This is an example for the familiar test Temperature. At the simplest level, we have a test with a result performed on a particular date/time.
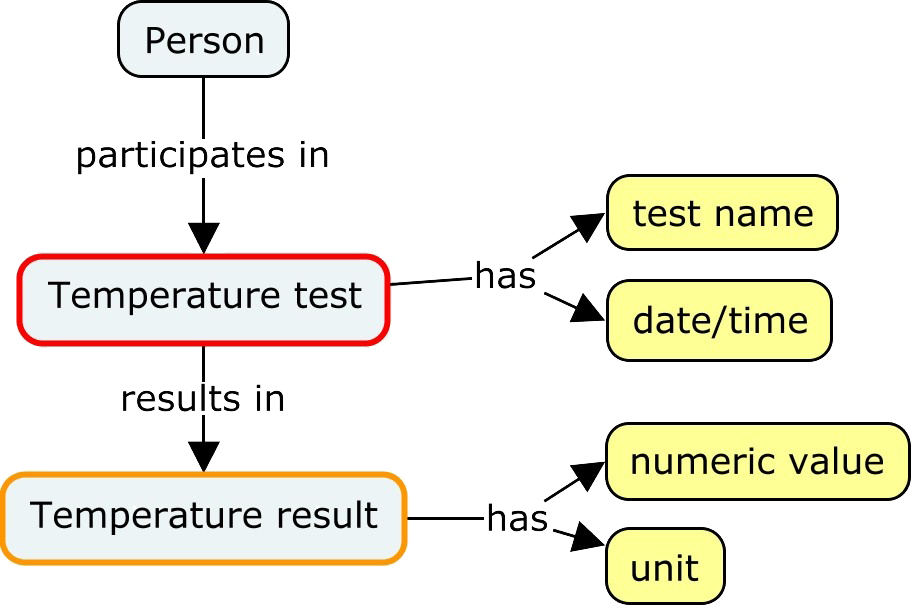
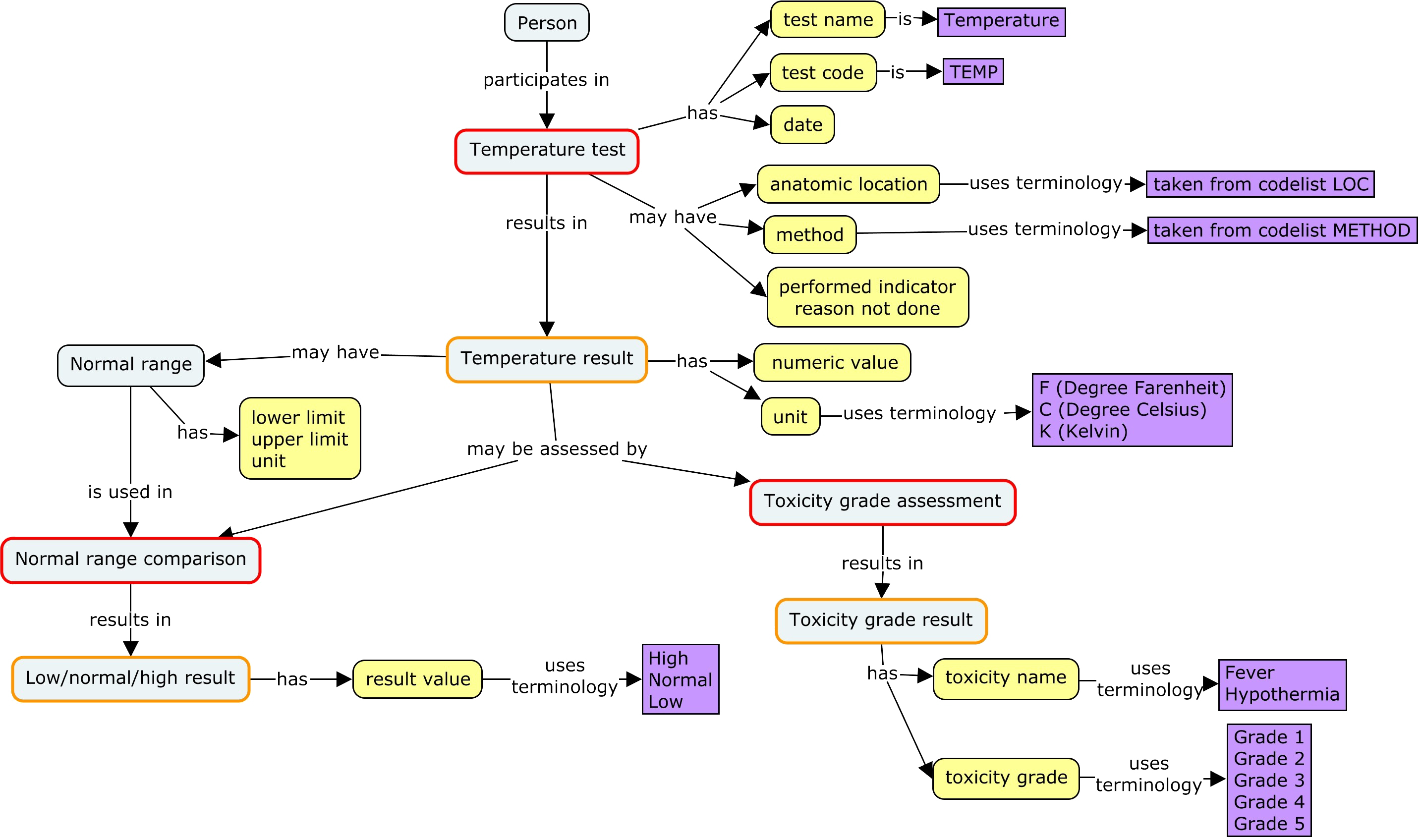
A medical record might include more information, such as
- additional details about the test, such as its anatomic location or method
- an indication that the test was not performed, and why
- comparison of the result to a normal range
- toxicity grading of the result
A structured medical record will use fixed terminology, rather than free text. In this diagram, the terminology is drawn from CDISC controlled terminology and the CTCAE code system.
SDTM was originally designed for data from clinical trials submitted to regulators. This means that the person is not identified by personally identifiable information, but by a study identifier assigned as part of the study in which they participated.
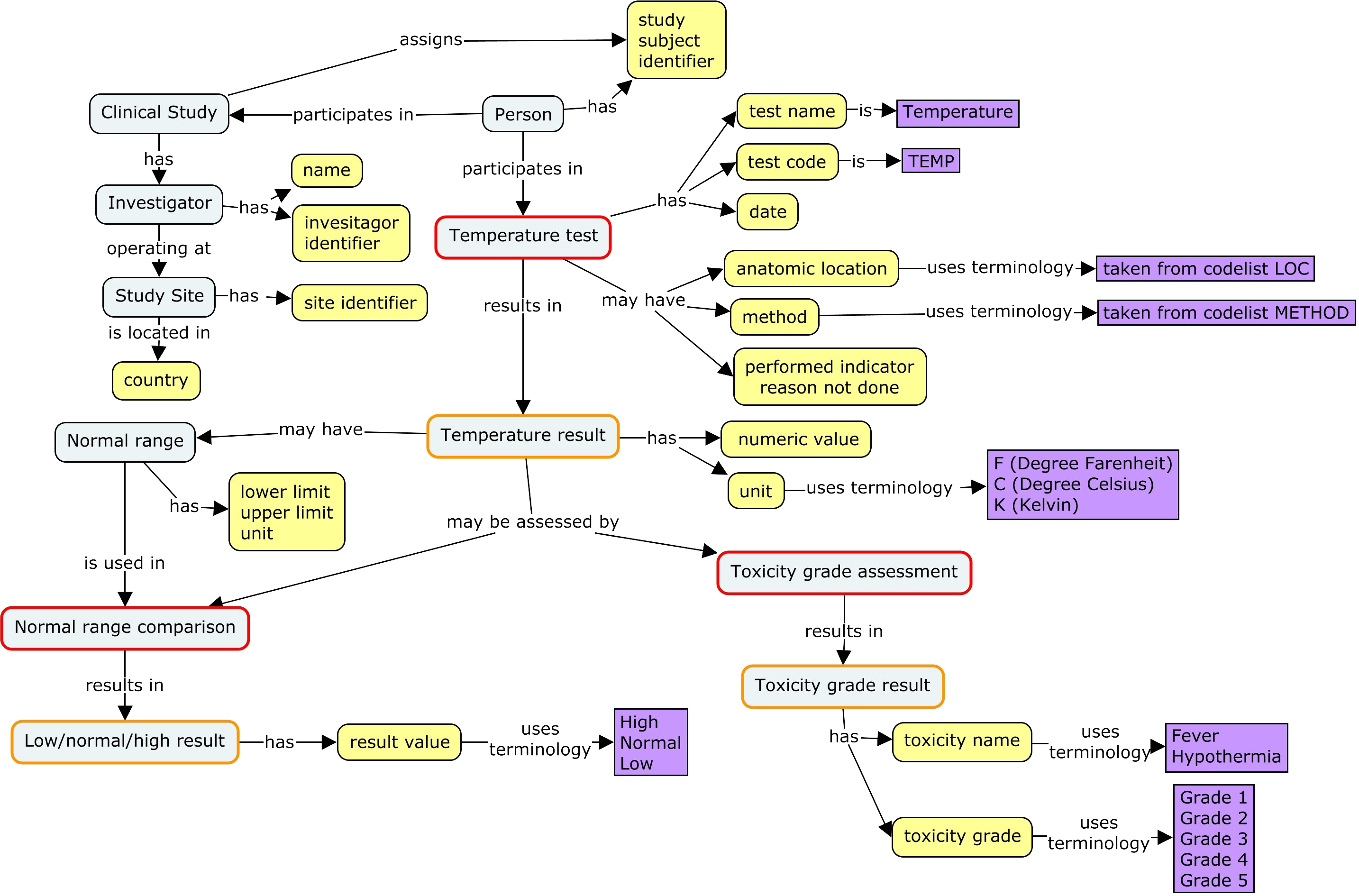
A test conducted for a clinical trial is usually performed according to the study protocol during a particular visit, and may be performed at a particular time point. SDTM timing variables may be used to represent the timing of the test
- As part of a particular epoch of the trial (e.g., screening, treatment, follow-up)
- As part of a visit
- As associated with a time point (e.g., at one of a series of tests performed at predetermined times before and after a study treatment)
- As a "study day", i.e., relative to a study-specified time point, usually the start of study treatment.
- Relative to a "disease milestone", such as a pre-specified adverse event which triggers the gathering of data.