SDTM Courses
Articles
Examples
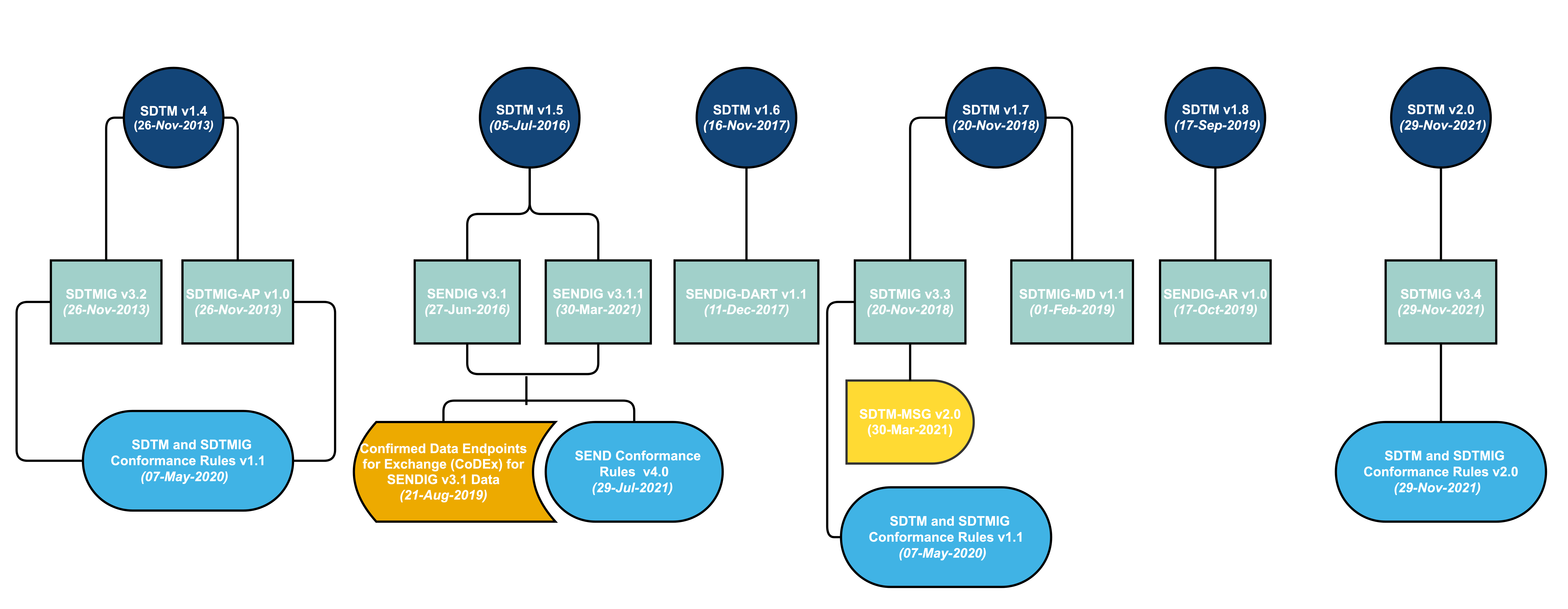
SDTM provides a standard for organizing and formatting data to streamline processes in collection, management, analysis and reporting. Implementing SDTM supports data aggregation and warehousing; fosters mining and reuse; facilitates sharing; helps perform due diligence and other important data review activities; and improves the regulatory review and approval process. SDTM is also used in non-clinical data (SEND), medical devices and pharmacogenomics/genetics studies.
For domains based on a general observation class, determining the SDTM class is the most important modeling decision point. Identifying the appropriate domain is dependent on understanding the general observation class.
The SDTM is a metadata model and SDTMIG domains classified as Interventions, Events, Findings, or Findings About are instantiations of an SDTM general observation class.
- Consistent representation of concepts in all domains in the same general observation class
- Users who become familiar with the SDTM root variable definitions understand a variable's meaning in SDTMIG domains.
- SDTMIG domains based on the same SDTM general observation class can be combined to look across topics (e.g., Medical History, Adverse Events, Clinical Events).
- Data repositories based on the conceptual model support warehousing standard and custom domains.
- Efficient creation of new or custom domains based on an SDTM general observation class
- Data represented in a custom domain can be easily migrated to a newly published domain of the same general observation class.
Every variable must have a clear definition to achieve structural standardization.
To be effective, concept definitions must not be ambiguous.
- A stakeholder who becomes familiar with the SDTM root variables and their definitions should understand their meaning in all IG domains.
- Implementers of IG domains know which variables to use.
- Users of IG domains know where to find data.
- Users of standardized study data should be able to find data without having to understand study-specific data collections or conventions.
Every data element (i.e., clinical study data element, nonclinical endpoint) should have a clear definition to achieve semantic standardization.
- A clinical study data element is a single observation associated with a subject in a clinical study. A data element in an eCRF represents the smallest unit of observation captured for a subject in a clinical investigation (C142437). Examples include birth date, white blood cell count, pain severity measure, and other clinical observations made and documented during a study.
- A nonclinical endpoint is a defined variable intended to reflect an outcome of interest that is analyzed to address a particular research question.
To be effective, concept definitions must not be ambiguous.
- A stakeholder who becomes familiar with CDISC Controlled Terminology should understand the meaning of a value within a record.
- Implementers of IG domains know what values to represent.
- Users of IG domains know what values they will find in the data.
A defined concept (i.e., clinical study data element, nonclinical endpoint) should be represented in the same domain.
Consistency and predictability in the data representation aid in both the development and the review process.
- Data are easy to find using SDTMIG domain definitions, assumptions, and examples.
- Users of standardized study data should be able to find data without having to understand study-specific data collections or conventions.
SDTM domains represent collected or received data that have been standardized to facilitate review and reporting. Standardization must not change the original meaning of the data.
Review is easier and more meaningful when data are in standardized format.
- Facilitates comparison of data collected in different formats
- Supports simple analyses using SDTM datasets
Be mindful of the impact of modeling changes to the user community. Minimize unnecessary or unproductive changes.
Change is costly and disruptive for end users, though some changes are necessary to correct an error/problem or to evolve the standard.
- Supports design stability and usefulness