generated data, but not pre-populated fields. |
| DM | Demographics domain |
| Domain | A domain is a collection of data points related by a common topic, such as adverse events or demographics. |
| DS | Disposition domain |
| DV | Protocol Deviations domain |
| eCRF | Electronic Case Report Form |
| EC | The European Commission (formally the Commission of the European Communities) is the executive branch of the European Union. |
| EDC | Electronic Data Capture |
| EG | ECG Test Results domain |
| EMEA | The European Medicines Agency. A decentralized body of the European Union, whose main responsibility is the protection and promotion of public and animal health through the evaluation and supervision of medicines for human and veterinary use. |
| Epoch | Interval of time in the planned conduct of a study. An epoch is associated with a purpose (e.g., screening, randomization, treatment, follow-up), which applies across all arms of a study. |
| EVS | Enterprise Vocabulary Services |
| EX | Exposure domain |
| FAQs | Frequently Asked Questions |
| FDA | Food and Drug Administration Part of the US Department of Health and Human Services Agency. The regulatory authority for all pharmaceuticals (including biologics and vaccines) and medical devices in the US. |
| GCDMP | Good Clinical Data Management Practices (GCDMP). SCDM publication on clinical data management processes |
| GCP | Good Clinical Practice |
| hERG | human Ether-a-go-go Related Gene |
| HITSP | Health Information Technology Standards Panel |
| HL7 | Health Level 7 |
| ICH | International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use |
| ICH E2A | ICH guidelines on Clinical Safety Data Management: Definitions and Standards for Expedited Reporting |
| ICH E2B | ICH guidelines on Clinical Safety Data Management: Data Elements For Transmission Of Individual Case Safety Reports |
| ICH E2C | ICH guidelines on Clinical Safety Data Management: Periodic Safety Update Reports For Marketed Drugs |
| ICH E3 | ICH guidelines on Structure and Content of Clinical Study Reports |
| ICH E4 | ICH guidelines on Dose-response Information to Support Drug Registration |
| ICH E5 | ICH guidelines on Ethnic Factors in the Acceptability of Foreign Clinical Data |
| ICH E6 (R1) | ICH guideline for Good Clinical Practice |
| ICH E9 | ICH guidelines on Statistical Principles for Clinical Trials |
| ICH E11 | ICH guidelines on Clinical Investigation of Medicinal Products in the Pediatric Population |
| ICH E14 | ICH guidelines on the Clinical Evaluation Of QT/QTc Interval |
| IE | Inclusion/Exclusion Criteria Not Met domain |
| IND | Investigational New Drug. IND application required by the US FDA before clinical trials of a new drug or new biological agent may be initiated. |
| IRB | Institutional Review Board. Under FDA regulations, an IRB is an appropriately constituted group that has been formally designated to review and monitor biomedical research involving human subjects |
| ISO 8601 | International Organization for Standardization document of character representation of dates, date/times, intervals, and durations of time |
| JIC | Joint Initiative Council |
| LB | Laboratory Test Results domain |
| MedDRA | Medical Dictionary for Regulatory Activities (new global standard medical terminology designed to supersede other terminologies (such as COSTART and ICD9) used in the medical product development process |
| MH | Medical History domain |
| NA | Not applicable |
| NCI | National Cancer Institute (NIH) |
| NCI EVS | National Cancer Institute (NIH) Enterprise Vocabulary Services |
| NCRR | The National Clinical Research Resources, a Collaborative Group Member |
| NDA | New Drug Application |
| NICHD | The National Institute of Child Health and Human Development, a Collaborative Group Member |
| NIH | National Institutes of Health |
| NLM | National Library of Medicine |
| Normalized | The organization of data such that only one observation (result) is presented per row. For example, PULSE, HEIGHT, WEIGHT would be presented as values in individual rows (with the column header VSTESTCD) with each result presented in another column (same row) called VSORRES. This is also called a vertical data structure. |
| ODM | Operational Data Model. Format for representing the study metadata, study data and administrative data associated with a clinical trial. |
| OTC | Over the counter |
| PE | Physical Examination domain |
| PK | Pharmacokinetics. The study of the absorption, distribution, metabolism, and excretion of a drug. |
| PhRMA | Pharmaceutical Research and Manufacturers Association |
| PRBC | Packed red blood cells |
|
Preprinted (pre-printed) | Items that are part of the original printing on a paper CRF. For example the unit required for a response, such as "years" for an age question. These data may or may not be stored in the database. |
|
Pre-populated (pre-populated) | Items that are part of the eCRF (or data collection device) that are not entered and cannot be modified. (Also see Preprinted). These data are stored in the study database. |
| PRN | As needed (Latin: pro re nata) |
| Protocol Deviation |
A variation from processes or procedures defined in a protocol. Deviations usually do not preclude the overall evaluability of subject data for either efficacy or safety, and are often acknowledged and accepted in advance by the sponsor. Good clinical practice recommends that deviations be summarized by site and by category as part of the report of study results so that the possible importance of the deviations to the findings of the study can be assessed. Compare to Protocol Violation. (See ICH E3). |
| Protocol Violation | A significant departure from processes or procedures that were required by the protocol. Violations often result in data that are not deemed evaluable for a per-protocol analysis, and may require that the subject(s) who violate the protocol be discontinued from the study. Compare to Protocol Deviation. |
| QD | Every day (Latin: quaque die) |
| QID | Four times daily (Latin: quater in die) |
| RCRIM | Regulated Clinical Research Information Management |
| RIM | Reference Information Model |
| SAP | Statistical Analysis Plan |
| SC | Subject Characteristics domain |
| SCDM | Society for Clinical Data Management, a Collaborative Group Member |
| SDS | Submission Data Standards. Also the name of the Team that created the SDTM and SDTMIG |
| SDOs | Standards Development Organizations |
| SDTM | Study Data Tabulation Model |
| SDTMIG | Study Data Tabulation Model Implementation Guide |
| SME | Subject Matter Expert |
| SOCs | System Organ Classes (from MedDRA) |
| Study Treatment | The drug, device, therapy, or process under investigation in a clinical trial which has an effect on outcome of interest in a study: e.g., health-related quality of life, efficacy, safety, pharmacoeconomics. Synonyms: intervention, therapeutic intervention, medical product. |
| SU | Substance Use domain |
| TA | Therapeutic area |
| TID | Three times daily (Latin: ter in die) |
| Uncoded | Not coded. Not having or showing a code. |
| UUID | Universally unique identifier |
| Variable Naming Fragment | A reusable pattern of characters in variable names that convey an equivalent meaning when applied to multiple variable names across classes and domains. |
| VS | Vital Signs domain |
| vs. | Versus; against; in contrast to or as the alternative of |
| WHO | World Health Organization |
| WHO ART | World Health Organization Adverse Reaction Terminology (WHO-ART) has been developed over more than 30 years to serve as a basis for rational coding of adverse reaction terms. |
| WHO DRUG (WHO Drug) | World Health Organization Drug Dictionary |
Appendix C: Revision History
Appendix C1: Changes from CDASH v1.1 to CDASHIG v2.0
Overview of Changes:
The release of the CDASHIG v2.0 entails significant changes from and supersedes prior versions of the CDASH standard. The most significant changes include:
- CDASH Model v1.0 was created to define the underlying structure of the CDASHIG domains with a direct relationship to the SDTM Model.
- CDASH Standard v1.1 and CDASH User Guide v.1.0 were consolidated to create CDASHIG v2.0.
- CDASHIG v2.0 is organized by class and domain to better align with the SDTMIG. Content changes include, but are not limited to:
- Reformatted the domain metadata to spreadsheet format
- Updated attributes for every CDASHIG variable
- Changed the structure of Question Text and Prompt to allow for more flexible implementation (e.g., verb tense, sponsor defined time periods, parameterized topic variables)
- Added CRF Examples for each domain, unless otherwise specified
- Added CDASH Draft Definitions to each of the variables. These are expected to be revised as the CDASH/SDTM Definitions Working Group releases final definitions.
The following table enumerates the changes in greater specificity.
| Classification | Type | Section | Domain | Variable | Description of Change |
| Major | Addition | All | All | All | Migrated source document from Microsoft Word to the CDISC Wiki. |
| Major | Addition | All | All | All | CDASH Standard v1.1 and CDASH User Guide v.1.0 were consolidated to create CDASHIG v2.0. |
| Major | Change | All | All | All | Structured the document so that it better aligns with the SDTMIG. Domains are organized by Class |
| Major | Deprecation | 8.1 | EC | ECPDOSE, ECPDOSU, ECPOCCUR | ECPDOSE, ECPDOSU, and ECPOCCUR were deprecated. |
| Major | Addition | 8 | All | STUDYID, SITEID, SUBJID | Common Identifier Variables were added to each of the Domains. |
| Major | Addition | 8 | All | N/A | Updated existing domains and added the following domains: CE, DD, EC, HO, MI, PC, PR, RP, and SR. |
| Major | Addition | 8 | All | N/A | Added CRF Examples for each domain. |
| Major | Addition | CDASHIG Metadata Table | All | N/A | Changed the structure of the metadata tables to be in Excel. |
| Major | Addition | CDASHIG Metadata Table | All | N/A | Added the following CDASH attributes to every CDASHIG Variable: Domain Class, Data Collection Scenario, Denormalized Options, CDASHIG Variable, DRAFT CDASH Definition, CDASH Core, SDTMIG Target, Mapping Instructions, SDTM Core, Controlled Terminology Codelist Name, Subset Controlled Terminology/CDASH Codelist Name, and Implementation Notes. |
| Major | Deprecation | CDASHIG Metadata Table | All | N/A | Removed the following CDASH Attributes from every CDASHIG Variable: SDTM or CDASH Variable Name, BRIDG, Definition, Codelist, Information for Sponsors, Core, Data Type |
| Major | Addition | CDASHIG Metadata Table | All | All | Changed the structure of Question Text and Prompt to allow for more flexible implementation (e.g., verb tense, sponsor defined time periods, parameterized topic variables). |
| Major | Addition | CDASHIG Metadata Table | All | All | Updated every attribute for every CDASHIG variable. |
| Major | Addition | CDASH Model | All | All | Developed CDASH Model v1.0 to support the CDASHIG and its associated metadata. |
Appendix D: Representations and Warranties, Limitations of Liability, and Disclaimers
CDISC Patent Disclaimers
It is possible that implementation of and compliance with this standard may require use of subject matter covered by patent rights. By publication of this standard, no position is taken with respect to the existence or validity of any claim or of any patent rights in connection therewith. CDISC, including the CDISC Board of Directors, shall not be responsible for identifying patent claims for which a license may be required in order to implement this standard or for conducting inquiries into the legal validity or scope of those patents or patent claims that are brought to its attention.
Representations and Warranties
"CDISC grants open public use of this User Guide (or Final Standards) under CDISC's copyright."
Each Participant in the development of this standard shall be deemed to represent, warrant, and covenant, at the time of a Contribution by such Participant (or by its Representative), that to the best of its knowledge and ability: (a) it holds or has the right to grant all relevant licenses to any of its Contributions in all jurisdictions or territories in which it holds relevant intellectual property rights; (b) there are no limits to the Participant's ability to make the grants, acknowledgments, and agreements herein; and (c) the Contribution does not subject any Contribution, Draft Standard, Final Standard, or implementations thereof, in whole or in part, to licensing obligations with additional restrictions or requirements inconsistent with those set forth in this Policy, or that would require any such Contribution, Final Standard, or implementation, in whole or in part, to be either: (i) disclosed or distributed in source code form; (ii) licensed for the purpose of making derivative works (other than as set forth in Section 4.2 of the CDISC Intellectual Property Policy ("the Policy")); or (iii) distributed at no charge, except as set forth in Sections 3, 5.1, and 4.2 of the Policy. If a Participant has knowledge that a Contribution made by any Participant or any other party may subject any Contribution, Draft Standard, Final Standard, or implementation, in whole or in part, to one or more of the licensing obligations listed in Section 9.3, such Participant shall give prompt notice of the same to the CDISC President who shall promptly notify all Participants.
No Other Warranties/Disclaimers. ALL PARTICIPANTS ACKNOWLEDGE THAT, EXCEPT AS PROVIDED UNDER SECTION 9.3 OF THE CDISC INTELLECTUAL PROPERTY POLICY, ALL DRAFT STANDARDS AND FINAL STANDARDS, AND ALL CONTRIBUTIONS TO FINAL STANDARDS AND DRAFT STANDARDS, ARE PROVIDED "AS IS" WITH NO WARRANTIES WHATSOEVER, WHETHER EXPRESS, IMPLIED, STATUTORY, OR OTHERWISE, AND THE PARTICIPANTS, REPRESENTATIVES, THE CDISC PRESIDENT, THE CDISC BOARD OF DIRECTORS, AND CDISC EXPRESSLY DISCLAIM ANY WARRANTY OF MERCHANTABILITY, NONINFRINGEMENT, FITNESS FOR ANY PARTICULAR OR INTENDED PURPOSE, OR ANY OTHER WARRANTY OTHERWISE ARISING OUT OF ANY PROPOSAL, FINAL STANDARDS OR DRAFT STANDARDS, OR CONTRIBUTION.
Limitation of Liability
IN NO EVENT WILL CDISC OR ANY OF ITS CONSTITUENT PARTS (INCLUDING, BUT NOT LIMITED TO, THE CDISC BOARD OF DIRECTORS, THE CDISC PRESIDENT, CDISC STAFF, AND CDISC MEMBERS) BE LIABLE TO ANY OTHER PERSON OR ENTITY FOR ANY LOSS OF PROFITS, LOSS OF USE, DIRECT, INDIRECT, INCIDENTAL, CONSEQUENTIAL, OR SPECIAL DAMAGES, WHETHER UNDER CONTRACT, TORT, WARRANTY, OR OTHERWISE, ARISING IN ANY WAY OUT OF THIS POLICY OR ANY RELATED AGREEMENT, WHETHER OR NOT SUCH PARTY HAD ADVANCE NOTICE OF THE POSSIBILITY OF SUCH DAMAGES.
Note: The CDISC Intellectual Property Policy can be found at: cdisc_policy_003_intellectual_property_v201408.pdf.