This diagram illustrates the steps that go into assessing the causality of an adverse event.
For certain kinds of adverse events, some steps are almost automatic (e.g., an infectious disease can't happen without a pathogen), but for other kinds of adverse events, there may be many possible causes, and the steps can be quite distinct.
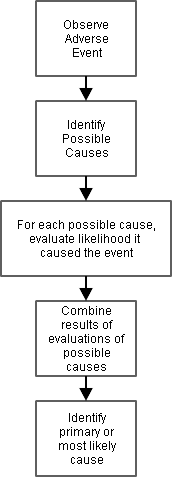
The process includes assessments of individual possible causes as well as summaries of the assessments of individual causes.
The SDTM includes, as standard event variables, assessments of two possible causes for an adverse event.
- The SDTM events class variable --REL is an assessment of the likelihood that study treatment is the cause of the event.
- The SDTM events class variable --RELNST is a description of the likelihood that a non-study treatment is the cause of the event.
However, it can be argued that other outcomes of causality assessments should be represented as findings about the adverse event. The rationale for this approach includes the following:
- Causality assessments may be done by multiple individuals, including the subject. A representation in the FA structure allows for an evaluator to be represented in the record.
- The possible causes considered may be different for different assessors.
- There are a great number of possible causes for adverse events. It would be impractical to create standard variables similar to --REL and --RELNST for all possible causes.